Since their discovery at the turn of the century by Ramon y Cajal (1), dendritic spines have fascinated scientists by their unusual structure and by their likely role in cognitive functions such as learning and memory. Spines are protrusions from dendrites having diverse shapes that range more than 100-fold in size. Larger spines have proportionately larger synapses and more diversity in subcellular organelles and molecular composition than do smaller spines. These differences in spine morphology and composition might account for differences in the function of the synapses located on them. Existing data suggest that spines are maintained by optimal activation. More spines form when neurons have less excitatory activation, and spines are lost when activation is too high, or when presynaptic axons degenerate (for review, see ref. 2). This pattern suggests that neurons may homeostatically regulate input by means of the number of axospinous synapses.
Recent imaging studies suggest that spines emerge or disappear depending on the age and activity of the neuron. Immature dendrites have more protrusions when the neurons have less global synaptic activation, or when there is more local activation. For example, reduced synaptic transmission results in more dendritic spines on neurons in the lateral geniculate nucleus (refs. 3 and 4; but see also ref. 5). Conversely, localized synaptic activation of cultured neurons can induce outgrowth of dendritic filopodia or spines in the vicinity of the activation (6, 7). In contrast, dendritic spines rapidly shorten and disappear from neurons exposed globally to high synaptic activity (8, 9). Mature dendrites also become increasingly spiny with a global reduction in synaptic activity (10, 11) and are lost from neurons after epileptic seizures (12).
New evidence from Korkotian and Segal (13) points to release of calcium from intracellular stores as a possible modulator of dendritic spine structure. When neurons in culture are exposed to caffeine, calcium is released from the intracellular stores into dendrites and spines (14). Under these conditions, most of the dendritic spines elongated about 33% over the subsequent 1–3 hr. Occasionally a new spine formed or a stubby protrusion developed a spine head.
This and other recent studies highlight both the power and limitations of imaging dendritic spines in culture. Individual dendritic spines are more readily imaged in culture than in acute brain slices (11) or in vivo (15). This imaging advantage is due in part to the relatively low spine density achieved in culture. There are 20 times more spines along dendrites in the adult brain than in culture; compare 4 spines per 20 μm in culture versus >4 spines per 1 μm in vivo (10). The dendrites are also more easily visualized in culture because there are many fewer neurons, glia, and processes to disperse the light. The low density of neurons and glia also means that drugs applied to neurons in culture can often have access to the neurons at a much higher concentration than would normally be obtained in vivo or even in brain slices maintained in vitro.
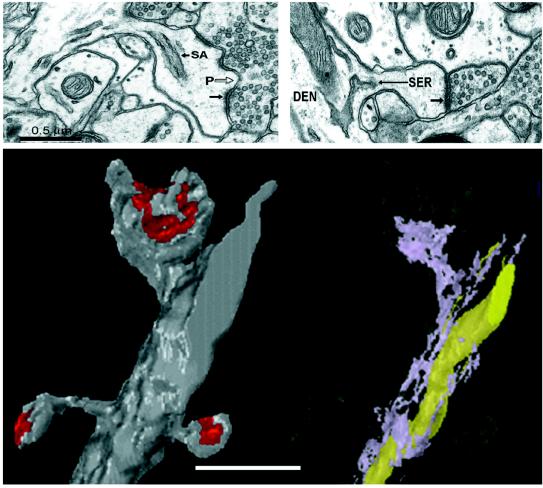
A particularly interesting finding by Korkotian and Segal (13) is that spines on cultured dendrites with a higher spine density were more likely to elongate in response to caffeine than spines on dendrites with a lower spine density. Smooth endoplasmic reticulum (SER) is a key organelle for the regulation of cytosolic calcium. In vivo, SER is in about 50% of dendritic spines (16). The amount of SER in a spine varies in proportion with its size (Fig. 1). About 80% of the large mushroom spines have SER, whereas only 20% of the small thin spines have SER in them. In addition, the amount of SER in the dendrite varies in proportion with the number of spines on the dendrite (16). Thus, caffeine may have a greater effect on spinier dendrites because they have more SER from which to release calcium. Similarly, caffeine might have a more profound effect on dendritic spines of mature neurons because these neurons are much spinier and are thus likely to have more SER. On the other hand, neurons as well as glial cells occurring in the complex intact neuropil might regulate caffeine in vivo, thereby reducing its bioavailability. In fact, it appears that the concentration of caffeine needed to release calcium from intracellular stores in vivo would be toxic, raising the question of whether caffeine can act in vivo through the release of calcium from intracellular stores, or through one of its other effects on synaptic transmission (17).
Figure 1.
Disposition of smooth endoplasmic reticulum (SER) in dendritic spines and dendrites of mature rat hippocampal neurons [modified from Spacek and Harris (16)]. (Upper Left) Electron micrograph through a large mushroom-shaped dendritic spine that has SER entering from the parent dendrite into its neck to form a spine apparatus (SA). The SA has stacks of SER separated by dense-staining fuzz between them. The postsynaptic density (small arrow) is perforated (P) by electron-lucent regions where only the plasmalemma is visible. (Upper Right) This small thin dendritic spine has a tubule of SER that enters its neck on this section and is continuous into the head of the spine on adjacent sections. The postsynaptic density (arrow) on its head is uniform. (Lower Left) A three-dimensional reconstruction of a short dendritic segment illustrating three dendritic spines and their postsynaptic densities (red). (Lower Right) The same dendrite has been rendered translucent and the SER inside is lavender. The top large mushroom spine has a spine apparatus, while the two thin spines have no SER in them. There is also a long mitochondrion (yellow) in the dendrite, which is another internal calcium store in dendrites. (Bar = 1 μm for Lower.)
Another strength of imaging neurons in vitro is that the dynamics of individual dendritic spines can be monitored over several hours. A recent study reports rapid spontaneous movements in spine heads labeled to visualize their actin cytoskeleton (18). By lowering the laser intensity on the confocal microscope, Korkotian and Segal (13) were able to visualize individual dendritic spines for at least 3 hr; however, they report no spontaneous “twitching” of individual dendritic spines in the control cultures. Perhaps visualization of actin is needed to detect rapid movements within the spine cytoskeleton that might not affect overall spine shape, at least on the time scale measured by Korkotian and Segal. Because their control cultures show no apparent spine movements, the slow elongations of dendritic spines after exposure to caffeine are unlikely to be spontaneous events.
It is difficult to know whether individual dendrites are of comparable health. This problem is exemplified by comparing the control dendrites of figure 2A in Korkotian and Segal (13) with those exposed to caffeine in figure 2 B, C, and D. Either caffeine serves to smooth the surface of the dendrites, or the control dendrite selected for this image is in a different state giving its membrane a more ragged appearance. As pointed out by Korkotian and Segal, a reasonably good indicator of dendrite health is the presence of spines and a rather uniform dendrite diameter, which this control dendrite has. When neurons become sick prior to death, the characteristic structural effect is for the dendrites to become varicose, and then to dump their contents, thereby losing their fluorescence. It remains to be determined whether other differences in the characteristics of the plasma membrane, such as the ragged edges, are important indicators of subtle differences in the capacity for spine plasticity.
Another difficulty in assessing spine characteristics while they are living has been that the parent dendrites can move in and out of focus. Even subtle movements can expose dendritic spines or make an individual dendritic spine appear to be elongating, when in fact its full length is being moved into view. Thus, subtle changes in dendrite or spine position might contribute to observed changes in spine shape.
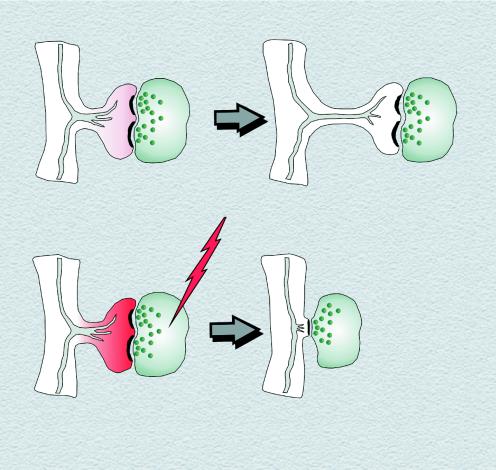
Despite these caveats, the finding that release of calcium from intracellular stores might have a direct effect on dendritic spine structure is especially interesting because changes in spine structure have long been thought to be an important mechanism of memory (19, 20). The increase in the intraspine calcium caused by release from intracellular stores is much less than the increase in the intraspine calcium resulting from high synaptic activity. Thus, a small elevation in intraspine calcium causes a spine to elongate, whereas high intraspine calcium from excessive activity causes a spine to collapse (Fig. 2).
Figure 2.
Model of spine elongation and shortening depending on different levels of intraspine calcium. (Upper) Release of calcium from intracellular stores of SER (gray tubules) leads to a small elevation in calcium (pink), which induces spine neck elongation. (Lower) High activation leads to excessive influx of calcium (red) that may be amplified by the release of calcium from intracellular stores. This high-calcium environment results in actin depolymerization and spine shortening or loss.
Currently, any link between spine elongation and memory is tenuous because it is not understood how spine elongation might influence memory. The main effect was to elongate the spine neck, thereby enhancing the degree of constriction occurring between the spine head (where the synapse is located) and the parent dendrite. This enhanced constriction could serve to sequester calcium and other molecules near the synapse, thereby facilitating subsequent synaptic plasticity. Further study will be needed to determine whether the altered spines permit enhanced synaptic plasticity and whether a direct link between these morphological effects on dendritic spines and memory processes can be established.
Acknowledgments
I thank John Fiala for insightful input on this commentary and Karen Szumowski for preparing the figures. The research from this laboratory is supported by National Institutes of Health Grants NS21184, NS33574, and MH/DA57351, with the latter funded jointly by the National Institute of Mental Health, the National Institutes on Drug Abuse, and the National Aeronautics and Space Administration (K.M.H.).
Footnotes
See companion article on page 12068 in issue 21 of volume 96.
References
- 1.Ramon y Cajal S. Cellule. 1891;7:124–176. [Google Scholar]
- 2.Harris K M. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- 3.Dalva M B, Ghosh A, Shatz C J. J Neurosci. 1994;14:3588–3602. doi: 10.1523/JNEUROSCI.14-06-03588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha M, Sur M. Proc Natl Acad Sci USA. 1995;92:8026–8030. doi: 10.1073/pnas.92.17.8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das S, Sasaki Y F, Rothe T, Premkumar L S, Takasu M, Crandall J E, Dikkes P, Conner D A, Rayudu P V, Cheung W, et al. Nature (London) 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- 6.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 7.Engert F, Bonhoeffer T. Nature (London) 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 8.Segal M. Neurosci Lett. 1995;193:73–76. doi: 10.1016/0304-3940(95)11665-j. [DOI] [PubMed] [Google Scholar]
- 9.Halpain S, Hipolito A, Saffer L. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirov S A, Sorra K E, Harris K M. J Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirov S A, Harris K M. Nat Neurosci. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Lee C L, Smith K L, Swann J W. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korkotian E, Segal M. Proc Natl Acad Sci USA. 1999;96:12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korkotian E, Segal M. Eur J Neurosci. 1998;10:2076–2084. doi: 10.1046/j.1460-9568.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 15.Svoboda K, Denk W, Kleinfeld D, Tank D W. Nature (London) 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- 16.Spacek J, Harris K M. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredholm B B, Battig K, Holmen J, Nehlig A, Zvartau E E. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 18.Fischer M, Kaech S, Knutti D, Matus A. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 19.Moser M B, Trommald M, Egeland T, Andersen P. J Comp Neurol. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Klintsova A Y, Greenough W T. Curr Opin Neurobiol. 1999;9:203–208. doi: 10.1016/s0959-4388(99)80028-2. [DOI] [PubMed] [Google Scholar]