Abstract
OBJECTIVE
Diabetes is a major predictor of death from heart disease and stroke; its impact on nonvascular mortality, including specific cancers, is less understood. We examined the association of diabetes with cause-specific mortality, including deaths from specific cancers.
RESEARCH DESIGN AND METHODS
A prospective cohort of 1,053,831 U.S. adults, without cancer at baseline, enrolled in the Cancer Prevention Study-II in 1982 and was followed for mortality until December 2008. At baseline, participants completed a self-administered questionnaire that included information on diabetes, smoking, physical activity, height, and weight. Multivariable-adjusted relative risks (RRs) (95% CI) were estimated using Cox proportional hazards regression.
RESULTS
During 26 years of follow-up, 243,051 men and 222,109 women died. In multivariable models that controlled for age, BMI, and other variables, diabetes was associated with higher risk of all-cause mortality (women RR 1.90 [95% CI 1.87–1.93]; men 1.73 [1.70–1.75]). Among women, diabetes was associated with higher risk of death from cancers of the liver (1.40 [1.05–1.86]), pancreas (1.31 [1.14–1.51]), endometrium (1.33 [1.08–1.65]), colon (1.18 [1.04–1.33]), and breast (1.16 [1.03–1.29]). Among men, diabetes was associated with risk of death from cancers of the breast (4.20 [2.20–8.04]), liver (2.26 [1.89–2.70]), oral cavity and pharynx (1.44 [1.07–1.94]), pancreas (1.40 [1.23–1.59]), bladder (1.22 [1.01–1.47]), colon (1.15 [1.03–1.29]), and (inversely) prostate (0.88 [0.79–0.97]). Diabetes was also associated with higher risks of death involving the circulatory system, respiratory system, digestive system, genitourinary system, and external causes/accidental deaths.
CONCLUSIONS
Diabetes is associated with higher risk of death for many diseases, including several specific forms of cancer.
The global prevalence of adults with diabetes is projected to reach 366 million in 2030, more than double the estimated prevalence for 2000 (1). Type 2 diabetes is the most frequent form of the disease. Microvascular conditions that affect eyesight, kidney function, and the nervous system are common among adults with prolonged diabetes (2). Men and women with diabetes also experience about twofold higher risks of death from macrovascular disease, including heart disease and stroke (2,3). Nonvascular conditions, such as depression and certain infections, are also more prevalent among adults with diabetes than among adults without diabetes (2). Emerging data support higher risks of death from cancers of the liver, pancreas, and colon among adults with diabetes, while the evidence with other malignancies is equivocal (4–7). Indeed, a recent consensus report sponsored by the American Cancer Society and the American Diabetes Association highlighted the need for large, systematic studies of associations between diabetes and cancer at specific organ sites (8).
In a previous analysis of one million U.S. adults enrolled in the Cancer Prevention Study-II (CPS-II), with mortality follow-up from 1982 through 1998, self-reported diabetes at baseline was associated with higher risks of death from colon, pancreatic, breast (women only), liver (men only), and bladder (men only) cancers (4). In the current analysis, we reassessed the association between diabetes at baseline and cause-specific mortality in CPS-II, with follow-up extended to December 2008, during which time 229,430 additional deaths occurred in the cohort since the end of 1998. The large sample size and extended follow-up period in this analysis allowed for the examination of rarer mortality outcomes, including cancers of the male breast as well as the oral cavity and pharynx. For the first time in this cohort, associations of diabetes with risk of death from noncancer end points are also presented to allow for direct comparisons across a broad spectrum of mortality outcomes.
RESEARCH DESIGN AND METHODS
In 1982, >77,000 volunteers enrolled ~1.2 million study participants into the CPS-II, a prospective cohort study designed to examine risk factors for cancer mortality (9). Study enrollment occurred in all 50 states, the District of Columbia, and Puerto Rico. All study participants were ≥30 years of age at enrollment, when they completed a four-page questionnaire that included information on personal identifiers and demographics, personal and family history of cancer, history of medical conditions, and various lifestyle and body size factors. The questionnaire for women is available online (http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-025728.pdf). The CPS-II study is approved by the Emory University Institutional Review Board.
From among the 1,184,418 men and women enrolled in CPS-II, we excluded from this analysis participants with missing data on height or weight (n = 29,323), BMI (body weight in kilograms divided by the square of height in meters) values <18.5 kg/m2 or >60 kg/m2 (n = 23,515), and prevalent cancer at baseline (n = 77,749). The final analytic cohort comprised 1,053,831 participants (467,143 men and 586,688 women). In analyses where endometrial cancer was the outcome, women who reported having had a hysterectomy were excluded (n = 150,824); similarly, for analyses of ovarian cancer, women who reported a history of hysterectomy or ovarian surgery were excluded (n = 165,142).
Assessment of diabetes and other study variables
Diabetes status was ascertained by a baseline questionnaire item that asked participants to place a check mark next to any diseases or conditions that had been diagnosed by a doctor. Participants were not asked to further describe type of diabetes, duration of disease, age at diagnosis, diabetes treatment(s), or severity of symptoms and complications.
As previously reported (10), the validity of self-reported diabetes in the CPS-II Nutrition Cohort (whose members were a subset of the earlier CPS-II baseline cohort, used in the current analysis) was assessed by reviewing medical records—primarily clinical notes—from a sample of colorectal cancer case patients. Medical records of 98 colorectal cancer case patients (49 with and 49 without self-reported type 2 diabetes) were reviewed by an investigator (P.T.C.) who was blinded to self-reported diabetes status. Of the 98 records selected, 80 were deemed usable (12 were excluded because of illegible hand writing on the medical record; 6 were excluded because the medical record only contained a pathology report). Self-reported diabetes was in good agreement with data extracted from medical records; of the 46 self-reports of diabetes from the questionnaires, 38 were confirmed by review of medical records (83%). The remaining eight self-reports of type 2 diabetes could have been either errors in self-reports or accurate self-reports that were not documented in the limited medical records available. Of the 34 participants who self-reported not having diabetes from the questionnaires, none of the medical records indicated a history of diabetes. Thus, the overall agreement between self-reports and medical records was 90%.
Other variables important to this analysis, ascertained from the baseline questionnaire, included age at enrollment and sex; body height and weight (used to estimate BMI); smoking history (age started, age stopped, cigarettes per day, and years smoked); highest level of education; amount of physical activity during work and leisure time; aspirin intake in the last month (frequency and duration in years); usual daily intake of beer, wine, and hard liquor (number of drinks per day and duration in years); and average weekly dietary intakes of red and processed meats (i.e., beef, pork, ham, hamburger, liver, sausage, bacon, and smoked meat) and vegetables (i.e., carrots, tomatoes, squash/corn, green leafy vegetables, raw vegetables, and cabbage/broccoli/brussels sprouts). The questionnaire for women also requested information on parity, age at menarche, menopausal status, postmenopausal hormone use, oral contraceptives, and age at first birth.
Mortality outcomes
Cause of death has been obtained for 99.3% of all known deaths in CPS-II. From the time of enrollment in 1982 to September 1988, cause of death was ascertained by personal inquiries made by volunteers to participants in 1984, 1986, and 1988; deaths during this period were confirmed by acquisition of medical records and/or death certificates. After September 1988, and up to 31 December 2008, underlying cause of death was ascertained by biennial linkage with the National Death Index, shown previously in this cohort to have high specificity and sensitivity compared with manual review of death certificates and records (11). By 31 December 2008, 46.0% of participants in CPS-II had died, 53.8% were still alive, and 0.2% were excluded from follow-up on 1 September 1988 because of insufficient data for National Death Index linkage.
The end point in this analysis is the single underlying cause of death, identified on the death certificate, and classified according to ICD-9 revision nosology (12). Contributing causes of death are not considered here. Specific outcomes of interest to this analysis were deaths from all cancers combined and deaths from specific cancer sites that accounted for at least 10 deaths among participants with diabetes: oral cavity or pharynx, esophagus, stomach, colon, rectum, liver, gallbladder, pancreas, larynx (men only), lung, connective tissue, melanoma, other skin (men only), male and female breast, endometrium, cervix, ovary, prostate, bladder, kidney or other urinary organs, brain or nervous system, lymphoma, multiple myeloma, and leukemia. Data regarding cancer stage, treatment, subsite, and histology were not available.
Noncancer causes of death were defined as deaths involving the circulatory system, respiratory system, digestive system, genitourinary system, external or accidental deaths, ill-defined causes, and other specific causes of death. Within each of these broader categories, more specific mortality outcomes that accounted for at least 10 deaths among participants with diabetes are also presented.
Statistical analysis
Age-adjusted and multivariable-adjusted relative risks (RRs) (95% CI) were calculated using Cox proportional hazards regression to estimate associations. Follow-up time began on the date of enrollment in 1982 and ended on the date of death or 31 December 2008—whichever came first. Participants who did not report having diabetes at baseline were the referent group. Models were adjusted for age at enrollment, in single years, by using the stratified Cox procedure with 1-year age strata. In addition to age, the multivariable-adjusted models included BMI (18.5 to <22.5, 22.5 to <25, 25 to <27.5, 27.5 to <30, 30 to <32.5, 32.5 to <35, 35 to <37.5, 37.5 to <40, and ≥40 kg/m2), education (less than high school, high school graduate, some college or vocational school, college graduate, graduate school, or unknown), physical activity (none, slight, moderate, heavy, or unknown), current use of aspirin (none, occasional, <15 times per month, 15 to <30 times per month, ≥30 times per month, or unknown), alcohol use (nondrinker, <1 drink per day, 1 drink per day, >1 drink per day, former drinker, or unknown), smoking status (never, current, former, ever cigar/pipe and never cigarette, or unknown), vegetable intake (servings per week: 0 to 10, >10 to 14, >14 to 18, >18 to 21, >21, or unknown), and red or processed meat intake (servings per week: 0 to 3, >3 to 5, >5 to 7, >7 to 9, >9, or unknown). More detailed categories of smoking status, derived from duration and smoking-intensity variables, made no material difference to the risk estimates, so we opted for the simpler covariable in this analysis. In sensitivity analyses among women, we also included parity, age at menarche, menopausal status, postmenopausal hormones, oral contraceptives, and age at first birth into each of the multivariable-adjusted models for the cancer-specific outcomes. These additional variables made no substantive differences to any of the risk estimates for any of the cancer-specific outcomes among women (<2%), so none of the women-specific variables were included in the final models. Similarly, when pre- and perimenopausal women were excluded in additional sensitivity analyses, the results again were not materially different. We did not adjust for prevalent health conditions (e.g., hypertension), except in sensitivity analysis, in order to avoid controlling for conditions that are intermediates on the causal pathway between diabetes and mortality. All results are presented separately by sex.
Life expectancy was calculated from adjusted survival curves using the corrected group prognosis method (13). This method first estimates survival curves for each level of a given adjustment variable, using Cox proportional hazards regression, and then estimates an average weighted survival curve from the group of individual survival curves. Coefficients from the average weighted survival models were then used to estimate life expectancy among study participants with and without diabetes; the difference between life expectancies in these two groups reflects potential years of life lost from diabetes, adjusted for covariables.
RESULTS
Participant characteristics at baseline
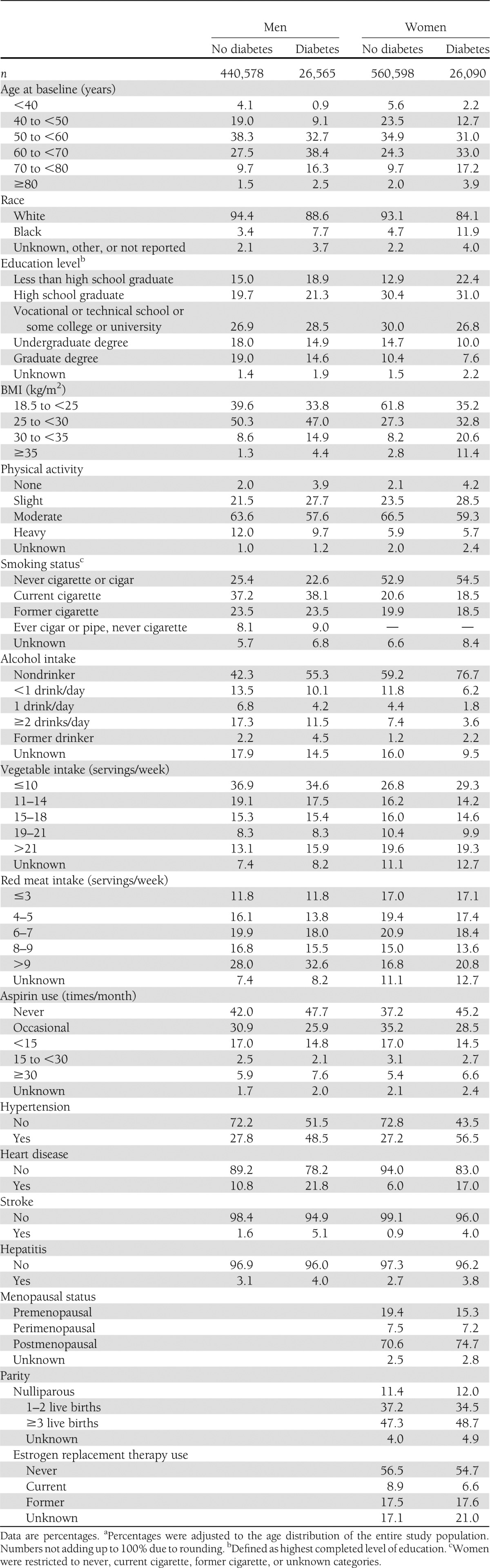
Baseline characteristics of the study participants are shown in Table 1 by sex and diabetes status. Overall, 5.7% of men and 4.4% of women reported having diabetes at baseline. Participants with diabetes were older at baseline and were more likely to report their race as nonwhite, although most (i.e., >90%) study participants were white. Age-adjusted frequencies showed that men and women with diabetes reported less education, higher BMI, less exercise, and less alcohol intake and were more likely to self-report hypertension, heart disease, and stroke.
Table 1.
Age-adjusted percentagesa of baseline characteristics by diabetes status and sex in the CPS-II cohort, 1982–2008
Diabetes and mortality
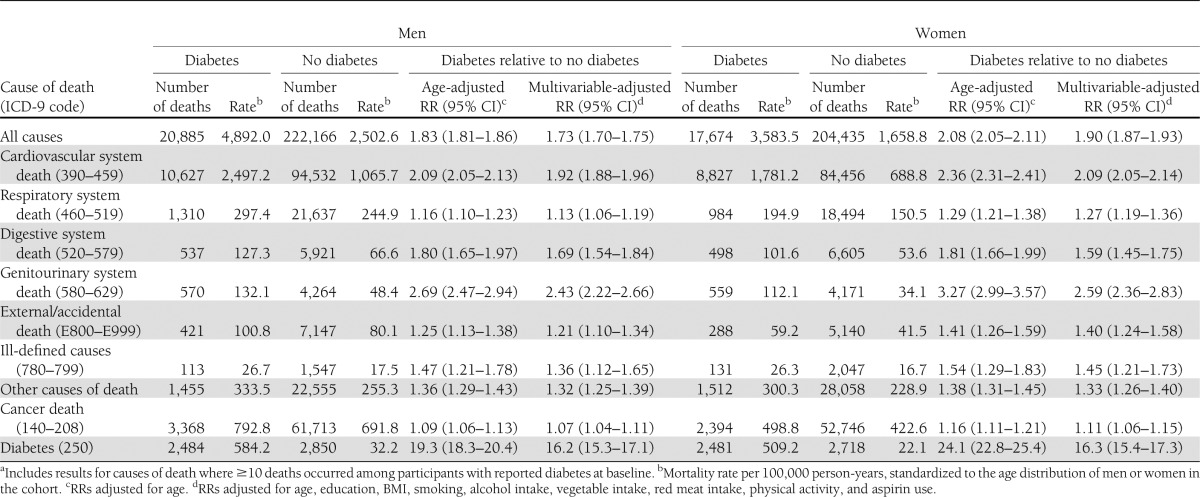
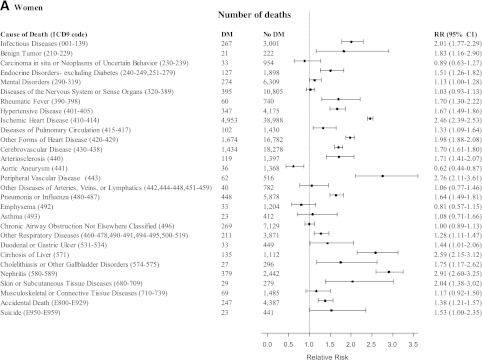
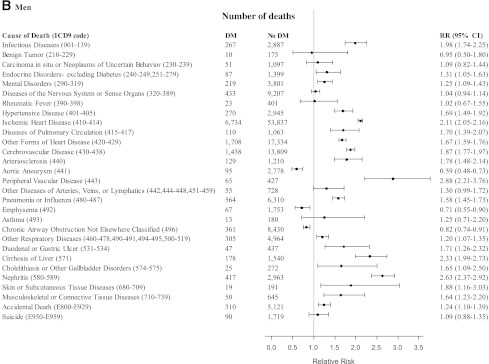
As shown in Table 2, diabetes was statistically significantly associated with higher risks of death involving all causes, all cancers, the cardiovascular system, the respiratory system, the digestive system, the genitourinary system, external causes and accidents, ill-defined causes, and all other specific causes of death. Fig. 1A and B describe, in further detail, the associations of diabetes with more specific noncancer mortality outcomes: RRs >2 were observed among women and men for the association of diabetes with risk of death from infectious diseases (men RR 1.98), ischemic heart disease, peripheral vascular disease, cirrhosis of the liver, nephritis, and skin or subcutaneous tissue diseases among women. Diabetes was associated with lower risks of death from aortic aneurysm, emphysema (men only), and chronic airway obstruction—not otherwise classified (men only).
Table 2.
Diabetes and risk of mortalitya: the CPS-II cohort, 1982–2008
Figure 1.
RRs (95% CI) for deaths from noncancer outcomes comparing female (A) and male (B) participants with diabetes (DM) with female and male participants without diabetes (No DM) at baseline, adjusting for age, education, BMI, smoking, alcohol intake, vegetable intake, red meat intake, physical activity, and aspirin use, in the CPS-II, 1982–2008. (Continued on p. 1841.)
After exclusion of participants with prevalent hypertension, heart disease, and stroke at baseline, associations of diabetes with all-cause mortality (women RR 1.85 [95% CI 1.79–1.90]; men 1.70 [1.66–1.74]) and cardiovascular disease mortality (women 2.09 [2.00–2.18]; men 1.94 [1.87–2.01]) were not materially different, still suggesting higher risks. RRs stratified by follow-up period (1982–1994 and 1995–2008) are shown in the online Supplementary Data (Supplementary Tables 1 and 2); generally, RRs in the latter period were attenuated compared with the earlier period, perhaps reflecting higher background mortality rates as the cohort aged.
Diabetes and cancer-specific mortality
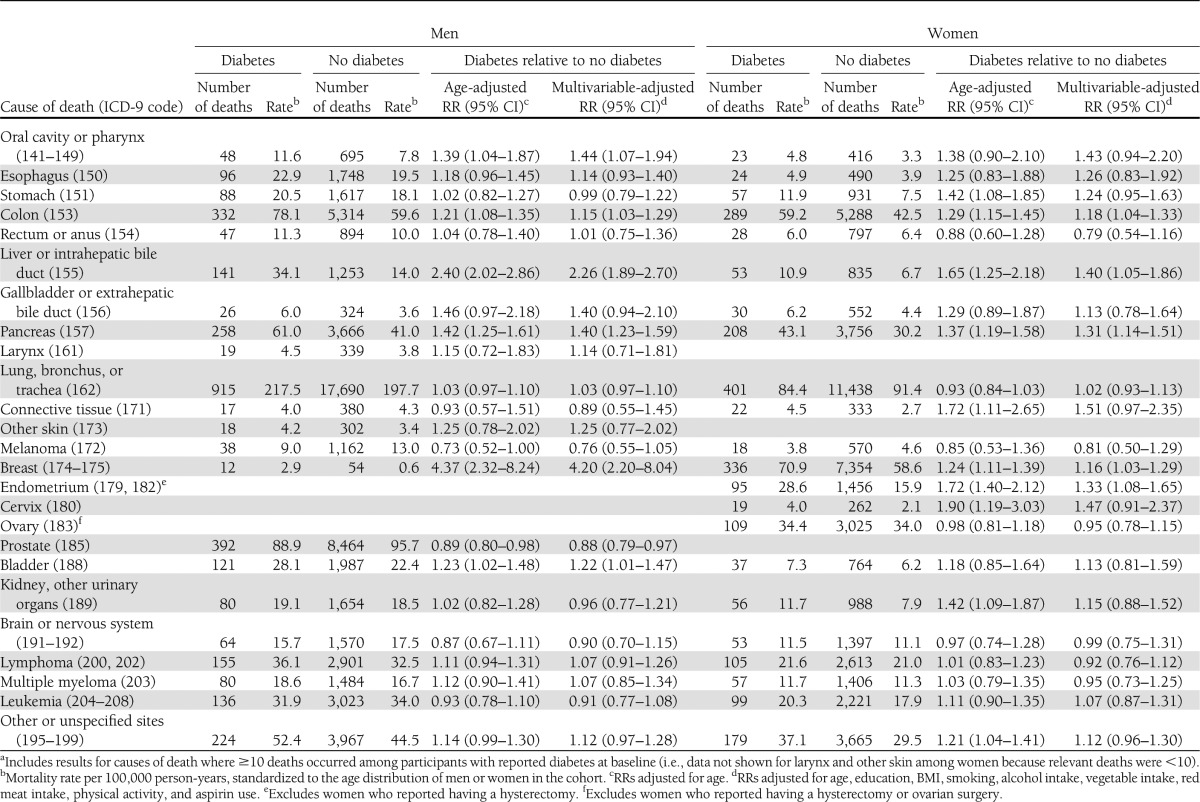
Among women, diabetes was associated with higher risk of death from cancers of the liver (40%), pancreas (31%), endometrium (33%), colon (18%), and breast (16%) (Table 3). Among men, diabetes was associated with higher risk of death from cancers of the breast (320%), liver (126%), oral cavity and pharynx (44%), pancreas (40%), bladder (22%), and colon (15%) (Table 3). Diabetes was associated with lower risk of death from prostate cancer (12%).
Table 3.
Diabetes and risk of cancer-specific mortalitya: the CPS-II Cohort, 1982–2008
Associations between diabetes and pancreatic cancer mortality were attenuated, but still suggested higher risks, after excluding the first 5 years (women RR 1.22 [95% CI 1.04–1.43]; men 1.33 [1.15–1.54]) and 10 years (women 1.12 [0.93–1.35]; men 1.28 [1.08–1.53]) of follow-up.
Impact of diabetes on potential years of life lost
We estimated the impact of diabetes on potential years of life lost. After adjustment for BMI and other important lifestyle factors, 50-year-old women and men with diabetes were expected to die an average of 5.1 and 5.3 years earlier than women and men without diabetes, respectively.
CONCLUSIONS
In this study of one million U.S. adults followed for up to 26 years, diabetes was associated with an almost doubling in the risk of all-cause mortality: men or women with diabetes at age 50 years were estimated to die an average of 5 years earlier than men and women without the disease. Importantly, this study included information on risk factors that are common to both diabetes and cancer (and other chronic diseases), including BMI, physical activity, cigarette smoking, alcohol, and diet. In a previous analysis of this cohort, diabetes was associated with higher risks of death from cancer of the colon, pancreas, breast (women only), liver (men only), and bladder (men only) (4). In this analysis, follow-up time was extended by 10 years, during which time 229,430 additional deaths occurred in the cohort. This updated analysis replicated all of the earlier findings and newly identified associations of diabetes with deaths from cancers of the breast (men), liver (women), endometrium, oral cavity and pharynx (men only), and prostate (inversely). For the first time in this cohort, we also present associations of diabetes with noncancer end points to allow for direct comparisons of RRs across a broad spectrum of mortality outcomes.
Cancers of the liver and pancreas are exceedingly fatal, with 5-year relative survival rates of 14 and 6%, respectively (14). Higher risks of these malignancies are often observed among men and women with diabetes (4–7), consistent with findings from this study. Liver is a target tissue for insulin, and the organ plays a central role in energy homeostasis, including glycogen storage. A recent meta-analysis of 25 prospective studies reported a twofold higher risk of liver cancer among men and women with diabetes (15); risk estimates persisted after controlling for alcohol intake, cirrhosis, and viral hepatitis B or C infection. Diabetes has been suggested as both a sequela and a cause of pancreatic cancer. A recent meta-analysis of 35 cohort studies reported that summary RRs of pancreatic cancer risk varied from 5.38 with <1 year of diabetes duration to 1.83 with >5 years of diabetes duration (16). Among patients without self-reported diabetes, postload plasma glucose was associated with higher risk of pancreatic cancer mortality; associations persisted after exclusion of the first 5 years of follow-up (17). Similarly, even after exclusion of the first 5 and 10 years of follow-up, results from this study suggested a higher risk for pancreatic cancer mortality among men and women with diabetes at baseline. Taken together, these data suggest that diabetes may be an early indicator of pancreatic cancer in some patients; however, diabetes and its related biomarkers are convincingly also associated with the etiology of pancreatic cancer.
Cancers of the lung, colorectum, female breast, and prostate comprise the four most common malignancies in the U.S. (14). Consistent with other prospective studies (6,18) and the conclusion from an expert report (8), we did not observe an association between diabetes and lung cancer mortality. Diabetes is consistently associated with higher risk of colorectal cancer incidence, although associations sometimes vary when stratified by subsite in the colorectum (19,20) and by sex (10,21). Studies of diabetes and colorectal cancer mortality are rarer (5–7); however, these studies are generally consistent with our findings of a higher risk of colon cancer mortality. A meta-analysis of five prospective studies suggested a 24% higher risk of breast cancer mortality among women with diabetes (22), which is also consistent with our findings.
Male breast cancer is exceptionally rare, and little is known about its etiology. A recent prospective study of 325,000 men suggested no association between diabetes and male breast cancer incidence (23), while two earlier case-control studies (24,25) and an administrative database study (26) suggested higher risk estimates, ranging from 1.3 to 2.6, of breast cancer incidence among men with diabetes. Thus, our finding of an RR of 4.20 for breast cancer mortality among men with diabetes is particularly interesting but warrants replication in other large studies.
We observed a lower risk of prostate cancer mortality for men with diabetes in this study, consistent with other primary incidence studies (27,28) and meta-analyses of prostate cancer incidence (29). The mechanisms to explain this inverse association are not known; however, observations from other studies that long-duration—but not short-duration—diabetes is associated with lower risk of prostate cancer incidence suggest that reductions in testosterone, or alterations in other steroid hormones, which occur with long-term diabetes (30), might be relevant to prostate cancer etiology.
Men with diabetes had a higher risk of bladder cancer mortality in this study, consistent with recent studies (5–7). Only one of these other studies stratified associations by sex; there was a higher risk of bladder cancer mortality among men, but results among women were not presented (5). Cigarette smoking is the primary risk factor for bladder cancer (31) and a weak risk factor for diabetes (32); thus, inclusion of smoking data in this study is a strength. We also observed a higher risk of oral cavity and pharyngeal cancer mortality among men with diabetes. To date, this relationship is understudied, although one recent study reported higher risk of head and neck cancer mortality among men and women who had diabetes, although the association was not statistically significant (7). Similar to bladder cancer, the main risk factor for oral cavity and pharyngeal cancer is cigarette smoking.
Endometrial cancer has a relatively good prognosis, with a 5-year relative survival rate of 84% (14). Thus, sufficiently large studies of endometrial cancer mortality and diabetes are rare (33), although a previous study suggested lower survival among women with diabetes after endometrial cancer diagnosis (34) and an earlier study from CPS-II also suggested higher risk of endometrial cancer mortality, although the result was not statistically significant (4). Critically, both of these earlier studies (4,34) and the current study were able to control for BMI, a major risk factor for endometrial cancer incidence (35) and mortality (36).
Macrovascular conditions, including heart disease, stroke, and peripheral vascular disease, are well-understood sequelae of diabetes (3,37). Our findings of higher risks of death from ischemic heart disease, arteriosclerosis, cerebrovascular disease, and peripheral vascular disease are supportive of these conclusions. We also noted higher risks of death from pneumonia or influenza, immune disorders, several digestive system diseases, and nephritis, consistent with the higher prevalence of impaired immunity, liver disease, and nephropathy among patients with advanced diabetes (2). Although speculative, our finding that diabetes was associated with higher risk of death from external causes and accidents might relate to reduced vision, slower reaction time, and decreased motor control or to acute complications from episodes of dysglycemia or ketoacidosis, as also previously reported (7). The lower risks of death from aortic aneurysm, emphysema (men only) and chronic airway obstruction not elsewhere classified (men only) among participants with diabetes in this study may relate to higher postenrollment smoking cessation among participants with diabetes than among study participants without diabetes.
Our study has several limitations, including the lack of data on diabetes type, treatment(s), duration, and severity of symptoms. However, given the age range of participants in CPS-II, the vast majority of participants with diabetes would be expected to have the type 2 form of the disease. Because we did not send follow-up questionnaires to participants in the baseline CPS-II cohort, we were unable to update diabetes status. Additionally, we relied on self-reported diabetes status, a potential source of misclassification; other studies, however, have suggested that self-reports are in good agreement with medical records, including results from CPS-II Nutrition (10), and direct measures of fasting glucose (38). Nonetheless, results from this study likely underestimate the true association between diabetes and cause-specific mortality, since we were generally more likely to misclassify participants with diabetes at the time of their death as not having diabetes than to misclassify in the opposite direction. While the prevalence of self-reported diabetes at baseline in 1982 in this study (women 4.4%; men 5.7%) is low compared with current diabetes prevalence estimates in the U.S. and other developed countries, these percentages are very comparable with National Health and Nutrition Examination Survey II (1976–1980) estimates of diagnosed (3.4%) and total (undiagnosed and diagnosed) (5.3%) diabetes from around the same period (39). Lastly, because the outcomes in this study are based on mortality data, they reflect the combined influence of diabetes on disease incidence and survival. Additional studies are warranted to quantify the specific, and potentially distinct, impacts of diabetes on cancer incidence versus cancer survival. In this context, we have recently shown that diabetes is associated with higher risk of colorectal cancer incidence among men but not women (10) and that diabetes was further associated with poorer prognosis among both men and women with colorectal cancer (40).
Specific strengths of this study include the large sample size and extended follow-up period, which allowed for examination of deaths from relatively rare cancer outcomes, including the oral cavity and pharynx, endometrium, and male breast. Other strengths of this study are the inclusion of important confounding lifestyle and demographic factors.
In conclusion, the broad range of deaths associated with diabetes in this study reflects the extensive nature of the disease. These findings support the need for coordinated, multidisciplinary care of men and women with diabetes, including age-appropriate cancer screening and early detection.
Acknowledgments
The American Cancer Society funds the creation, maintenance, and updating of the CPS-II Cohort. All of the authors were employed by the American Cancer Society during the course of the study.
Staff in the Epidemiology Research Program of the American Cancer Society designed and conducted the study, including collection, analysis, interpretation, and presentation of the manuscript. No staff at the American Cancer Society, other than study investigators, reviewed or approved the manuscript.
No potential conflicts of interest relevant to this article were reported.
P.T.C. developed the study concept and design, acquired data, analyzed and interpreted data, drafted the manuscript, performed statistical analysis, obtained funding, and supervised the study. C.C.N. developed the study concept and design, drafted the manuscript, analyzed and interpreted data, and performed statistical analysis. A.V.P. acquired data, analyzed and interpreted data, critically revised the manuscript for important intellectual content, and obtained funding. E.J.J. acquired data, analyzed and interpreted data, critically revised the manuscript for important intellectual content, performed statistical analysis, and obtained funding. S.M.G. acquired data, analyzed and interpreted data, critically revised the manuscript for important intellectual content, obtained funding, and supervised the study. P.T.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0002/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 3.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004;159:1160–1167 [DOI] [PubMed] [Google Scholar]
- 5.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005;293:194–202 [DOI] [PubMed] [Google Scholar]
- 6.Lam EK, Batty GD, Huxley RR, et al. Asia Pacific Cohort Studies Collaboration Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol 2011;22:730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin 2010;60:207–221 [DOI] [PubMed] [Google Scholar]
- 9.Stellman SD, Garfinkel L. Smoking habits and tar levels in a new American Cancer Society prospective study of 1.2 million men and women. J Natl Cancer Inst 1986;76:1057–1063 [PubMed] [Google Scholar]
- 10.Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010;139:1138–1146 [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among cancer prevention study II participants. Am J Epidemiol 1993;137:235–241 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization International Classification for Diseases. 9th ed. Geneva, World Health Org., 1985 [Google Scholar]
- 13.Ghali WA, Quan H, Brant R, et al. APPROACH (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease) Investigators Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA 2001;286:1494–1497 [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 2012;130:1639–1648 [DOI] [PubMed] [Google Scholar]
- 16.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer 2011;47:1928–1937 [DOI] [PubMed] [Google Scholar]
- 17.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000;283:2552–2558 [DOI] [PubMed] [Google Scholar]
- 18.Zhou XH, Qiao Q, Zethelius B, et al. DECODE Study Group Diabetes, prediabetes and cancer mortality. Diabetologia 2010;53:1867–1876 [DOI] [PubMed] [Google Scholar]
- 19.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol 2011;106:1911–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limburg PJ, Anderson KE, Johnson TW, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev 2005;14:133–137 [PubMed] [Google Scholar]
- 21.Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol 2006;101:1872–1879 [DOI] [PubMed] [Google Scholar]
- 22.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–862 [DOI] [PubMed] [Google Scholar]
- 23.Brinton LA, Richesson DA, Gierach GL, et al. Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst 2008;100:1477–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DB, Jimenez LM, McTiernan A, et al. Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol 1992;135:734–748 [DOI] [PubMed] [Google Scholar]
- 25.Ewertz M, Holmberg L, Tretli S, Pedersen BV, Kristensen A. Risk factors for male breast cancer—a case-control study from Scandinavia. Acta Oncol 2001;40:467–471 [DOI] [PubMed] [Google Scholar]
- 26.Brinton LA, Carreon JD, Gierach GL, McGlynn KA, Gridley G. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical care system database. Breast Cancer Res Treat 2010;119:185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer 2009;124:1398–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez C, Patel AV, Mondul AM, Jacobs EJ, Thun MJ, Calle EE. Diabetes and risk of prostate cancer in a prospective cohort of US men. Am J Epidemiol 2005;161:147–152 [DOI] [PubMed] [Google Scholar]
- 29.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2056–2062 [DOI] [PubMed] [Google Scholar]
- 30.Rice D, Brannigan RE, Campbell RK, Fine S, Jack L Jr, Nelson JB, et al. Men's health, low testosterone, and diabetes: individualized treatment and a multidisciplinary approach. Diabetes Educ 2008;34(Suppl. 5):97S–112S [DOI] [PubMed]
- 31.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laaksonen MA, Knekt P, Rissanen H, et al. The relative importance of modifiable potential risk factors of type 2 diabetes: a meta-analysis of two cohorts. Eur J Epidemiol 2010;25:115–124 [DOI] [PubMed] [Google Scholar]
- 33.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia 2007;50:1365–1374 [DOI] [PubMed] [Google Scholar]
- 34.Folsom AR, Anderson KE, Sweeney C, Jacobs DR., Jr Diabetes as a risk factor for death following endometrial cancer. Gynecol Oncol 2004;94:740–745 [DOI] [PubMed] [Google Scholar]
- 35.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578 [DOI] [PubMed] [Google Scholar]
- 36.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–1638 [DOI] [PubMed] [Google Scholar]
- 37.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes—a review. Diabet Med 2010;27:4–14 [DOI] [PubMed] [Google Scholar]
- 38.Margolis KL, Lihong Qi, Brzyski R, et al. Women Health Initiative Investigators Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA 2005;293:1868–1874 [DOI] [PubMed] [Google Scholar]
- 40.Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 2012;30:53–59 [DOI] [PubMed] [Google Scholar]