Abstract
OBJECTIVE
The aim of this study was to evaluate HbA1c as an alternative criterion for impaired glucose tolerance (IGT) or type 1 diabetes (T1D) in high-risk subjects <21 years of age.
RESEARCH DESIGN AND METHODS
Subjects <21 years of age who participated in the prospective DPT-1, TEDDY, TRIGR, and Type 1 Diabetes TrialNet Natural History (TrialNet) studies and had an HbA1c within 90 days of an OGTT with a 2-h plasma glucose (2-hPG) measure were included. An OGTT of 140–199 mg/dL defined IGT, and an OGTT with 2-hPG ≥200 mg/dL or fasting plasma glucose ≥126 mg/dL defined diabetes. HbA1c ≥5.7% defined IGT, and HbA1c ≥ 6.5% defined diabetes. Receiver-operating characteristic curve analysis was used to assess diagnostic accuracy of HbA1c compared with OGTT.
RESULTS
There were 587 subjects from DPT-1, 884 from TrialNet, 91 from TEDDY, and 420 from TRIGR. As an indicator for IGT, HbA1c sensitivity was very low across the studies (8–42%), and specificity was variable (64–95%). With HbA1c ≥6.5% threshold used for T1D diagnosis, the sensitivity was very low and specificity was high (sensitivity and specificity: DPT-1 24 and 98%, TrialNet 28 and 99%, TEDDY 34 and 98%, and TRIGR 33 and 99%, respectively). The positive predictive value of HbA1c ≥6.5% for the development of T1D was variable (50–94%) across the four studies.
CONCLUSIONS
HbA1c ≥6.5% is a specific but not sensitive early indicator for T1D in high-risk subjects <21 years of age diagnosed by OGTT or asymptomatic hyperglycemia. Redefining the HbA1c threshold is recommended if used as an alternative criterion in diagnosing T1D.
Elevated HbA1c has been proposed by the joint American Diabetes Association (ADA), International Diabetes Federation, and European Association for the Study of Diabetes International Expert Committee (IEC) as an alternative criterion for the diagnosis of diabetes (1). HbA1c reflects a 90-day moving average of blood glucose concentrations, weighted more heavily toward the last 30 days, and may be more stable in the presence of significant day-to-day glycemic variation (2). The recommended thresholds are ≥5.7% for impaired glucose tolerance (IGT) and ≥6.5% for diagnosis of diabetes on two tests (1). These thresholds were established based on research conducted in adults with type 2 diabetes (T2D) who demonstrated significant correlations with HbA1c ≥6.5% and higher prevalence of retinopathy and nephropathy (1,3). While recent research has substantiated rising HbA1c as a predictor of type 1 diabetes (T1D) in both young (age <15 years) (4) and adult (median age >50 years) populations (5), data demonstrating appropriate HbA1c thresholds for diagnosing T1D are lacking.
In >50% of children (6), diagnosis is made when characteristic symptoms (polyuria, polydipsia, and weight loss) are associated with elevated blood glucose. The most widely used screening test in children is random plasma glucose (RPG) with characteristic symptoms, diagnostic if ≥200 mg/dL (11.1 mmol/L) and confirmed in a laboratory. Asymptomatic cases are rare and usually picked up on screening of urine (glucosuria or ketonuria) or blood (hyperglycemia) or among subjects known to be positive for islet autoantibodies. In these cases, fasting plasma glucose (FPG) and a 2-h oral glucose tolerance test (OGTT) are recommended and diagnostic if abnormal on two separate days. It is in these individuals that HbA1c has gained appeal as a potential diagnostic indicator that would preclude the need for fasting tests and provide diagnostic data (1).
Although there are many proponents for including HbA1c as an alternative criterion for diagnosing diabetes, full consensus has not yet been achieved (7). Most studies evaluating HbA1c against the gold standard of the OGTT were carried out in subjects at increased risk for T2D (7). In contrast, few studies have assessed the sensitivity and specificity of HbA1c in children and adolescents: the age-group with the highest incidence of T1D.
The purpose of this study was to determine the utility of HbA1c as an alternative criterion in diagnosing IGT and T1D in high-risk subjects <21 years of age.
RESEARCH DESIGN AND METHODS
The population for this study was derived from four prospective studies: Diabetes Prevention Trial–Type 1 (DPT-1) (8), Type 1 Diabetes TrialNet Natural History (TrialNet) (9), The Environmental Determinants of Diabetes in the Young (TEDDY) (10), and Trial to Reduce IDDM in the Genetically at Risk (TRIGR) (11). All subjects <21 years of age with an HbA1c and OGTT drawn within 90 days of each other were eligible for the primary analysis. IGT and T1D were defined using the joint IEC criteria: OGTT 2-h plasma glucose (2-hPG) between 140 and 199 mg/dL (7.8 and 11.0 mmol/L) was classified as IGT, and FPG ≥126 mg/dL (≥7.0 mmol/L) or OGTT 2-hPG ≥200 mg/dL (≥11.1 mmol/L) was classified as T1D (1). The primary objective was to assess how well HbA1c performed in diagnosing T1D and IGT compared with the OGTT. To do this, the maximum HbA1c collected within a 90-day window before or after the OGTT was used; the two measures were paired to assess the sensitivity and specificity. For those diagnosed with T1D or IGT, the diagnostic HbA1c and OGTT pair was used, and for those without T1D/IGT the maximum HbA1c-OGTT pair across the follow-up period was used. Secondary analysis evaluated the subjects diagnosed with T1D who did not have an OGTT within the 90-day window in TEDDY and DPT-1 but had HbA1c collected within 7 days for TEDDY and 90 days for DPT-1 of diagnosis based on symptoms, FPG, RPG, and/or asymptomatic hyperglycemia (i.e., the diagnostic HbA1c was used). These subjects may have been clinically different but met the IEC criteria. The details for these four studies have previously been described (8,9,10,11).
DPT-1
Autoantibody-positive subjects 3–20 years of age who had a first- or second-degree relative with T1D were enrolled from February 1994 to October 2002 and followed until September 2003. HbA1c and OGTT were captured every 6 months once positive autoantibodies were confirmed. All sample assays were performed at a central core laboratory.
TEDDY
Subjects with high-risk HLA genotypes were enrolled at 3 months of age starting in September 2004 and followed for up to 6 years of age (January 2011). Their HbA1c and FPG were collected at first appearance of autoantibodies starting as early as the 12-month visit. If the subject remained antibody positive, HbA1c and FPG were collected every 3 months. Collection of these measures was stopped if the subject reverted to antibody negative and remained negative for at least 12 months. HbA1c samples were evaluated at a central core laboratory and OGTT at a local laboratory.
TRIGR
TRIGR is a randomized, double-blind clinical trial with two groups (treatment and control). For this analysis, only subjects from the control group were eligible. Subjects with a first-degree relative with T1D were enrolled at birth between May 2002 and February 2007 and followed until January 2011. HbA1c and OGTT measures were collected regardless of autoantibody status. HbA1c was measured at 12 and 18 months and then annually between 2 and 10 years of age. OGTTs were performed at 6 years of age and if there was a suspicion of diabetes. All samples were assayed at site-specific local laboratories.
TrialNet
Autoantibody-positive subjects 1–20 years of age with a first-, second-, or third-degree relative with T1D were enrolled starting in February 2004 and followed until March 2011. HbA1c and OGTT were performed every 6 months for the autoantibody-positive subjects. All laboratory assays were performed at a central core laboratory.
Laboratory methods
HbA1c.
DPT-1, TrialNet, and TEDDY studies used single-core laboratories that processed HbA1c using the National Glycohemoglobin Standardization Program (NGSP)-certified method. DPT-1 and TrialNet samples were processed at the Northwest Lipids Research Laboratory in Seattle, Washington. TEDDY samples were processed at the Diagnostic Diabetes Laboratory in Columbia, Missouri. Both laboratories measured HbA1c using ion-exchange high-performance liquid chromatography with G7 and G8 autoanalyzers (imprecision coefficient of variation <1.3%) (TOSOH Bioscience, San Francisco, CA) standardized using the Diabetes Control and Complications Trial (DCCT) reference method. TRIGR processed the samples at site-specific laboratories using high-performance liquid chromatography (TOSOH, Siemens, or Bayer DCA), immunoturbidimetry (TINA-Quant; Roche Diagnostics), spectrophotometry, inhibition of latex agglutination, liquid high-pressure boronate chromatography, cation exchange chromatography, or automated boronate affinity assays. There were 77 TRIGR sites in North America, Europe, and Australia. The U.S. and Canadian NGSP reference ranges and most European sites used International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) or DCCT ranges. The Swedish, Czech Republic, and Australian clinical centers used the Mono S normal ranges or IFCC reference ranges. These measures were converted to NGSP reference ranges for comparison in the analyses [Czech/Swedish: NGSP% = (0.9148 × IFCC) + 2.152; Swedish Mono S: NGSP% = (0.923 × Swedish %) + 1.345] (12).
OGTT.
DPT-1 and TrialNet evaluated OGTT samples using a photometric measurement of d-glucose absorbance using Modular P autoanalyzer (Roche Diagnostics, Indianapolis, Indiana). TRIGR evaluated OGTT samples at each of the 77 sites using one of the following: Olympus AU5400 IZASA SA (Beckman Coulter); Synchron UniCel DXc600; Beckman Coulter LX20 Pro; Ortho Vitros; Roche P autoanalyzer; Konelab Prime 60 J, Tremo Bio, Meriux; Olimps AV640; Double P Molecular System, Roche; and Onetouch Ultra, LifeScan. The TEDDY OGTT samples were processed at each local site using an enzymatic determination of hexokinase (Cobas 6000; Roche Diagnostics) or ADVIA Hexokinase at the Finland sites, Hemocue at the Swedish sites, a calibrated Freestyle Lite meter at the Georgia and Washington sites, and the YSI 2300 STAT analyzer at the Colorado site.
Statistical analysis
Data were analyzed using the Statistical Analysis System software (Version 9.2, SAS Institute, Cary, NC). Characteristics of subjects from each of the four studies were summarized and compared as follows: categorical variables were analyzed using Pearson χ2 or Fisher exact tests. Continuous variables were tested using ANOVA for differences in means. Receiver-operating characteristic (ROC) curves were used to evaluate the diagnostic performance of the recommended HbA1c cut points for IGT (HbA1c ≥5.7%) and T1D (HbA1c ≥6.5%). Optimal thresholds for HbA1c versus OGTT were identified using three methodologies: 1) minimum distance to the point closest to (0,1) on the ROC curve (13); 2) Youden index, i.e., maximum of the sum of the sensitivity and specificity (14); and 3) positive likelihood ratio, where the ratio of the sensitivity to 1 − specificity provides the greatest positive predictive value used to determine a cut point (15). Prognostic accuracy was evaluated in each study with ROC analysis adjusted for censoring (the C-statistic). The area under the curve was calculated using the trapezoid rule.
RESULTS
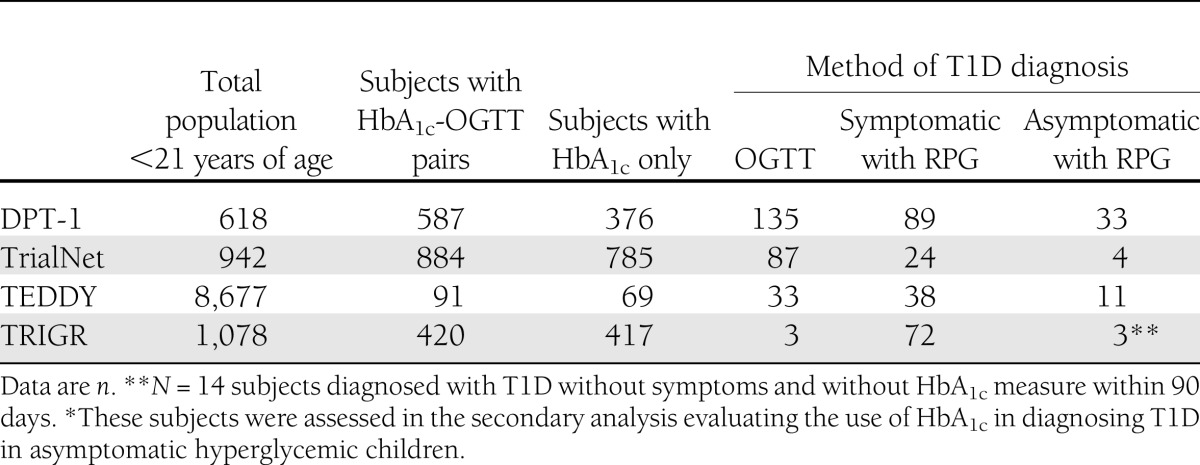
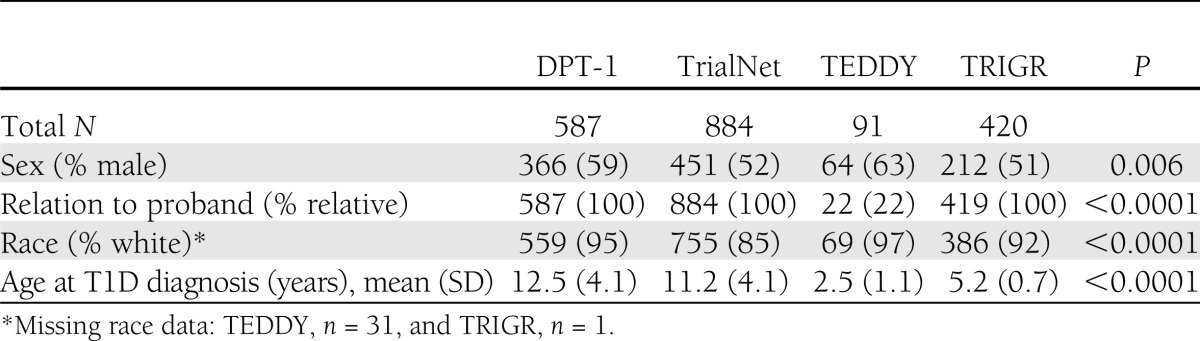
There were 587 subjects from DPT-1, 884 from TrialNet, 91 from TEDDY, and 420 from TRIGR. Based on OGTT, 135 were diagnosed with T1D in DPT-1, 87 in TrialNet, 33 in TEDDY, and 3 in TRIGR. Table 1 provides the total number of subjects <21 years of age by study and those eligible to participate in this analysis. The majority of subjects (TrialNet 98%, DPT-1 87%, TEDDY 100%, and TRIGR 99%) for the primary analysis had an HbA1c within 30 days of the paired OGTT. Of these, the vast majority (TrialNet 92%, DPT-1 70%, TEDDY 76%, and TRIGR 96%) had a HbA1c -OGTT pair on the same day. Baseline characteristics and P values describing the significant differences across the groups are shown in Table 2.
Table 1.
Eligible population by study and T1D method of diagnosis
Table 2.
Characteristics of the study group populations with paired HbA1c-OGTT measures
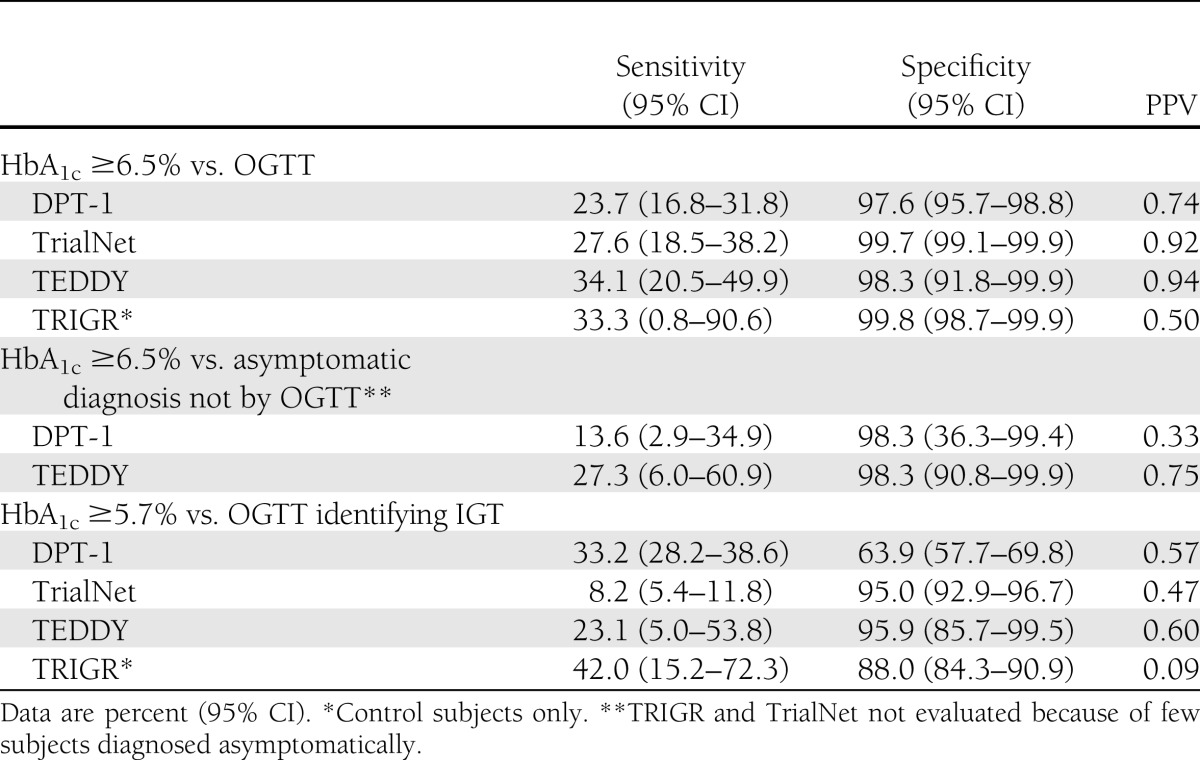
Sensitivity and specificity of HbA1c cut points for IGT (≥5.7%), T1D, and T1D-diagnosed asymptomatic hyperglycemia (≥6.5%) compared with OGTT by study group are presented in Table 3. Sensitivities and specificities were similar across the four studies for HbA1c ≥6.5% (range 23.7–33.3 sensitivity and 97.6–99.8 specificity). Positive predictive values (PPVs) were variable; probability was 50–94% that a subject had T1D when HbA1c was ≥6.5%. Similarly, the HbA1c ≥6.5% threshold was not sensitive (27.3% TEDDY; 13.6% DPT-1), but it was specific (98.3% for both TEDDY and DPT-1) in asymptomatic hyperglycemic (no OGTT data) subjects diagnosed with T1D. TRIGR and TrialNet were not included in this analysis, since few asymptomatic subjects were diagnosed. PPVs were variable, ranging from 33 to 75% for the two studies. HbA1c ≥5.7% sensitivities were low (8–42%), and specificities were variable (63–96%) in identifying IGT across the studies. PPVs were variable and poor with 9–60% likelihood of IGT if the HbA1c was ≥5.7%. In addition, the diagnostic value of HbA1c versus OGTT for both T1D and IGT was not statistically different by age across the four studies.
Table 3.
Sensitivity, specificity, and PPV of HbA1c cut point versus OGTT or asymptomatic diagnosis by study group
Given that few subjects were diagnosed with T1D by OGTT in TRIGR, the number of false positives (based on the eventual development of T1D with HbA1c ≥6.5% versus the OGTT) was assessed to further evaluate the diagnostic utility of HbA1c. There was only one false positive, and this subject did not develop T1D during the follow-up.
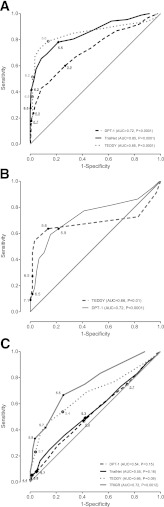
Lastly, ROC curves (Fig. 1A–C) comparing the sensitivity (true positive rate) versus 1 − specificity (false positive rate) suggested lower thresholds than the current thresholds for T1D (Fig. 1A), asymptomatic T1D (Fig. 1B), and IGT (Fig. 1C). ROC could only be performed for TEDDY and DPT-1 because of too few asymptomatic subjects diagnosed in TrialNet and TRIGR. We evaluated three statistical approaches to identify more optimal HbA1c thresholds for the diagnosis of T1D, asymptomatic T1D, and IGT (shown in Fig. 1A–C). The alternative thresholds identified for this high-risk population were only slightly more sensitive, with a reduction in specificity.
Figure 1.
A–C: ROC curves of various HbA1c thresholds for diagnosing T1D vs. OGTT (A), those diagnosed asymptomatically (B), and IGT vs. OGTT (C) by study group. AUC, area under the curve.
CONCLUSIONS
The alternative criterion proposed by the joint IEC to use HbA1c ≥6.5% to diagnose T1D is premature given contradictory reports on its utility in pediatric and adolescent populations (16,17). DPT-1, TrialNet, TEDDY, and TRIGR are among the largest high-risk T1D studies worldwide. Assessment of the value of HbA1c as a diagnostic tool in subjects <21 years of age in these four studies demonstrated that the current HbA1c threshold of 6.5% is not a sensitive indicator of T1D diagnosis in high-risk asymptomatic hyperglycemic individuals and in those diagnosed with T1D by OGTT. This study demonstrated that many subjects diagnosed with T1D by OGTT have an HbA1c <6.5%, which suggests that this HbA1c cutoff is too high as an early indicator of T1D. All four studies provided evidence that HbA1c ≥6.5% does not always mean that a subject has T1D, as shown by varying PPVs. Additionally, HbA1c ≥5.7% was not a sensitive indicator of IGT. These findings provide evidence that HbA1c ≥6.5% is not an adequate alternative criterion for diagnosing T1D in high-risk subjects <21 years of age.
Studies of HbA1c and diabetes in pediatric populations have been sparse, and their findings have been contradictory. A recent study from the Diabetes Incidence Registry (DIARY) Group Baden Wuerttemberg concluded that HbA1c is a reliable measure for diagnosing T1D in youth (17); however, these findings were based on cross-sectional data, which only included youth with blood glucose >200 mg/dL (11.1 mmol/L). Those with blood glucose <200 mg/dL were not assessed. They noted that HbA1c ≥6.35% was optimal in their sample. A larger study using National Health and Nutrition Examination Survey data (16) evaluated the utility of HbA1c as a diagnostic tool for diabetes (predominantly T2D) in an adolescent population and concluded that HbA1c, compared with FPG, is not a sensitive indicator of diabetes in adolescence (75% [95% CI 30–95]). This finding was consistent with the current study. HbA1c may be a promising alternative criterion for diagnosing T2D, which develops more slowly over time and is characterized by a more stable increase in hyperglycemia because of insulin resistance. T1D arises more rapidly, and children tend to have more bouts of transient hyperglycemia that may not be identified by HbA1c while still meeting the IEC definition for diabetes as measured by OGTT or FPG.
Multiple HbA1c measures over time may show promise as an early indicator in individuals at high risk. In the Diabetes Autoimmunity Study in the Young (DAISY) (4), an increase in HbA1c over time was predictive of progression to T1D. Stene et al. (4) reported that a 0.4% point increase in HbA1c corresponded to a fivefold increase in T1D risk with modest increases starting as early as 5 years prior to diagnosis, followed by a sharp increase 6 months prior to diagnosis.
This analysis is not without limitations. Our primary limitation is that our population was all high risk, which accounts for 15–20% of all cases of T1D. Secondly, both the TEDDY and TRIGR studies did not conduct routine OGTTs; TRIGR only performed OGTTs on subjects ≥6 years of age, so it is possible that these subjects were further along in T1D progression and may have had higher HbA1c levels as a result. The TRIGR study did not use a reference laboratory for processing HbA1c and OGTT samples, and TEDDY used local laboratories for OGTT sample evaluations. Although these two studies used methods that adhere to a more practical manner of monitoring high-risk subjects, the TEDDY results were very similar to those found in both DPT-1 and TrialNet. The current study used the diagnostic HbA1c in asymptomatic hyperglycemic subjects because two measures of HbA1c were not uniformly collected in TEDDY at or postdiagnosis. A second HbA1c is primarily used as a confirmation test, with the implication that it would reduce the number of false positives. However, our false-positive rate was very low (Table 3). Given this, if a second HbA1c failed to confirm diabetes then the sensitivity would actually be less than we have shown. Lastly, a 90-day window for the HbA1c-OGTT pair was used to determine eligibility; however, the vast majority of the HbA1c measures were conducted within 30 days of the OGTT. Although the HbA1c is a 90-day moving average, it has been shown to be more heavily weighted toward the last 30 days; thus, we extended our analysis to assess the effect those few subjects who had an HbA1c-OGTT pairing outside of the 30-day window had on our findings by only including those with a 30-day window and found there were no differences in sensitivity, specificity, or PPV.
The diagnostic performance of various diabetes indicators is in need of careful evaluation. Redefining a lower threshold for HbA1c will be more optimal for diagnosing diabetes. Further understanding of the relationship between known markers of clinical disease (HbA1c, FPG, random glucose, and OGTT) and T1D symptoms and complications with a focus on when to initiate treatment is needed. Until then, the OGTT, a sensitive indicator of diabetes and an early marker of impaired glucose homeostasis (18), is the better diagnostic option for T1D.
Acknowledgments
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreements U01 DK061010, U01 DK061016, U01 DK061034, U01 DK061036, U01 DK061040, U01 DK061041, U01 DK061042, U01 DK061055, U01 DK061058, U01 DK084565, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085505, and U01 DK085509 and contract HHSN267200800019C; the National Center for Research Resources through Clinical Translational Science awards UL1 RR024131, UL1 RR024139, UL1 RR024153, UL1 RR024975, UL1 RR024982, UL1 RR025744, UL1 RR025761, UL1 RR025780, UL1 RR029890, UL1 RR031986, and P30 DK017047 and General Clinical Research Center Award M01 RR00400; the Juvenile Diabetes Research Foundation International; and the ADA. The TEDDY Study Group is funded by grants DK 63829, 63861, 63821, 63865, 63863, 63836, and 63790 and UC4DK095300 and contract HHSN267200700014C from the NIH National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of Allergy and Infectious Diseases; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Institute of Environmental Health Sciences; Juvenile Diabetes Research Foundation; and Centers for Disease Control and Prevention. The TRIGR study group is supported by grants HD040364, HD042444, and HD051997 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Diabetes and Digestive and Kidney Diseases; Canadian Institutes of Health Research; the Juvenile Diabetes Research Foundation International; the Commission of the European Communities (specific RTD program “Quality of Life and Management of Living Resources,” contract QLK1-2002-00372 “Diabetes Prevention”); and the European Foundation for the Study of Diabetes/Juvenile Diabetes Research Foundation/Novo Nordisk Focused Research Grant.
The study formulas were provided free of charge by Mead Johnson Nutrition. No other potential conflicts of interest relevant to this article were reported.
K.V. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. D.C., D.B., and C.A.B. researched data. H.R. reviewed and edited the manuscript. L.L. and M.H. researched data and reviewed and edited the manuscript. M.J.R and D.A.S reviewed and edited the manuscript. J.P.K. contributed to discussion and reviewed and edited the manuscript. K.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 12–16 September 2011.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0111/-/DC1.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, JDRF, or ADA and does not reflect the views of the Commission of the European Communities or in any way anticipate the Commission’s future policy in this area.
References
- 1.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008;93:2447–2453 [DOI] [PubMed] [Google Scholar]
- 3.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 4.Stene LC, Barriga K, Hoffman M, et al. Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes 2006;7:247–253 [DOI] [PubMed] [Google Scholar]
- 5.Cheng P, Neugaard B, Foulis P, Conlin PR. Hemoglobin A1c as a predictor of incident diabetes. Diabetes Care 2011;34:610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elding Larsson H, Vehik K, Bell R, et al. TEDDY Study Group. SEARCH Study Group. Swediabkids Study Group. DPV Study Group. Finnish Diabetes Registry Study Group Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34:2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med 2007;24:333–343 [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Trial--Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 9.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. TrialNet Natural History Committee. Type 1 Diabetes TrialNet Study Group The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 10.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akerblom HK, Krischer J, Virtanen SM, Berseth C, Becker D, Dupre J, et al. The Trial to Reduce IDDM in the Genetically at Risk (TRIGR) study: recruitment, intervention and follow-up. Diabetologia 2011;54:627–633 [DOI] [PMC free article] [PubMed]
- 12.Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC Working Group on HbA1c Standardization IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 2004;50:166–174 [DOI] [PubMed] [Google Scholar]
- 13.Holmes WC. A short, psychiatric, case-finding measure for HIV seropositive outpatients: performance characteristics of the 5-item mental health subscale of the SF-20 in a male, seropositive sample. Med Care 1998;36:237–243 [DOI] [PubMed] [Google Scholar]
- 14.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35 [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247:2543–2546 [PubMed] [Google Scholar]
- 16.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952, e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehehalt S, Gauger N, Blumenstock G, et al. DIARY-Group Baden-Wuerttemberg Hemoglobin A1c is a reliable criterion for diagnosing type 1 diabetes in childhood and adolescence. Pediatr Diabetes 2010;11:446–449 [DOI] [PubMed] [Google Scholar]
- 18.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011;34:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]