Abstract
OBJECTIVE
Circulating levels of NH2-terminal probrain natriuretic peptide (NT-proBNP), a marker of acute heart failure, are associated with increased risk of cardiovascular disease (CVD) in the general population. However, there is little information on the potential role of NT-proBNP as a biomarker of vascular complications in type 1 diabetic patients. We investigated whether serum NT-proBNP levels were associated with micro- and macrovascular disease in type 1 diabetic subjects.
RESEARCH DESIGN AND METHODS
A cross-sectional nested case-control study from the EURODIAB Prospective Complications Study of 507 type 1 diabetic patients was performed. Case subjects (n = 345) were defined as those with one or more complications of diabetes; control subjects (n = 162) were those with no evidence of any complication. We measured NT-proBNP levels by a two-site sandwich electrochemiluminescence immunoassay and investigated their associations with complications.
RESULTS
Mean NT-proBNP levels were significantly higher in case than in control subjects. In logistic regression analyses, NT-proBNP values >26.46 pg/mL were independently associated with a 2.56-fold increased risk of all complications. Odds ratios of CVD (3.95 [95% CI 1.26–12.35]), nephropathy (4.38 [1.30–14.76]), and distal symmetrical polyneuropathy (4.32 [1.41–13.23]) were significantly increased in patients with NT-proBNP values in the highest quartile (>84.71 pg/mL), independently of renal function and known risk factors. These associations were no longer significant after inclusion of TNF-α into the model.
CONCLUSIONS
In this large cohort of type 1 diabetic subjects, we found an association between NT-proBNP and diabetic micro- and macrovascular complications. Our results suggest that the inflammatory cytokine TNF-α may be involved in this association.
NH2-terminal probrain natriuretic peptide (NT-proBNP) is the inactive molecule resulting from cleavage of brain natriuretic peptide prohormone (1). Circulating NT-proBNP levels are used for screening, diagnosis, and prognostic assessment of patients with acute decompensated heart failure (2) and correlate with left ventricular dilatation, remodelling, and dysfunction (3).
Recent studies have shown that NT-proBNP is strongly associated with risk of cardiovascular disease (CVD) in both high-risk patients with established CVD and the general population (4,5). Diabetes is associated with a greatly increased risk of vascular complications, which cannot be completely accounted for by conventional risk factors. However, there is relatively little information on the potential role of NT-proBNP as a biomarker of micro- and macrovascular complications in type 1 diabetes. A prospective study has shown that NT-proBNP levels are elevated in type 1 diabetic patients with overt diabetic nephropathy and that NT-proBNP is an independent predictor of the excess overall and cardiovascular mortality in these patients (6). In addition, a recent cross-sectional study in long-term surviving type 1 diabetic patients reported that higher NT-proBNP values were independently associated with altered monofilament test, history of macrovascular disease, and albuminuria, assessed by measurement of albumin only in a spot urine sample (7).
Whether NT-proBNP is a marker of early microvascular complications remains, thus, unclear. This hypothesis is, however, supported by the observation that enhanced cellular stretch and hypoxic conditions, which play a key role in the pathogenesis of diabetic microangiopathy, are also a potent inducer of NT-proBNP in cardiomyocytes (8,9). Moreover, in type 1 diabetes, circulating levels of inflammatory cytokines are independently associated with vascular complications (10), and recent studies have shown that cardiac NT-proBNP production is modulated by tumor necrosis factor (TNF)-α both in vitro and in vivo (11,12).
The aim of the current study was to test whether high serum NT-proBNP levels increased odds ratios (ORs) of micro- and macrovascular complications in a nested case-control sample of type 1 diabetic individuals from the EURODIAB Prospective Complications Study (PCS) and whether markers of inflammation, cellular stress, and endothelial injury play a role in potential associations.
RESEARCH DESIGN AND METHODS
The EURODIAB PCS (1997–1999) is a follow-up of the EURODIAB IDDM Complications Study (1989–1991), which was designed to explore risk factors for diabetes complications in 3,250 randomly selected people with type 1 diabetes, aged 15–60 years, attending 31 diabetes centers in 16 European countries (13,14).
A cross-sectional nested case-control study was designed at the follow-up examination (10,15–19). Case subjects were selected to have the greatest complication burden possible in order to provide sufficient numbers for subgroup analyses. Thus, case subjects were all those with CVD, proliferative retinopathy, or micro- and macroalbuminuria at follow-up. Control subjects were selected to be completely free of complications. This design allowed us to compare individuals with single or multiple complications with individuals free of complications, according to the study question, as efficiently as possible. Applying these criteria yielded 345 case and 162 control subjects with full data on complications and samples available for analyses.
Patient evaluation for the presence of cardiovascular risk factors (hypertension, BMI, waist-to-hip ratio (WHR), smoking, cholesterol, triglycerides, and HbA1c) has previously been described (10,19). Retinopathy was graded according to the EURODIAB protocol (20). Albumin excretion rate (AER), assessed on two 24-h urine collections by immunoturbidimetric method, was categorized as normoalbuminuria (<20 μg/min), microalbuminuria (20–200 μg/min), and macroalbuminuria (≥200 μg/min). Estimated glomerular filtration rate (eGFR) was determined using the four-component abbreviated equation from the Modification of Diet in Renal Disease study (21). Subjects with an eGFR <60 ml/min/1.73m2 were defined as having chronic kidney disease. Pulse pressure and proportional pulse pressure were defined as follows, respectively: systolic blood pressure − diastolic blood pressure and 100 × (systolic blood pressure − diastolic blood pressure/systolic blood pressure). Distal symmetrical polyneuropathy (DSP) was diagnosed based on 1) presence of one or more neuropathic symptoms, 2) absence of two or more ankle or knee reflexes, and 3) abnormal vibration perception threshold, measured by centrally calibrated biothesiometers (Biomedical, Newbury, OH) on the right big toe and on the right medial malleolus. CVD was defined as physician-diagnosed myocardial infarction, angina, coronary artery bypass graft, or stroke and/or ischemic changes on centrally Minnesota-coded electrocardiogram. Left ventricular hypertrophy (LVH) was defined by the electrocardiogram (ECG) Cornell voltage-duration product criterion (RaVL + SV3 × QRS duration) >2,623 mm × ms in men and >1558.7 mm × ms in women as we have previously described (22). Data on ECG-LVH were available in 467 subjects (92% subjects: 90.7% case and 92.5% control).
Serum NT-proBNP levels were measured by a two-site sandwich electrochemiluminescence immunoassay (Elecsys proBNP II; Roche Diagnostic, Mannheim, Germany), using a Modular Analytics Evo analyzer with an E170 module (Roche). Briefly, samples were incubated with both a biotinylated monoclonal anti–NT-proBNP antibody and a monoclonal anti–NT-proBNP antibody labeled with a ruthenium complex to form a sandwich complex. Streptavidin-coated microparticles were added to bind the complex to the solid phase via interaction of biotin with streptavidin. Microparticles were magnetically captured onto the surface of the analyzer electrode, and application of a voltage to the electrode induced chemiluminescent emission, which was measured by a photomultiplier. The intra-assay coefficient of variation was <3.0%, and total coefficient of variation ranges were between 2.2 and 5.8% in low and high ranges of NT-proBNP.
Soluble vascular cell adhesion molecule (sVCAM)-1, soluble E-selectin (s-E-selectin), interleukin (IL)-6, TNF-α (10), heat shock protein 27 (HSP27) (17), and anti-HSP70 antibodies (16) were measured by commercially available ELISA (R&D Systems, Oxon, U.K.) (HSP27; Calbiochem, San Diego, CA) (anti-HSP70 antibodies; Stressgen Biotechnologies) and plasma levels of C-reactive protein (CRP) using a highly sensitive in-house ELISA (10). Plasma homocysteine was determined with an automated fluorescence polarization immunoassay on an Abbott IMx analyzer (Abbott Laboratories) (23).
Statistical analysis
Variables distributed normally are presented as means (SD), while variables with skewed distribution were analyzed after logarithmic transformation (NT-proBNP, triglycerides, AER, creatinine, CRP, IL-6, TNF-α, sVCAM, s-E-selectin, homocysteine, HSP27, and anti-HSP70) and results presented as geometric means (interquartile range). Logistic regression analysis was used to estimate the ORs of NT-proBNP for any complication (AER ≥20 μg/min, retinopathy, neuropathy, and CVD), independently of confounders and known risk factors. Both backward and forward strategies, examining all potentially explanatory variables, were used to select models. The likelihood ratio test was used to compare nested models examining the role of age, sex, diabetes duration, BMI, WHR, HbA1c, blood pressure, lipids, AER, CRP, IL-6, TNF-α, homocysteine, s-E-selectin, sVCAM, HSP27, anti-HSP70, smoking, and ECG-LVH. Analyses were hypothesis oriented and did not use stepwise regression (24). Variables were retained in the final model if they added significantly to the likelihood of models or to the estimated coefficients of predictors. In light of the hypothesis of a different role of NT-proBNP in the pathogenesis of different complications, logistic regression models were also fitted separately for each complication. To assess pattern of ORs across increasing NT-proBNP values, NT-proBNP values were categorized by the quartile distribution in control subjects. We tested for linear trends across quartiles by entering a single ordinal term into the models.
RESULTS
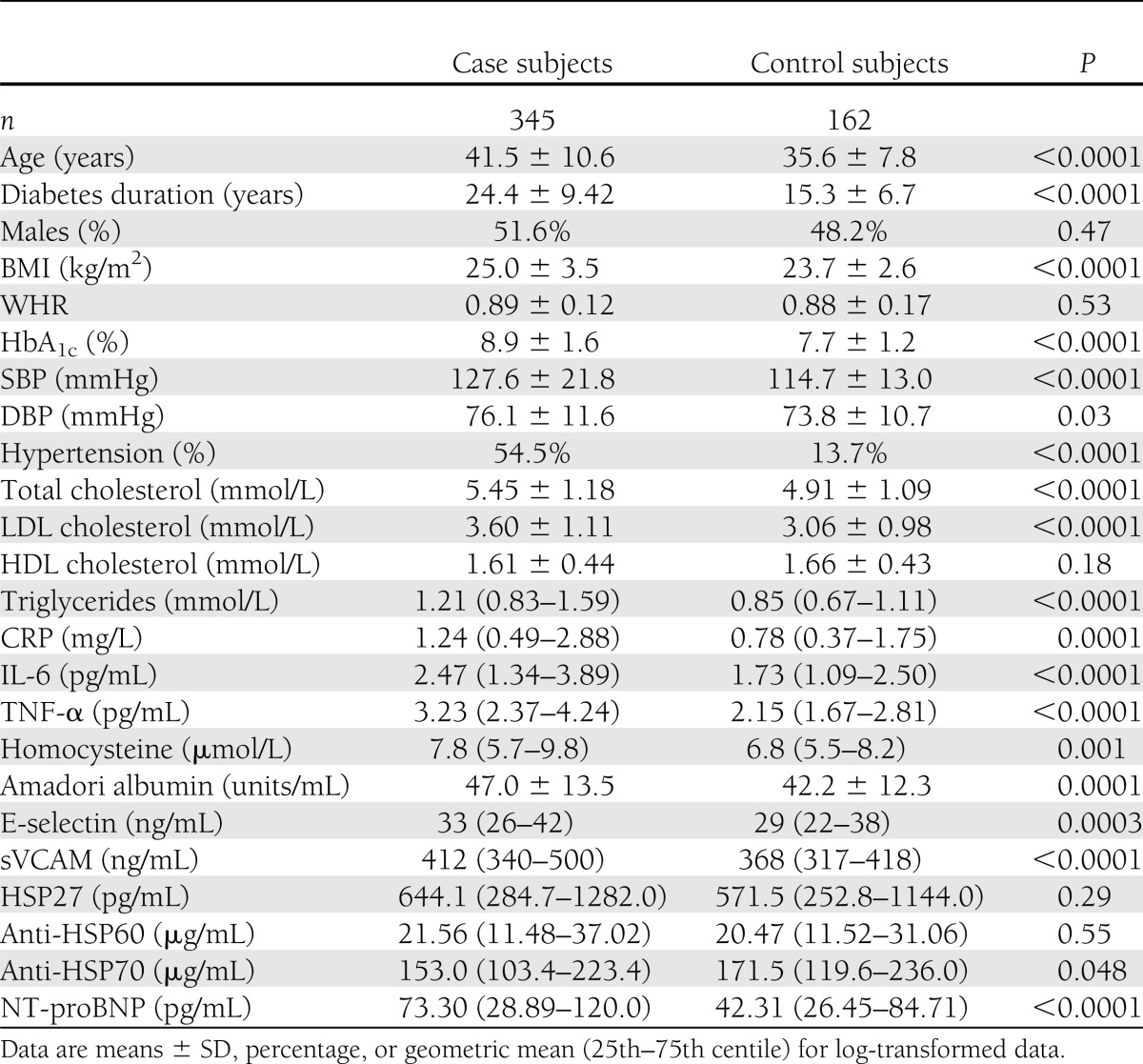
The study population (n = 507) had a mean age of 39.6 years, a diabetes duration of 21.5 years, and an equal proportion of men and women. As we have previously reported (16,17), those with vascular complications had a more adverse risk factor profile than control individuals (Table 1). Of the 345 case subjects, nephropathy was present in 193 (23.3% microalbuminuria and 32.8.3% macroalbuminuria), retinopathy in 276 (background 39.3% and proliferative 42.9%), and DSP in 196 (54.8%). Most people, however, had more than one complication; indeed, 175 (50.7%) individuals had both AER ≥20 μg/min and retinopathy; 121 (35.1%) had both AER ≥20 μg/min and DSP; and 114 (33.0%) had AER ≥20 μg/min, DSP, and retinopathy. CVD was present in 139 subjects (40.3%), all of whom also had at least one microvascular complication. Electocardiogram-LVH was present in 21 case (6%) and 4 control subjects (2.5%).
Table 1.
Characteristics of the 507 type 1 diabetic subjects recruited in cross-sectional nested case-control study of the EURODIAB PCS
Serum NT-proBNP was measurable in all the 507 samples, with right skewed distribution of values. The average age augmented progressively through increasing quartiles of NT-proBNP in both case and control subjects (case, 34.9, 39.0, 40.4, and 45.3 years, P < 0.0001; control, 33.2, 36.2, 38.2, and 39.5 years, P < 0.0001). Among case subjects, NT-proBNP values were similar in men and in women (66.2 vs. 81.6 pg/mL, P = 0.14), while they were significantly higher in women than in men among control subjects (60.6 vs. 28.7 pg/mL, P < 0.0001), and this was not modified by further adjustment for age, diabetes duration, blood pressure, AER, glycemic control, and BMI. In case subjects, the prevalence of both ECG-LVH and coronary heart disease (CHD) increased progressively through NT-proBNP quartiles (ECG-LVH 3.4, 5.1, 4.2, and 9.3%, P = 0.32; CHD 26.2, 22.7, 26.3, and 55.2%, P < 0.001). This trend reached statistical significance for CHD (P = 0.004) and was still present after adjustment for age and sex (OR 1.40 [95% CI 1.11–1.76]).
NT-proBNP levels were significantly (P < 0.0001) higher in case than in control subjects (Table 1), and results were unchanged after adjustment for age and sex (73.44 vs. 42.42 pg/mL, P < 0.0001). In case subjects with DSP (P < 0.001), micro- and macroalbuminuria (P < 0.0001), retinopathy (P = 0.005), or CVD (P < 0.001), age-and sex-adjusted NT-proBNP levels were also greater than in control subjects.
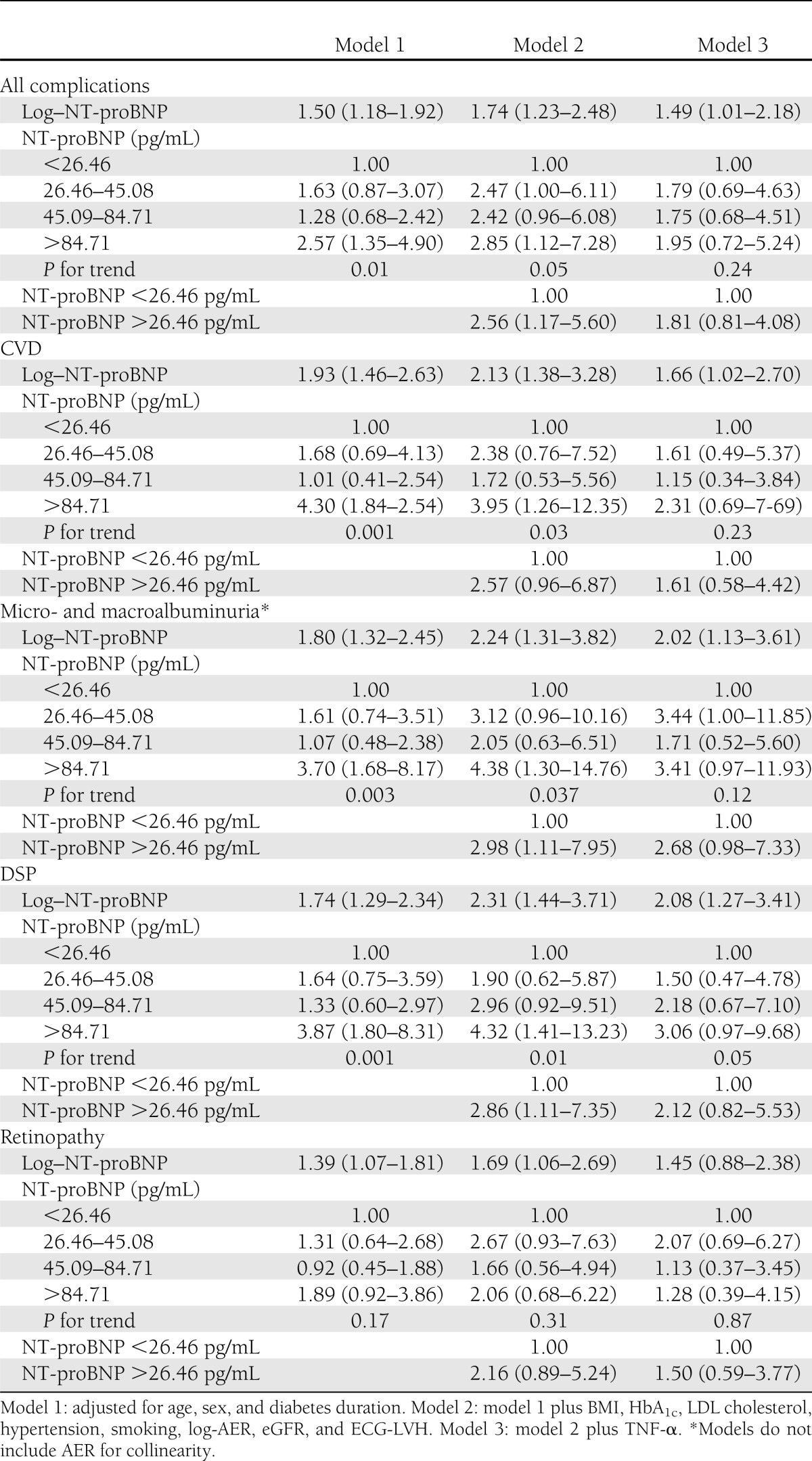
In logistic regression analyses (Table 2), models adjusted for age, sex, and diabetes duration (model 1) showed that, relative to subjects in the lowest NT-proBNP quartiles (<26.46 pg/mL), those in the highest quartiles (>84.71 pg/mL) had significantly increased ORs for all complications as well as for each complication examined separately, except retinopathy. BMI had a negative confounding effect on all these associations, and OR for retinopathy reached statistical significance (2.18 [95% CI 1.03–4.60]) only after further adjustment for BMI.
Table 2.
ORs (95% CI) for diabetes complications by serum NT-proBNP values in the nested case-control study within the EURODIAB PCS
In model 2, further adjustment was performed for main risk factors (BMI, HbA1c, hypertension, LDL cholesterol, AER, and smoking) and potential confounders (eGFR and ECG-LVH). Despite full adjustment, all three upper NT-proBNP quartiles showed significantly increased ORs for all complications, and values of NT-proBNP >26.46 pg/mL were independently associated with a 2.56-fold increased risk with respect to values of ≤26.46 pg/mL. Analyses performed separately for each complication showed that NT-proBNP values in the highest quartile were independently associated with a 3.95-fold increased OR of CVD, a 4.38-fold increased OR of nephropathy, and a 4.32-fold increased OR of DSP, which were statistically significant. Inclusion of CRP, IL-6, sVCAM, s-E-selectin, homocysteine, HSP27, anti-HSP70, and study center did not add significantly to the models or to the estimated coefficients of determinants. Adjustment for pulse pressure or proportional pulse pressure and exclusion of patients treated with diuretics (1 control and 36 case subjects) did not modify observed associations.
In contrast, after further adjustment for TNF-α (Table 2, model 3), NT-proBNP was no longer significantly associated with all complications and CVD. As regards nephropathy and DSP, trends in ORs across NT-proBNP quartiles were not significant and ORs, comparing the highest with the lowest quartile of NT-proBNP, were only marginally significant. Further inclusion into model 3 of either CRP or IL-6 did not add significantly to the models or to the estimated coefficients of predictors. TNF-α itself was strongly associated with diabetes complications. Indeed, in model 2, ORs for log–TNF-α, prior to NT-proBNP inclusion, were 8.41 (95% CI 8.41–21.16) for all complications, 7.31 (2.63–20.28) for CVD, 7.97 (2.71–23.39) for micro- and macroalbuminuria, 8.72 (2.84–26.82) for DSP, and 8.36 (2.64–26.51) for retinopathy. These ORs were virtually unmodified by NT-proBNP inclusion into the model.
CONCLUSIONS
In this cross-sectional sample of type 1 diabetic patients from the EURODIAB PCS, we have provided evidence of an association between NT-proBNP and vascular complications that is dependent on circulating levels of the inflammatory cytokine TNF-α.
Serum levels of NT-proBNP were higher in case than in control subjects, and logistic regression analysis showed that values of NT-proBNP >26.46 pg/mL were associated with a 2.56-fold increased risk of all complications, independently of renal function and risk factors, including ECG-LVH. This finding extends to type 1 diabetic patients emerging evidence in the general population showing that NT-proBNP is a novel vascular risk factor (4,5).
NT-proBNP values in the highest quartile distribution of the control subjects were associated with increased OR of CVD independently of confounders and known risk factors. This association is not surprising, as several studies have described it both in the general population and in type 2 diabetic patients (4,5,25). Furthermore, a prospective study has shown that NT-proBNP is an independent predictor of excess cardiovascular mortality in type 1 diabetic patients with overt nephropathy (6). The biological link between NT-proBNP and CVD is not fully understood; however, possible mechanisms have been proposed, such as activation of the cardiac natriuretic system by ischemic injury (26) and release of NT-proBNP by atherosclerotic plaques (27).
NT-proBNP levels were also strongly associated with nephropathy and DSP. A previous study has shown elevated NT-proBNP levels in type 1 diabetic patients with micro- and macroalbuminuria (28). Furthermore, an association of NT-proBNP with both nephropathy and DSP has recently been reported in a selected group of long-term surviving type 1 diabetic patients (7). However, potential for selection bias attributed to the study design and lack of adjustment for BMI and renal function were important limits of this study (7). Therefore, our data provide the first convincing evidence of an association between NT-proBNP and both DSP and nephropathy.
An original finding of our study is the suggestion that TNF-α may explain the relationship between NT-proBNP and vascular disease, particularly with macrovascular complications. Indeed, in our analyses NT-proBNP was no longer independently associated with diabetic micro- and macrovascular complications after further adjustment for TNF-α. Furthermore, the strong association between TNF-α and diabetes complications was left unchanged by NT-proBNP inclusion into the model. In type 1 diabetic patients, acute hyperglycemia induces an inflammatory response with a rise in TNF-α levels (29). In addition, a previous study performed on the cross-sectional sample of type 1 diabetic patients from the EURODIAB PCS has shown that TNF-α is independently associated with micro- and macrovascular complications (10). Finally, TNF-α has pleiotropic effects in cytokine-mediated inflammation underlying vascular disease. Therefore, the link between type 1 diabetes, TNF-α, and vascular disease is well established. The role of NT-proBNP in this setting is less clear. However, in patients with rheumatoid arthritis, TNF-α blockade by treatment with adalimumab for 16 weeks decreased NT-proBNP levels by ~18%, indicating that TNF-α can modulate NT-proBNP. Changes in NT-proBNP levels were independently related to changes in pulse pressure, an index of arterial stiffness (11), raising the possibility that TNF-α modulates left ventricular overload and, hence, NT-proBNP production by affecting arterial stiffness. However, in our study inclusion of pulse pressure into the models did not modify the results. A nonhemodynamic and direct effect of TNF-α on cardiac NT-proBNP production can be proposed, as in cultured neonatal rat cardiomyocytes TNF-α elicits a significant dose- and time-dependent increase in brain natriuretic peptide mRNA and protein expression via a P38-dependent mechanism (12). This is the first study providing evidence of a link between NT-proBNP, TNF-α, and vascular disease. Given the increasing interest in NT-proBNP as a prognostic marker for cardiovascular risk stratification (30), our results are of clinical relevance, though further studies are required to establish whether these findings also apply to the general population and whether they hold true in prospective studies.
In control subjects, NT-proBNP values were twofold higher in women than in men, in agreement with several reports in the general population (31,32). Experimental studies suggest that androgens have an inhibitory effect on natriuretic peptide secretion, providing a potential underlying mechanism (33). By contrast, in case subjects there was no sex-related difference in NT-proBNP levels. This is in line with a previous study in type 1 diabetic patients with diabetic nephropathy (6) but at variance with data from Grauslund et al. (7) in long-term surviving type 1 diabetic patients. Differences in age, diabetes duration, and study design may explain these conflicting results.
There are certain limitations to our study. This is a cross-sectional study, and this restricts our ability to assess temporal relationships between NT-proBNP, TNF-α, and vascular complications and to identify underlying causal biological mechanisms. However, no previous data on NT-proBNP in a large group of type 1 diabetes patients exist and this study may serve as a reasonable starting point to explore the association between NT-proBNP and TNF-α. Patients with acute heart failure were not formally excluded; however, information on current treatment for heart disease was collected at the follow-up interview and exclusion of patients treated with diuretics did not modify observed associations. ECG-based diagnosis of LVH may have resulted in underestimation of the real prevalence of LVH because of poor diagnostic sensitivity and specificity of the method, but the feasibility of studying these large numbers of subjects by echocardiography was limited. A recent study has also shown that in type 1 diabetic patients, NT-proBNP is associated with left ventricular mass even in the absence of LVH as assessed by cardiovascular magnetic resonance imaging (34). This association, however, was specifically observed in patients with diabetic nephropathy, and in our study high NT-proBNP levels increased the ORs of complications even after adjustment for AER, eGFR, and LVH risk factors, reducing the probability of a major confounding effect of left ventricular mass. Our definition of CVD may have underestimated the real number of patients with CVD. Indeed, patients with either asymptomatic peripheral vascular disease or silent myocardial ischemia/coronary artery disease and with no resting ECG abnormalities may have been falsely allocated to the control group. However, in diabetic patients silent myocardial ischemia/coronary artery disease are strongly associated with both peripheral vascular disease and microvascular complications, particularly nephropathy (35), and control subjects were selected to be free of these complications. This makes it unlikely that asymptomatic CVD significantly affected both group allocation and results. In addition, myocardial ischemia is a known inducer of NT-proBNP; therefore, potential misclassification in both case and control subjects would have led to an underestimation of the true association of NT-proBNP with CVD. The number of control subjects was lower than the overall number of case subjects, thus reducing the power of analyses; comparisons between control and case subjects with single complications allowed a more favorable case-to-control ratio, but multiple comparisons within the same case-control study base might have caused significant results due to chance. Although serum samples were adequately stored, the possibility of protein degradation cannot be excluded; however, random misclassification would have biased downward our estimates, without affecting significant associations. Unlike previous studies, a key strength here is the ability to account for confounding by other risk factors and complications, and the large sample size provides sufficient power for these analyses. In addition, our patients were from a representative sample of people with type 1 diabetes across Europe, and our results, therefore, are likely to be generalizable.
In conclusion, our results provide evidence of an association between NT-proBNP and micro- and macrovascular complications in type 1 diabetic patients that may be explained by TNF-α. Further studies are required to determine causal relationships and elucidate underlying mechanisms.
Acknowledgments
This work was supported by the University of Turin, the European Community, and the Wellcome Trust.
No potential conflicts of interest relevant to this article were reported.
G.G. and G.B. researched data and wrote the manuscript. F.B. wrote the manuscript. N.C. researched data and reviewed and edited the manuscript. C.S., S.P., M.M., M.L., M.T., and D.R.W. researched data. C.D.S contributed to the discussion and reviewed and edited the manuscript. G.M., J.H.F., and P.C.P. contributed to the discussion. G.G. and G.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all the investigators and all the patients who took part. A full list of the EURODIAB Investigators can be found in the Supplementary Data online.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0089/-/DC1.
References
- 1.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 2003;362:316–322 [DOI] [PubMed] [Google Scholar]
- 2.Kim HN, Januzzi JL., Jr Natriuretic peptide testing in heart failure. Circulation 2011;123:2015–2019 [DOI] [PubMed] [Google Scholar]
- 3.Groenning BA, Nilsson JC, Sondergaard L, et al. Detection of left ventricular enlargement and impaired systolic function with plasma N-terminal pro brain natriuretic peptide concentrations. Am Heart J 2002;143:923–929 [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–663 [DOI] [PubMed] [Google Scholar]
- 5.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation 2009;120:2177–2187 [DOI] [PubMed] [Google Scholar]
- 6.Tarnow L, Hildebrandt P, Hansen BV, Borch-Johnsen K, Parving HH. Plasma N-terminal pro-brain natriuretic peptide as an independent predictor of mortality in diabetic nephropathy. Diabetologia 2005;48:149–155 [DOI] [PubMed] [Google Scholar]
- 7.Grauslund J, Nybo M, Green A, Sjølie AK. N-terminal pro brain natriuretic peptide reflects long-term complications in type 1 diabetes. Scand J Clin Lab Invest 2010;70:392–398 [DOI] [PubMed] [Google Scholar]
- 8.Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail 2005;11(Suppl.):S81–S83 [DOI] [PubMed] [Google Scholar]
- 9.Casals G, Ros J, Sionis A, Davidson MM, Morales-Ruiz M, Jiménez W. Hypoxia induces B-type natriuretic peptide release in cell lines derived from human cardiomyocytes. Am J Physiol Heart Circ Physiol 2009;297:H550–H555 [DOI] [PubMed] [Google Scholar]
- 10.Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes—the EURODIAB Prospective Complications Study. Diabetologia 2005;48:370–378 [DOI] [PubMed] [Google Scholar]
- 11.Peters MJ, Welsh P, McInnes IB, et al. Tumour necrosis factor alpha blockade reduces circulating N-terminal pro-brain natriuretic peptide levels in patients with active rheumatoid arthritis: results from a prospective cohort study. Ann Rheum Dis 2010;69:1281–1285 [DOI] [PubMed] [Google Scholar]
- 12.Ma KK, Ogawa T, de Bold AJ. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol 2004;36:505–513 [DOI] [PubMed] [Google Scholar]
- 13.The EURODIAB IDDM Complications Study Group Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia 1994;37:278–285 [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi N, Sjoelie AK, Porta M, et al. EURODIAB Prospective Complications Study Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 2001;24:284–289 [DOI] [PubMed] [Google Scholar]
- 15.Schram MT, Chaturvedi N, Schalkwijk C, et al. EURODIAB Prospective Complications Study Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2003;26:2165–2173 [DOI] [PubMed] [Google Scholar]
- 16.Gruden G, Bruno G, Chaturvedi N, et al. EURODIAB Prospective Complications Study Group ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/ macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med 2009;266:527–536 [DOI] [PubMed] [Google Scholar]
- 17.Gruden G, Bruno G, Chaturvedi N, et al. EURODIAB Prospective Complications Study Group Serum heat shock protein 27 and diabetes complications in the EURODIAB prospective complications study: a novel circulating marker for diabetic neuropathy. Diabetes 2008;57:1966–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burt D, Bruno G, Chaturvedi N, et al. Anti-heat shock protein 27 antibody levels and diabetes complications in the EURODIAB study. Diabetes Care 2009;32:1269–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaturvedi N, Schalkwijk CG, Abrahamian H, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Circulating and urinary transforming growth factor beta1, Amadori albumin, and complications of type 1 diabetes: the EURODIAB prospective complications study. Diabetes Care 2002;25:2320–2327 [DOI] [PubMed] [Google Scholar]
- 20.Aldington SJ, Kohner EM, Meuer S, Klein R, Sjølie A-K. Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM complications study. Diabetologia 1995;38:437–444 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 22.Giunti S, Bruno G, Veglio M, et al. Eurodiab IDDM Complications Study Electrocardiographic left ventricular hypertrophy in type 1 diabetes: prevalence and relation to coronary heart disease and cardiovascular risk factors: the Eurodiab IDDM Complications Study. Diabetes Care 2005;28:2255–2257 [DOI] [PubMed] [Google Scholar]
- 23.Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocyst(e)ine with the Abbott IMx analyzer. Clin Chem 1995;41:991–994 [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 1998 [Google Scholar]
- 25.Bhalla MA, Chiang A, Epshteyn VA, et al. Prognostic role of B-type natriuretic peptide levels in patients with type 2 diabetes mellitus. J Am Coll Cardiol 2004;44:1047–1052 [DOI] [PubMed] [Google Scholar]
- 26.Goetze JP, Gore A, Møller CH, Steinbrüchel DA, Rehfeld JF, Nielsen LB. Acute myocardial hypoxia increases BNP gene expression. FASEB J 2004;18:1928–1930 [DOI] [PubMed] [Google Scholar]
- 27.Casco VH, Veinot JP, Kuroski de Bold ML, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem 2002;50:799–809 [DOI] [PubMed] [Google Scholar]
- 28.Siebenhofer A, Ng LL, Plank J, Berghold A, Hödl R, Pieber TR. Plasma N-terminal pro-brain natriuretic peptide in Type 1 diabetic patients with and without diabetic nephropathy. Diabet Med 2003;20:535–539 [DOI] [PubMed] [Google Scholar]
- 29.Gordin D, Forsblom C, Rönnback M, et al. Acute hyperglycaemia induces an inflammatory response in young patients with type 1 diabetes. Ann Med 2008;40:627–633 [DOI] [PubMed] [Google Scholar]
- 30.Wannamethee SG, Welsh P, Lowe GD, et al. N-terminal pro-brain natriuretic Peptide is a more useful predictor of cardiovascular disease risk than C-reactive protein in older men with and without pre-existing cardiovascular disease. J Am Coll Cardiol 2011;58:56–64 [DOI] [PubMed] [Google Scholar]
- 31.Lam CS, Cheng S, Choong K, et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol 2011;58:618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254–258 [DOI] [PubMed] [Google Scholar]
- 33.Hwu CM, Tsai SC, Lau CP, et al. Increased concentrations of atrial and plasma atrial natriuretic peptide in castrated male rats. Life Sci 1993;52:205–212 [DOI] [PubMed] [Google Scholar]
- 34.Astrup AS, Kim WY, Tarnow L, et al. Relation of left ventricular function, mass, and volume to NT-proBNP in type 1 diabetic patients. Diabetes Care 2008;31:968–970 [DOI] [PubMed] [Google Scholar]
- 35.Sultan A, Piot C, Mariano-Goulart D, Rasamisoa M, Renard E, Avignon A. Risk factors for silent myocardial ischemia in high-risk type 1 diabetic patients. Diabetes Care 2004;27:1745–1747 [DOI] [PubMed] [Google Scholar]