Abstract
Background:
Aged garlic extract (AGE) and coenzyme Q10 (CoQ10) have been shown to affect multiple cardiovascular risk factors. The current study evaluates the effect of AGE combined with CoQ10 on inflammatory markers and progression of coronary atherosclerosis compared with placebo.
Methods and Results:
In this placebo-controlled, double-blind, randomized trial, 65 intermediate risk firefighters (age 55 ± 6 years) were treated with a placebo capsule or a capsule containing AGE and CoQ10 (AGE+CoQ10, 1200 and 120 mg, respectively) daily for 1 year. All participants underwent coronary artery calcium (CAC) scanning and C-reactive protein (CRP) at baseline and at 12 months. At 1 year, mean CAC progression was significantly lower in AGE+CoQ10 (32 ± 6 vs. 58 ± 8, P = 0.01) than placebo. Similarly, CRP were significantly decreased in AGE+CoQ10 compared with placebo (-0.12 ± 0.24 vs. 0.91 ± 0.56 mg/L, P < 0.05). After adjustment for age, gender, conventional cardiac risk factors, and statin therapy, AGE+CoQ10 was associated with 3.99 fold (95% 1.3–12.2, P = 0.01) lack of CAC progression compared with the placebo.
Conclusion:
AGE+CoQ10 are associated with beneficial effects on inflammatory markers and reduced progression of coronary atherosclerosis.
Keywords: Aged garlic extract, atherosclerosis, coenzyme Q10, inflammatory markers
INTRODUCTION
Atherosclerosis is a complex phenomenon involving deposition of lipoproteins into the arterial intima, oxidative modification of lipids, recruitment of leukocytes into the lesion, uptake of oxidized lipids into macrophages to create foam cells, release of multiple factors involved in the atherosclerosis process including IL-1, TNF-α, and TGF-β, and migration of smooth muscle cells into intima.[1] Aged garlic extract (AGE) has been shown to reduce the progression of coronary atherosclerosis, improve vascular function, and have favorable effect on oxidative biomarkers.[2,3] AGE has effects on coronary atherosclerosis by preventing smooth muscle cell transformation and proliferation, preventing entry of lipids into arterial wall and macrophages, and also directly suppressing atherosclerosis.[4,5] AGE has been shown to have beneficial effects on arterial function by inhibiting endothelial cell damage, transforming smooth muscle cells, and inhibiting the damage of nitric oxide synthesis.[6,7] AGE helps to modulate cardiovascular risk factors by lowering blood pressure, inhibiting platelet aggregation and adhesion, lowering cholesterol, preventing low-density lipoproteins (LDL) oxidation and smoking-induced oxidative damage.[4,8–10]
There is accumulating evidence that suggests the role of inflammation in atherosclerosis process. C-reactive protein (CRP) is a nonspecific marker of inflammation, measured by levels of highly sensitive CRP (hsCRP), which may be increased in many pathological processes. CRP is also a mild predictor of future cardiovascular events and is intermittently used for risk assessment of selected asymptomatic population.[11–13]
Coenzyme Q10 (CoQ10) is present in all cellular membranes and acts as an electron carrier from complex I and complex II to complex III and the translocation of protons in the mitochondrial respiratory chain.[14] CoQ10 has been shown to have antioxidant properties by preventing oxidative modification of LDL, helping regeneration of α-tocopherol from the tocopheroxyl radical and as a scavenger for free radicals.[15–16] CoQ10 concentration has been shown to be lower in patients with coronary artery disease and the LDL/CoQ10 ratio has been shown to be a coronary risk factor.[17–19]
Both AGE and CoQ10 may help modify multiple cardiovascular risk factors for coronary atherosclerosis disease. This randomized placebo-controlled, double-blinded trial was designed to test the hypothesis that the combined formulation of AGE and CoQ10 will have beneficial effect on atherosclerosis by reducing the progression of atherosclerosis, measured by coronary artery calcium (CAC). Furthermore, the combined formulation will also have positive effect on inflammatory biomarker profiles.
MATERIALS AND METHODS
This placebo-controlled, double-blind randomized trial was designed to look at the combined effect of a commercially available AGE and CoQ10 supplement (Kyolic 110, Wakunaga Nutritional Supplement, CA, USA) on coronary atherosclerosis plaque burden and inflammatory biomarkers. The firefighter participants were enrolled at Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. The Investigational Review Board of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center approved this research project. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing mentioned in the International Journal of Cardiology.[20]
Study population
Sixty five firefighter participants who had CAC score >10 at baseline were included in the study. Subjects were required to be on stable concomitant medications for at least 12 weeks prior to randomization and agree to refrain from supplemental or significant dietary garlic or CoQ10. Subjects also signed written informed consent approved by the Institutional Review Board of Los Angeles Biomedical Research Institute. Exclusion criteria for the study included any known hypersensitivity to AGE or CoQ10 therapy, any unstable medical, psychiatric, or substance abuse disorder that may interfere with continuation in the study, weight ≥325 pounds, bleeding disorder, history of myocardial infarction, stroke or life-threatening arrhythmia within prior 6 months, resting hypotension (systolic < 90 mmHg) or hypertension (resting blood pressure >170/110), heart failure NYHA class III or IV, history of malignancy within the last 5 years (other than skin cancer) or evidence of active cancer which would require concomitant cancer chemotherapy, serum creatinine >1.4 mg/dl, triglycerides > 400 at baseline visit, diabetic subjects with HbA1C >12%, drug or alcohol abuse or current intake of more than 14 standard drinks per week and current enrollment in another placebo-controlled trial. Conditions interfering with accurate assessment of coronary calcification (metal clips, bypass patients, intracoronary stents) and drug absorption (partial ileal bypass or malabsorption syndrome) were exclusion criteria. Current use of anticoagulants (except for antiplatelet agents), chronic renal or liver failure, and hematological or biochemical values at baseline visit outside the reference ranges considered as clinically significant in the opinion on the investigator were also part of exclusion criteria.
The participants were assigned to AGE plus CoQ10 or placebo in a double-blinded manner, using numbered containers assigned to a computer-generated randomization chart by the nurse coordinator. All participants, personnel administering the intervention and the physicians involved in the study were blinded to the randomization. A capsule of placebo or commercially available AGE (1200 mg) plus CoQ10 (120 mg) was given daily for the duration of 1 year. All participants were educated on a low-cholesterol diet and instructed to avoid any direct form of garlic and CoQ10 supplementation. All participants underwent CAC and CRP assessment at baseline and 12 months.
After randomization, participants were followed up at 3, 6, 9, and 12 months to assess their compliance with medication, blood pressure, and weight measurement.
Coronary artery calcium measurement
CAS studies were performed with 64-multidetector computed tomography scanner (Light Speed VCT scanner, GE Healthcare, Milwaukee, Wisconsin). Electrocardiographic triggering was performed at 75% of the R-R interval. The coronary arteries were imaged with at least 48 consecutive nonenhanced images at 2.5 mm slice thickness. Subjects underwent similar repeat examination at the end of the study protocol.
CAC score measurements were performed on noncontrast studies by an experienced reader blinded to the patient's clinical information. CAC was defined as a plaque of at least three contiguous pixels (area 1.02 mm 2) with a density of >130 Hounsfield units. The lesion score was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield unit within this area, as described by Agatston et al.[21] The density factor were derived in the following manner: 1 for lesions with peak attenuation of 130–199, 2 for lesions with peak attenuation of 200–299, 3 for lesions with peak attenuation of 300–399, and 4 for lesions with peak attenuation of >400. Total calcium score was determined by summing individual lesion scores from each of the four main coronary arteries (left main coronary, left anterior descending coronary, left circumflex coronary, and right coronary arteries).
Statistical analysis
All continuous data are presented as a mean value ± SD, and all categorical data are reported as a percentages or absolute numbers. The power analysis was based on our previously published studies evaluating the effect of garlic and supplements on coronary atherosclerosis.[2,3] Student's t tests and chi-square tests were used to assess differences between groups. Comparisons of all parameters between the active therapy and placebo were made with the Student's t test. Trends of all parameters during follow-up periods were examined using matched pair t tests. The primary end point was the effect of AGE+CoQ10 on absolute change in CAC from baseline and secondary end points included the effect of AGE+CoQ10 on inflammatory biomarkers. The associations between changes in the two treatment groups over 1 year between groups (active therapy and placebo) for risk factors, including lipid profile, CAC, and CRP were analyzed by logistic regression analyses. These analyses were adjusted for demographics, age, gender, and traditional cardiac risk factors. Odds ratios were calculated for median annual change of lack of CAC progression, decrease in CRP, and decrease in BMI.
RESULTS
Demographic and baseline clinical information of the AGE + CoQ10 and placebo group participants are given in Table 1. The CONSORT (Consolidated Standards of Reporting Trials) statement is given in Figure 1. These participants were enrolled during 2009–2010 period. Initially, 65 participants were enrolled in the study. Five participants withdrew their consent during the course of the trial. Two participants were removed from the trial due to diagnosis of medical conditions that were exclusion criteria for the study (bleeding disorder and cancer). Eight participants dropped out due to relocation or unable to follow up with the scheduled visits. Finally, 24 and 26 participants from AGE + CoQ10 group and the placebo group were analyzed in the final analysis, respectively. Safety information was collected as part of the trial and none of the participants had significant adverse reaction mandating removal from the study.
Table 1.
Baseline demographic and clinical characteristics of study participants and absolute change at 1 year follow-up
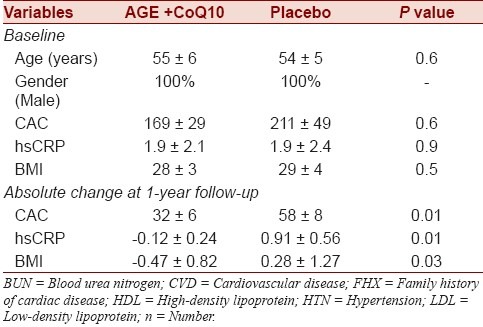
Figure 1.
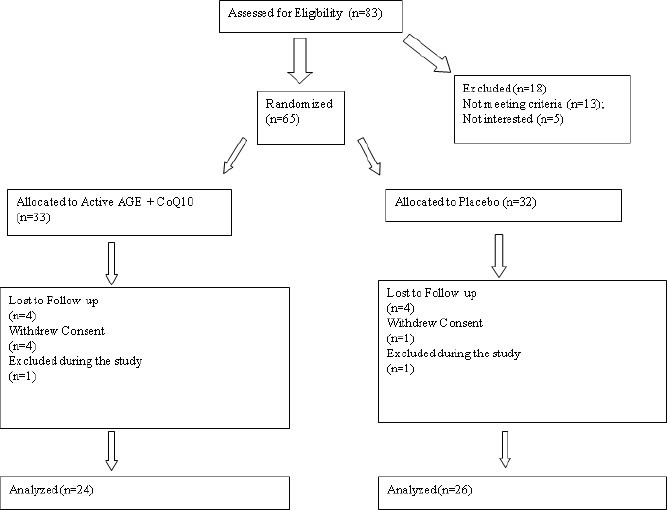
CONSORT statement (Consolidated Standards of Reporting Trials) flow chart
There were no significant differences between placebo and AGE+CoQ10 groups at baseline based on conventional cardiac risk factors. The mean CAC scores (169 ± 29 vs. 211±49, P > 0.05) and CRP (1.9 ± 2.1, 1.9 ± 2.4, P > 0.05) at baseline for AGE+CoQ10 and placebo groups were not significantly different. The absolute change in CAC at 1 year follow-up in AGE+CoQ10 and placebo groups was 32 ± 6 and 58 ± 8 (P = 0.01), respectively. At 1 year, mean CAC progression was significantly lower in AGE+CoQ10 (32 ± 6 vs. 58 ± 8, P = 0.01) than placebo.
Similarly, CRP were significantly decreased in AGE+CoQ10 compared with placebo (-0.12 ± 0.24 vs. 0.91 ± 0.56 mg/L, P < 0.05).
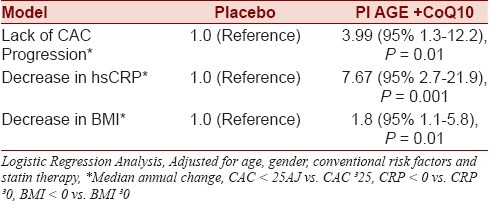
Table 2 shows the results of logistic regression analysis looking at the odds of various parameters in AGE+CoQ10 group compared with placebo taken as reference group. The results detail median annual changes in these parameters and the results were adjusted for age, gender, diabetes, hypertension, hyperlipidemia, family history of CAD, smoking status, and statin therapy. After adjustment for age, gender, diabetes, hypertension, hyperlipidemia, family history of CAD, smoking status, and statin therapy, AGE+CoQ10 was associated with 3.99 odds (95% 1.3– 12.2, P = 0.01) for lack of CAC progression.
Table 2.
Results of logistic regression analysis
DISCUSSION
This study demonstrates that AGE+CoQ10 reduce the progression of CAC and has a marked beneficial effect on inflammation over a 1 year follow-up period. These data are consistent with prior studies documenting reduced progression of CAC by AGE alone[2] and AGE supplemented with B vitamins, folic acid, and L-arginine.[3,22]
CAC not only provides information regarding cardiovascular risk but it can also be used to track progression of atherosclerosis over time.[13,23–25] In the current study, the annual progression rate for CAC in the placebo group was significantly higher compared with AGE + CoQ10 group (58 ± 8 vs. 32 ± 6, P = 0.01). In our prior study of AGE supplemented with B vitamins, folic acid, and L-arginine where all patients were treated with statins, a procession rate of 6.8% was noted compared to 26.5% in the placebo arm.[3] In the current study, only 24.24% of patients in the AGE+CoQ10 were taking statins compared with 31.25% of patients in the placebo group. Despite of the low use of statins in current study, the rate of CAC progression was significantly less in AGE+CoQ10 compared to placebo. AGE+CoQ10 worked to slow CAC progression in both participants taking statins and those not taking statins, thus the benefit of this supplement is clearly independent, but incremental to, statin use. In addition to lower rate of progression of CAC, participants in AGE+CoQ10 group had lower CRP levels compared with placebo group.
CoQ10 is a coenzyme present in the inner mitochondrial membranes enzyme complexes, as a component of the mitochondrial respiratory chain, involved in oxidative phosphorylation.[14] CoQ10 may be helpful in atherosclerosis prevention and progression by acting as an antioxidant and helping lowering blood pressure.[15–16,26] Studies have shown significantly lower concentration of CoQ10 in patients with coronary artery disease compared with control patients. These studies also showed ratio of CoQ10 and LDL to be lower in patients with coronary artery disease compared with control patients.[17–18] Singh et al.[27] studied the effect of oral CoQ10 supplementation after acute myocardial infarction. After 1 year follow-up, total cardiac events (24.6 vs. 45.0, P < 0.02) including nonfatal infarctions and cardiac deaths were significantly reduced in the treatment group compared to control group. Wittig et al.[28] studied the antiatherogenic effects of CoQ10 supplementation in apolipoprotein E gene knockout mice fed on a high fat diet. CoQ10 supplementation significantly reduced aortic atherosclerotic lesion size and markers of lipid peroxidation in Apolipoprotein E gene knockout mice compared to controls.
Limitations
This was a relatively small randomized trial and larger trials are needed to confirm the findings. Furthermore, this study was too small to evaluate for reduction of outcomes with this formulation. Since the formulation given to participants on active therapy contained AGE and CoQ10 in a single capsule, we cannot assess the effects on AGE and CoQ10 on the CAC progression separately. CAC progression was defined by the absolute change in CAC scores that may be affected by the interscan variability. We included only firefighters in this study. Firefighters are usually healthy group of population, but under more stress, and studies have demonstrated increased cardiovascular events in this population.[29]
CONCLUSION
This is the first study to our knowledge evaluating the effect of combination of AGE and CoQ10 on coronary atherosclerosis and inflammatory biomarkers. Participants taking AGE and CoQ10 combination were found to have significant improvements in CAC and CRP, suggesting improvement in cardiovascular health. These findings were incremental to, and independent of, statin use, which also have beneficial effects on both atherosclerosis and CRP. The current study and the prior two studies documenting the effect of AGE on CAC and inflammatory biomarkers sets the stage for an outcomes based study to assess whether this approach may reduce CVD events.
ACKNOWLEDGMENTS
This study was supported by a grant from Wakunaga of America to Dr. Budoff, the manufacturer of AGE and CoQ10 used in this research. None of the other authors have relationships to disclose.
Funding Sources: This study was supported by a grant from Wakunaga of America to Dr. Budoff, the manufacturer of AGE and CoQ10 used in this research.
Disclosures: This study was supported by a grant from Wakunaga of America to Dr. Budoff, the manufacturer of AGE and CoQ10 used in this research. None of the other authors have relationships to disclose.
Footnotes
Source of Support: Wakunaga of America to Dr. Budoff, the manufacturer of AGE and CoQ10
Conflict of Interest: None declared.
Clinical Trials Registry Information: This trial has been registered with ClinicalTrials.gov. (ClinicalTrials.gov Identifier: NCT00860847)
REFERENCES
- 1.Fauci A BE, Kasper D. Harrison's Principles of Internal Medicine. McGraw-Hill Companies Inc; 2008. The Pathogenesis, Prevention, and Treatment of Atherosclerosis. [Google Scholar]
- 2.Budoff MJ, Takasu J, Flores FR, Niihara Y, Lu B, Lau BH, et al. Inhibiting progression of coronary calcification using Aged Garlic Extract in patients receiving statin therapy: a preliminary study. Prev Med. 2004;39:985–91. doi: 10.1016/j.ypmed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Ahmadi N, Gul KM, Liu ST, Flores FR, Tiano J, et al. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–7. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JH, Efendy JL, Smith NJ, Campbell GR. Molecular basis by which garlic suppresses atherosclerosis. J Nutr. 2001;131:1006S–9S. doi: 10.1093/jn/131.3.1006S. [DOI] [PubMed] [Google Scholar]
- 5.Gonen A, Harats D, Rabinkov A, Miron T, Mirelman D, Wilchek M, et al. The antiatherogenic effect of allicin: possible mode of action. Pathobiology. 2005;72:325–34. doi: 10.1159/000091330. [DOI] [PubMed] [Google Scholar]
- 6.Ho SE, Ide N, Lau BH. S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine. 2001;8:39–46. doi: 10.1078/0944-7113-00005. [DOI] [PubMed] [Google Scholar]
- 7.Morihara N, Sumioka I, Moriguchi T, Uda N, Kyo E. Aged garlic extract enhances production of nitric oxide. Life Sci. 2002;71:509–17. doi: 10.1016/s0024-3205(02)01706-x. [DOI] [PubMed] [Google Scholar]
- 8.Steiner M, Khan AH, Holbert D, Lin RI. A double-blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am J Clin Nutr. 1996;64:866–70. doi: 10.1093/ajcn/64.6.866. [DOI] [PubMed] [Google Scholar]
- 9.Okuhira M HH, Sandhu R, Steiner M. Modication of Cardiovascular Risk Factors Aged Garlic Extract (AGE) Phytomedicine. 2000;7:49–50. [Google Scholar]
- 10.Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP-induced platelet aggregation in humans. J Nutr. 2000;130:2662–5. doi: 10.1093/jn/130.11.2662. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 13.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 15.Ernster L, Forsmark-Andree P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71:S60–5. doi: 10.1007/BF00226842. [DOI] [PubMed] [Google Scholar]
- 16.Kagan VE, Serbinova EA, Koynova GM, Kitanova SA, Tyurin VA, Stoytchev TS, et al. Antioxidant action of ubiquinol homologues with different isoprenoid chain length in biomembranes. Free Radic Biol Med. 1990;9:117–26. doi: 10.1016/0891-5849(90)90114-x. [DOI] [PubMed] [Google Scholar]
- 17.Yalcin A, Kilinc E, Sagcan A, Kultursay H. Coenzyme Q10 concentrations in coronary artery disease. Clin Biochem. 2004;37:706–9. doi: 10.1016/j.clinbiochem.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Hanaki Y, Sugiyama S, Ozawa T, Ohno M. Coenzyme Q10 and coronary artery disease. Clin Investig. 1993;71:S112–5. doi: 10.1007/BF00226850. [DOI] [PubMed] [Google Scholar]
- 19.Tomasetti M, Alleva R, Solenghi MD, Littarru GP. Distribution of antioxidants among blood components and lipoproteins: significance of lipids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. Biofactors. 1999;9:231–40. doi: 10.1002/biof.5520090218. [DOI] [PubMed] [Google Scholar]
- 20.Shewan LG, Coats AJS. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi N, Tsimikas S, Hajsadeghi F, Saeed A, Nabavi V, Bevinal MA, et al. Relation of oxidative biomarkers, vascular dysfunction, and progression of coronary artery calcium. Am J Cardiol. 2010;105:459–66. doi: 10.1016/j.amjcard.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 23.Gopal A, Nasir K, Liu ST, Flores FR, Chen L, Budoff MJ. Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol. 2007;117:227–31. doi: 10.1016/j.ijcard.2006.04.081. [DOI] [PubMed] [Google Scholar]
- 24.Thurlbeck WM, Haines JR. Bronchial dimensions and stature. Am Rev Respir Dis. 1975;112:142–5. doi: 10.1164/arrd.1975.112.1.142. [DOI] [PubMed] [Google Scholar]
- 25.Budoff MJ, Yu D, Nasir K, Mehrotra R, Chen L, Takasu J, et al. Diabetes and progression of coronary calcium under the influence of statin therapy. Am Heart J. 2005;149:695–700. doi: 10.1016/j.ahj.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Digiesi V, Cantini F, Oradei A, Bisi G, Guarino GC, Brocchi A, et al. Coenzyme Q10 in essential hypertension. Mol Aspects Med. 1994;15(Suppl):s257–63. doi: 10.1016/0098-2997(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 27.Singh RB, Neki NS, Kartikey K, Pella D, Kumar A, Niaz MA, et al. Effect of coenzyme Q10 on risk of atherosclerosis in patients with recent myocardial infarction. Mol Cell Biochem. 2003;246:75–82. [PubMed] [Google Scholar]
- 28.Witting PK, Pettersson K, Letters J, Stocker R. Anti-atherogenic effect of coenzyme Q10 in apolipoprotein E gene knockout mice. Free Radic Biol Med. 2000;29:295–305. doi: 10.1016/s0891-5849(00)00311-7. [DOI] [PubMed] [Google Scholar]
- 29.Kunadharaju K, Smith TD, DeJoy DM. Line-of-duty deaths among U.S. firefighters: an analysis of fatality investigations. Accid Anal Prev. 2011;43:1171–80. doi: 10.1016/j.aap.2010.12.030. [DOI] [PubMed] [Google Scholar]


