Abstract
Despite their paracrine activites, cardiomyogenic differentiation of bone marrow (BM)-derived mesenchymal stem cells (MSCs) is thought to contribute to cardiac regeneration. To systematically evaluate the role of differentiation in MSC-mediated cardiac regeneration, the cardiomyogenic differentiation potential of human MSCs (hMSCs) and murine MSCs (mMSCs) was investigated in vitro and in vivo by inducing cardiomyogenic and noncardiomyogenic differentiation. Untreated hMSCs showed upregulation of cardiac tropopin I, cardiac actin, and myosin light chain mRNA and protein, and treatment of hMSCs with various cardiomyogenic differentiation media led to an enhanced expression of cardiomyogenic genes and proteins; however, no functional cardiomyogenic differentiation of hMSCs was observed. Moreover, co-culturing of hMSCs with cardiomyocytes derived from murine pluripotent cells (mcP19) or with murine fetal cardiomyocytes (mfCMCs) did not result in functional cardiomyogenic differentiation of hMSCs. Despite direct contact to beating mfCMCs, hMSCs could be effectively differentiated into cells of only the adipogenic and osteogenic lineage. After intramyocardial transplantation into a mouse model of myocardial infarction, Sca-1+ mMSCs migrated to the infarcted area and survived at least 14 days but showed inconsistent evidence of functional cardiomyogenic differentiation. Neither in vitro treatment nor intramyocardial transplantation of MSCs reliably generated MSC-derived cardiomyocytes, indicating that functional cardiomyogenic differentiation of BM-derived MSCs is a rare event and, therefore, may not be the main contributor to cardiac regeneration.
Introduction
In the last decades there has been no significant breakthrough in either the surgical/interventional or pharmacological treatment of acute or late phase myocardial infarction [1–3]. These conventional therapeutic strategies fail to achieve a complete regeneration of the infarcted, necrotic, and finally scarred remodeled myocardial tissue. Intensive investigations on cardiac cell biology resulted in a shift from the paradigm of the heart as a terminally differentiated postmitotic organ to a self-renewing organ [4,5]. Despite the impressive intrinsic regenerative properties of the heart mediated by, for example, resident adult cardiac progenitor cells (CPCs) [6–9], the clinical observation of fatal outcome after myocardial infarction suggests that endogenous CPCs do not effectively respond in all cases to ischemic injury. Reorganization of the damaged myocardium obviously represents the best repair mechanism; however, the natural remodeling processes of the postinfarcted heart may lead to undesired changes in the ultrastructure of the myocardium on the ionic/genomic level, the cellular level, and the extracellular matrix level [10]. Therefore, it is reasonable to assume that the transplantation of cells that are able to home to the damaged or surrounding area and contribute to the formation of a regenerative tissue that is functionally superior to the remodeled cardiac tissue will be a significant step toward therapy of cardiac diseases. To date, various animal studies and clinical studies have shown evidence that the application of bone marrow (BM)-derived mesenchymal stem cells (MSCs) can improve cardiac function after myocardial infarction [11,12]. The mechanisms of their beneficial effects on the cardiac tissue are still insufficiently elucidated. Considering MSC plasticity, these cells may have the potential to differentiate into cardiomyocytes, a lineage also belonging to the mesodermal germ layer. The host myocardium and, in particular, the (peri)infarcted zone may be sufficient to induce in vivo differentiation of MSCs into cardiomyocytes. In vitro and in vivo differentiation of MSCs into functional cardiomyocytes has been reported but is still controversial [13–17]. To evaluate the role of differentiation in MSC-mediated cardiac regeneration, we have systematically investigated the cardiomyogenic differentiation potential of human MSCs (hMSCs) and murine MSCs (mMSCs) in vitro and in vivo by comparing 7 different approaches of cardiomyogenic differentiation with a noncardiomyogenic differentiation approach, and assessed survival and cardiomyogenic differentiation after intramyocardial transplantation.
Materials and Methods
Isolation and culture of BM-derived hMSCs and mMSCs
The study was approved by the local institutional review board (ethical committee) and animal welfare committee. Human BM (hBM) was obtained with informed consent from randomly chosen donors without metabolic or neoplastic disease (n=4; 3 male, 1 female; age 36.25±9.74 years standard deviation) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd) under sterile conditions during orthopedic operations. From each donor, the MSCs were isolated, characterized, and included as a separate MSC population into the study. hMSCs from whole BM were isolated using the density gradient technique as described previously [18], split at subconfluency, and replated at subsequent passages at a density of 1,500 cells/cm2. mMSCs were isolated from the BM of female C57/black6 mice (age, 6 weeks) by plastic adherence of whole BM mononuclear cells and were cultured with α-MEM (BioWhittaker) supplemented with 10% fetal calf serum (FCS; Lonza) and antibiotics/antimycotics (containing 100 U/mL Penicillin, 100 μg/mL streptomycin sulfate, and 0.25 μg/mL amphotericin B) from Sigma.
Characterization of hMSCs and mMSCs by in vitro differentiation and fluorescence-activated cell sorting analysis
MSCs are functionally characterized by in vitro differentiation assays [19]. Basic characterization included the evaluation of the differentiation potential into 3 mesenchymal lineages at passage 2 and 4: specifically into the adipogenic, osteogenic, and chondrogenic lineages as described previously [20]. The differentiated MSCs were stained with Oil Red O for adipogenesis, alkaline phosphatase for osteogenesis, and with Alcian Blue for chondrogenesis. Flow cytometric analysis was performed with a FACScan (BD Biosciences) using BD CellQuest Pro software and (secondary) phycoerythrin (PE)-labeled antibodies (anti-human CD10, CD29, CD34, CD44, CD45, CD71, CD73, CD90, CD105, CD106, CD243, CD309, GD2 and anti-mouse CD4, CD8a, CD9, CD11b, CD11c, CD14, CD29, CD31, CD34, CD43, CD44, CD45, CD71, CD80, CD86, CD105, CD106, CD117, CD135, CD140a, CD140b, CD144, CD184, CD195, and Sca-1) were used. All antibodies were from BD except for anti-human CD243 (Millipore) and anti-mouse CD34 (Serotec).
Cardiomyogenic differentiation assays
To evaluate the cardiomyogenic differentiation potential of hMSCs in vitro, 2 strategies were used.
Treatment of the hMSCs with 6 cardiomyogenic differentiation protocols
hMSCs were treated at passage 1 with cardiomyogenic differentiation media according to the following protocols: (1) exposure to 10 μM 5-azacytidine for 48 h [21] followed by culturing in normal medium (=AZA), (2) continous exposure to 5 ng/mL fibroblast growth factor 2 (FGF2)+10 ng/mL platelet-derived growth factor+10 ng/mL vascular endothelial growth factor [22] (=VEGF), (3) continuous exposure to Dulbecco's modified Eagle's medium (DMEM) containing 1 mg/mL bovine insulin+0.55 mg/mL human transferrin+10−4 M l-ascorbic acid+10−9 M dexamethasone+0.47 μg/mL linolic acid+50 mg/mL bovine serum albumin+0.5 μg sodium selenite in 38% MCDB-solution and 60% DMEM-low glucose [23] (=ITAD), (4) exposure to 1 ng/mL FGF2+0.2 ng/mL bone morphogenetic protein 2 (BMP2) for 72 h followed by culturing in normal medium [24] (=FGF+BMP), (5) exposure to 2% dimethylsulfoxide (DMSO) for 96 h followed by culturing in normal medium (=DMSO), and (6) exposure to 150 ng/mL noggin for 72 h [25] followed by culturing in normal medium (=noggin).
Co-cultivation of hMSCs with cardiomyocytes derived from murine pluripotent cells (mcP19) or murine fetal cardiomyoctes
For co-cultivation with hMSCs, murine pluripotent embryonic carcinoma mP19 cells were purchased from ATCC (CRL-1825™) and cardiomyogenic differentiation was performed as described previously [26]. Briefly, mP16 cells were cultivated in 2% DMSO in Petri dishes. Embryoid bodies were transferred to 24-well plates (Corning Life Sciences) and cardiomyogenic differentiation was assessed by spontaneous beating of cardiomyocytes derived from murine pluripotent cells (mcP19) (Supplementary Video S1). After the development of beating cell clusters, mcP19 were mitotically inactivated by treatment with 10 μg/mL mitomycin c for 1 h, then trypsinized, and seeded in 6-well plates in a density of 2×104 cells/cm2. hMSCs were added to the mcP19 at a density of 1×103 cells/cm2. hMSCs were also cultivated in monoculture under the same conditions. Both, mono- and co-cultures were cultivated for 10 days until fixation and immunocytochemical staining for cardiomyogenic differentiation.
Murine fetal cardiomyocytes (mfCMCs) were isolated as previously described with a few modifications [27]. Timed pregnant C57BL/6 mice were sacrificed 14 days after fertilization (E14) and the fetal mice were removed and decapitated. The fetal hearts were cut into small pieces and incubated in 0.2% (w/v) papain (Sigma) and 0.05% (w/v) DNase I (Sigma) in DMEM/F-12 medium (PAA) for 1 h at 37°C. The enzyme reaction was blocked with FCS [final concentration 10% (v/v)] and tissue was disrupted by trituration using a fire-polished tip. The cell suspension was washed twice in Hank's balanced salt solution by centrifugation at 200 g at room temperature, and the cell pellet was resuspended in culture medium containing α-MEM supplemented with 10% (v/v) FCS. For co-culture experiments, dissociated cardiomyocytes (40,000 cells/cm2) were seeded together with hMSCs (10,000 cells/cm2) on collagen I precoated 12-well culture plates. Cardiomyogenic differentiation was assessed after 7 days of co-culturing and after 10 days of co-culturing, adipogenic or osteogenic differentiation was induced on the co-cultures and MSC-monocultures (control) for 21 more days before fixation and staining for cardiomyogenic and noncardiomyogenic differentiation. Before fixation, the mono- and co-cultures under adipogenic differentiation were videotaped. In all co-culture assays, hMSCs were immunocytochemically identified by anti-human nuclei antibody.
Cell growth kinetics of undifferentiated hMSCs and hMSCs under cardiomyogenic differentiation
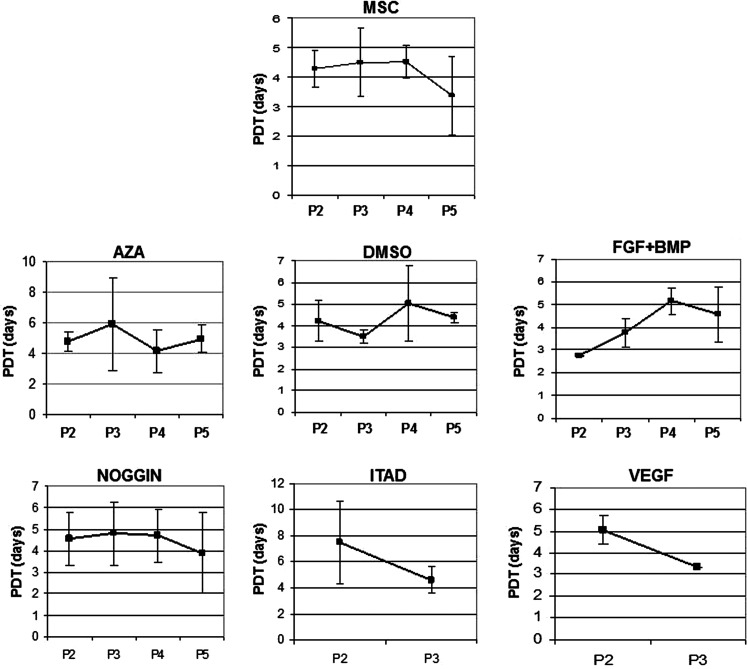
To assess the cell growth kinetics, the population doubling times (PDTs) of undifferentiated hMSCs and hMSCs treated with cardiomyogenic media of the same passage were calculated using the following formula: (PDT) tg=log2 * Δt/logN – logN0 | Δt=t–t0, N0=plating cell number, N=cell harvest number [28]. The PDT was determined in quadruplicates with the use of CASY®2 Cell Counter and Analyzer System (Model TT; Innovatis AG).
Surface epitope expression of untreated hMSCs, untreated mMSCs, and hMSCs treated with cardiomyogenic differentiation media
To assess their surface epitope expression under differentiation, untreated hMSCs and hMSCs treated with cardiomyogenic media of the same passage were analyzed by flow cytometry using (secondary) PE-labeled antibodies (anti-human CD10, CD31, CD45, CD71, CD73, CD90, CD105, CD106, CD243, CD309, and GD2).
In vitro expression of cardiomyogenic mRNA by hMSCs
To evaluate the cardiomyogenic in vitro differentiation potential of hMSCs by hMSCs, the mRNA expression of cardiomyogenic genes (cardiac troponin I, cardiac actin, and myosin light chain) in untreated hMSCs of the same passage was analyzed and quantified by quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR). To minimize the influence of in vitro culturing time (passage), the induction of cardiomyogenic genes by the differentiation media was calculated by relating the expression of cardiomyogenic genes of treated hMSCs to the expression of cardiomyogenic genes of untreated hMSCs of the same passage.
RNA isolation and reverse transcription
In each passage 1×105 hMSCs were frozen in RLT buffer and stored at −80°C for subsequent RNA isolation, which was performed using the RNeasy Mini Kit (Qiagen), including DNA digestion with the RNase-Free DNase Set (Qiagen). From each sample, 1 μg of the total RNA was reverse transcribed with the Transcriptor First Strand cDNA Synthesis Kit (Roche), using anchored-oligo (dT)18 primers. The resultant cDNA was stored at −20°C and diluted by 1:10 for subsequent use in qRT-PCR. Total RNA from human heart (Stratagene) was reverse transcribed and used as positive control in all TaqMan PCRs.
TaqMan qRT-PCR for quantification of cardiomyogenic mRNA
For determination of expression of MPGs, TaqMan PCR assays were established, using the LightCycler® TaqMan® Master Kit (Roche), variable hydrolysis probes from the Universal ProbeLibrary (Roche), and the LightCycler Instrument (Roche), according to the manufacturer's instructions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene, and primers (high-performance liquid chromatography quality) were ordered from Metabion International AG. For detailed descriptions of primer sequences, probe numbers, and PCR product lengths, see Supplementary Table S1. The temperature profile for all targets was 10 s denaturation at 95°C, 20 s primer annealing at 60°C, and 1 s extension time at 72°C for 45 cycles per run. PCR efficiency and sensitivity for all targets was assessed by generation of regression lines with standard dilutions over 8 powers of 10. PCR specificity was confirmed by running non-RT control samples and by gel electrophoresis of PCR products, using the FlashGel system (Lonza) and 2.2% agarose cassettes. For relative quantification, the expression of all target genes was normalized to the expression of GAPDH in the respective sample, including efficiency correction [29].
Transplantation of mMSCs into infarcted hearts
Anesthesia and ventilatory support
All protocols are in accordance with the German guidelines for use of live animals and were approved by the Institutional Animal Care and Use Committee of the Tübingen University Hospital and the local authorities. Transplantation experiments were performed with 6-week-old C57BL/6 female mice. Anesthesia was induced (70 mg/kg body weight i.p.) and maintained (10 mg/kg/h) with pentobarbital sodium. Mice were placed on a temperature-controlled heated table (Effenberger) with a rectal thermometer probe attached to thermal feedback controller to maintain body temperature at 37.5°C. After induction of anesthesia, mice were secured in a supine position, with the upper and lower extremities attached to the table with removable tape. The trachea was surgically exposed, and tracheal intubation was performed. Briefly, a blunt polyethylene cannula (Insyte 22g; Beckton Dickinson) was inserted into the trachea without direct visualization of the larynx, while the tongue of the animal was pulled out by a pair of forceps. Correct tube placement was confirmed by direct visualization of the cannula within the previously exposed trachea above the carina. The tracheal tube was connected to a mechanical ventilator (Servo 900C; Siemens) with pediatric tubing, and the animals were ventilated by using a pressure-controlled ventilation mode (peak inspiratory pressure of 15 mbar, frequency 105 breaths/min). Fluid replacement was performed with normal saline (0.1 mL/h i.p.).
Technique of coronary artery occlusion
All operations were performed under an upright dissecting microscope (Olympus SZX12) as described previously [30]. After left anterior thoracotomy, exposure of the heart and dissection of the pericardium, the mouse was placed on the right side, and the left foreleg extended in cranial direction. For the purpose of inducing myocardial ischemia, the left coronary artery (LCA) was visually identified before ligation by close inspection of the heart without the microscope from the side, while applying pressure to the heart using a cotton stick. Once visually identified, an 8-0 nylon suture (Prolene; Ethicon) was placed around the LCA. In short, the LCA suture was threaded through a small piece of plastic tube (PE-10 tubing) with blunt edges, and 2 small weights (1 g) were attached to each end. With the weights freely hanging, the LCA was immediately occluded. The LCA occlusion was terminated once the weights were supported. Successful LCA occlusion was confirmed by an immediate color change of the vessel from light red to dark violet, and the change of color of myocardium supplied by the vessel from bright red to white. During reperfusion, the color returned to normal when the hanging weights were supported and the LCA was reperfused. During the entire procedure, the heart was hydrated with a wet piece of absorbent cotton.
Administration of mMSCs and postoperative animal care
mMSCs were trypsinized, labeled with PKH26 as described previously [31], and kept on ice until transplantation. One minute before the end of myocardial ischemia (30 min), 0.5×106 mMSCs in 50 μL saline were injected intramuscularly into the apex cordis. Thereafter, the reperfusion was visually verified and the chest was closed by 7.0 suture (PDS II; Ethicon). Disconnection of the ventilator was performed after the resumption of normal breathing. The mice were housed separately in microisolators for 14 days and supplied with water containing tetracycline (100 mg/L). After 14 days, the mice were sacrificed and the hearts were flushed with saline using a plastic catheter inserted into the abdominal aorta. Thereafter, the hearts were frozen immediately in Tissue-Tek (Sakura Finetek) at −80°C.
Evaluation of in vitro and in vivo expression of cardiomyogenic proteins
The in vitro and in vivo expression of cardiomyogenic proteins by MSCs was evaluated by immunocytochemical and immunohistochemical staining.
Cells and 5 μm cryosections were fixed in 4% paraformaldehyde for 20 min, washed with phosphate-buffered saline (PBS), and incubated over night at 4°C with the following primary antibodies: mouse antialpha actinin (1:800; Sigma), mouse anticardiac actin (1:50; Abcam), rabbit antialpha atrial natriuretic peptide (ANP, 1:400; Chemicon), mouse antislow muscle myosin (1:400; Chemicon), mouse antiheavy chain cardiac myosin (1:400; Biozol), mouse anticardiac troponin I (1:400; Chemicon), and mouse anti-human nuclei (1:200; Millipore) suspended in PBS including 5% FCS and 0.1% Triton ×100 (Sigma) in case of cells or 0.5% Triton ×100 in case of tissue. After washing with PBS, the secondary antibodies conjugated to Alexa 594 and 488 fluorochromes (dilution 1:200; Invitrogen) were incubated for 1 h at room temperature. After washing with PBS and mounting with mounting medium (VectaShield; Vectorlabs) containing 4,6-diamidino-2-phenylindole, the slides were covered with cover slips and analyzed using an Axiovert 200 immunofluorescence microscope and Axiovision software (Zeiss).
Real-time video imaging
Real-time video imaging was performed using a digital camcorder (GR-DVL200; JVC) through the ocular of the microscope (Axiovert 200) at 800× total optical magnification.
Statistics/data analysis
Statistical significance was assessed for the mRNA expression using the Relative Expression Software tool (REST; www.gene-quantification.de/rest.html). The statistical analyses of the fluorescence-activated cell sorting experiments and of the growth kinetics were performed using the 1-way analysis of variance. The data are presented in mean±standard error of means, and P<0.05 was considered significant (*).
Results
Characterization of hBM-MSCs
All hMSC populations could be effectively differentiated into the adipogenic, osteogenic, and chondrogenic lineage (Supplementary Fig. S1A–C). All hMSC populations highly expressed CD73, CD90, and CD105, whereas the hematopoietic markers CD14 and CD34 were not expressed (Supplementary Fig. S1D).
hMSCs upregulate cardiomyogenic mRNA during in vitro cultivation
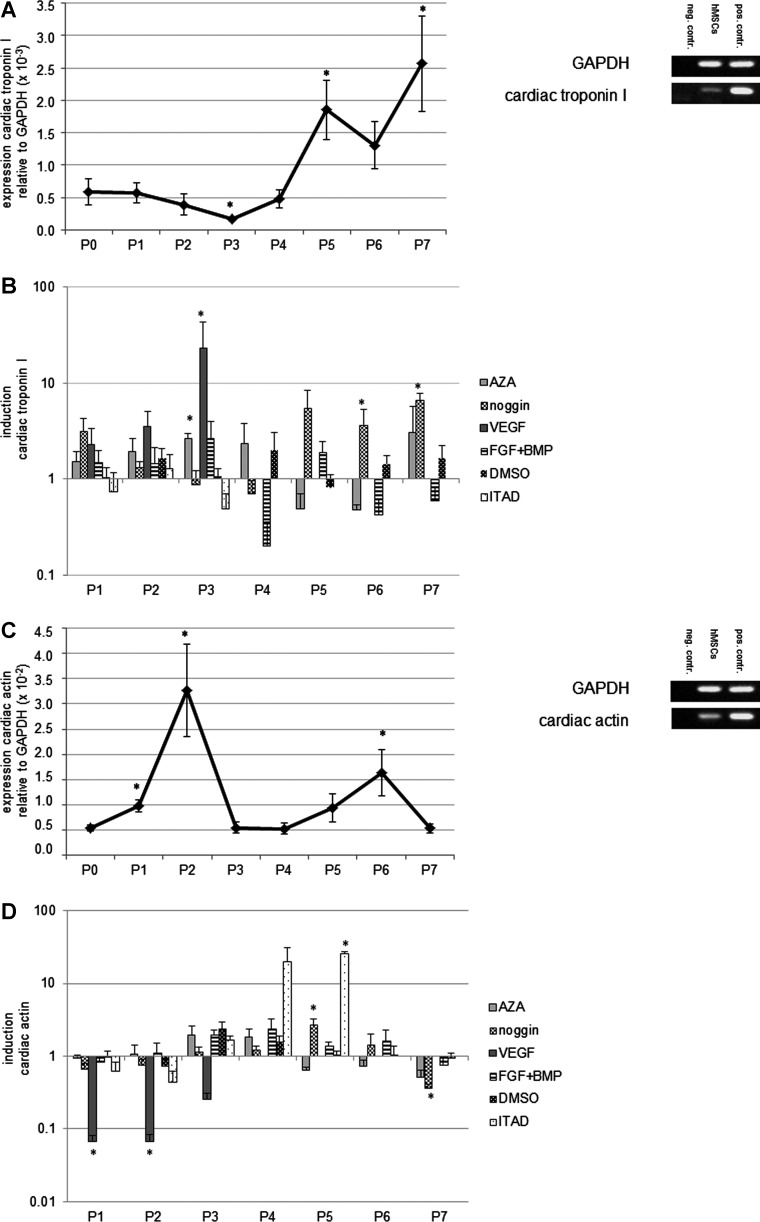
Untreated hMSCs showed upregulation of cardiac troponin I, cardiac actin, and myosin light chain during their in vitro cultivation in normal medium (intrinsic expression). For each cardiomyogenic gene, maximal mRNA expression could be identified at certain points in culture (Fig. 1A, C, E). Treatment with cardiomyogenic differentiation media led to an enhanced expression of cardiomyogenic mRNA compared to the untreated hMSCs of the respective passages (defined as induction) (Fig. 1B, D, F). Interestingly, treatment with differentiation media not only resulted in induction of cardiomyogenic gene mRNA but also decreased the expression of distinct cardiomyogenic gene mRNA with respect to the time course. In general, treatment with ITAD resulted in the most effective induction of cardiomyogenic gene mRNAs in hMSCs.
FIG. 1.
Expression of cardiomyogenic genes in untreated human mesenchymal stem cells (hMSCs) and induction of cardiomyogenic genes in hMSCs treated with cardiomyogenic differentiation media. Untreated hMSCs showed upregulation of cardiac troponin I, cardiac actin, and myosin light chain during their in vitro cultivation in normal medium (intrinsic expression). For each cardiomyogenic gene, distinct passages with maximal mRNA expression could be identified (A, C, E). Statistically significant differences of mRNA expression compared to P0 are indicated by an asterisk. Treatment with cardiomyogenic differentiation media led to an enhanced expression of cardiomyogenic gene mRNA compared to the untreated hMSCs of the respective passages, which was defined as induction (B, D, F). Statistically significant differences of mRNA induction compared to the untreated control of the respective passages are indicated by an asterisk. Error bars show standard error of the mean (SEM); asterisks indicate statistically significant differences (P<0.05) compared to P0 (expression) or to the untreated control of the respective passage (induction).
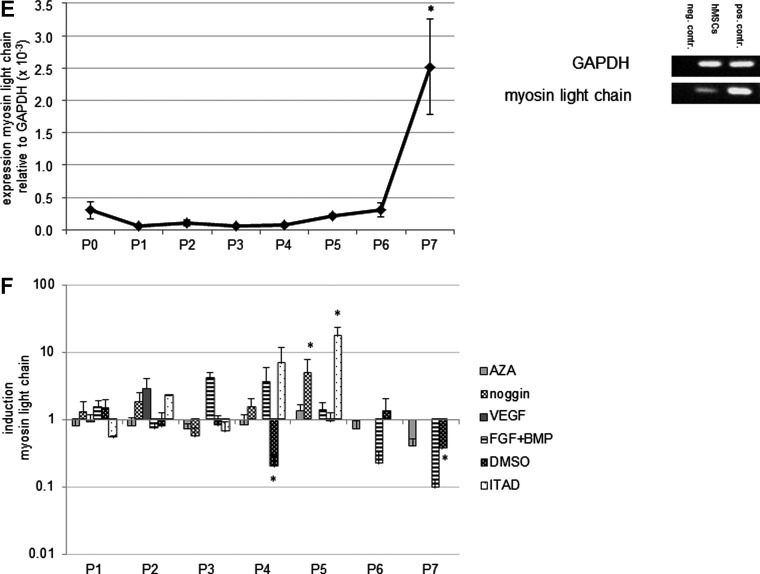
hMSCs upregulate cardiomyogenic proteins during in vitro cultivation
Untreated hMSCs showed an increased expression of cardiac troponin I, ANP, cardiac alpha sarcomeric actin, and slow muscle myosin protein during their in vitro cultivation in normal medium (intrinsic expression). Treatment with cardiomyogenic differentiation media did not have an obvious effect on the expression of cardiomyogenic proteins compared to the untreated hMSCs (Fig. 2A–D). At passage 4, slow muscle myosin-positive myotubal-like structures could be identified in hMSC cultures treated with ITAD (Fig. 2D), but no beating cells were present.
FIG. 2.
Cardiomyogenic phenotype of untreated hMSCs and hMSCs treated with cardiomyogenic differentiation media. Untreated hMSCs showed an increased expression of cardiac troponin I (A), atrial natriuretic peptide (ANP) (B), cardiac alpha sarcomeric actin (C), and slow muscle myosin (D) protein during their in vitro cultivation in normal medium (intrinsic expression) for up to 7 passages. Lacking passages indicate the growth arrest of the cells. Treatment with cardiomyogenic differentiation media did not have an obvious effect on the expression of cardiomyogenic proteins compared to the untreated hMSCs. At passage 4, slow muscle myosin positive myotube like structures, but no beating cells, could be identified in hMSC cultures treated with ITAD (D). Cardiac troponin I, ANP, cardiac alpha sarcomeric actin, and slow muscle myosin: green; 4,6-diamidino-2-phenylindole (DAPI): blue; scale bars: 20 μm.
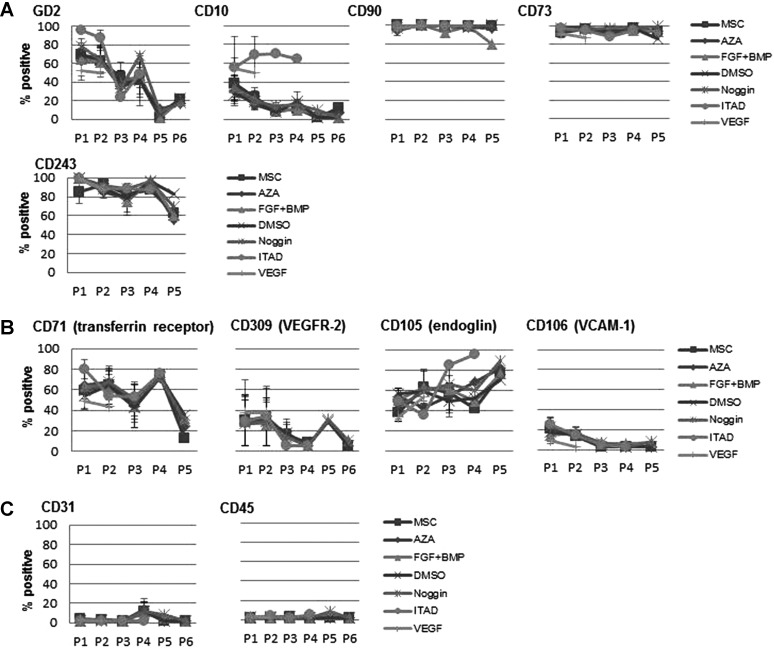
Treatment with cardiomyogenic differentiation media does not significantly change the surface epitope pattern of hMSCs
At the beginning of the in vitro analyses (P1) the hMSCs were positive for CD10, CD29, CD44, CD71, CD73, CD90, CD105, CD106, CD243, CD309, and GD2 and negative for CD34 and CD45 [exempt for 1 MSC population (5.1% CD45+ cells)]. During the prolonged in vitro culture the surface epitope expression of treated and untreated hMSCs showed a similar trend of changes (likely due to donor-to-donor variations, no statistically significant differences were detectable): the surface expression of GD2 and CD71 decreased for treated and untreated hMSCs, whereas the surface expression of CD73, CD90, CD105, CD106, CD243, CD309, and CD45 for treated and untreated hMSCs did not change. A slight but statistically nonsignificant increase of CD31-positive cells could be observed. Treatment with ITAD led to an enhanced expression of CD10 and CD105 for 1 hMSC population, whereas the expression of CD10 decreased for untreated hMSCs and hMSCs treated with other differentiation media (Fig. 3A–C).
FIG. 3.
Surface epitope pattern of untreated hMSCs and hMSCs treated with cardiomyogenic differentiation media. The expression of stem cell associated markers (A), functional receptors (B), and lineage markers (C) on hMSCs was not significantly changed upon treatment with cardiomyogenic differentiation media compared to normal media. Error bars show SEM.
Treatment with cardiomyogenic differentiation media does not significantly affect the growth kinetics of hMSCs
Except for a slight but statistically nonsignificant increase in the PDT of hMSCs treated with FGF+BMP, no obvious differences of the growth kinetics of the treated and untreated hMSCs could be detected (Fig. 4).
FIG. 4.
Growth kinetics of untreated MSCs and MSCs treated with cardiomyogenic differentiation media as shown in population doubling times (PDTs) in days. Except for a slight but statistically not significant increase in the PDT of hMSCs treated with fibroblast growth factor+bone morphogenetic protein (FGF+BMP), and no significant differences of the growth kinetics of the treated and untreated hMSCs could be detected. Error bars show SEM.
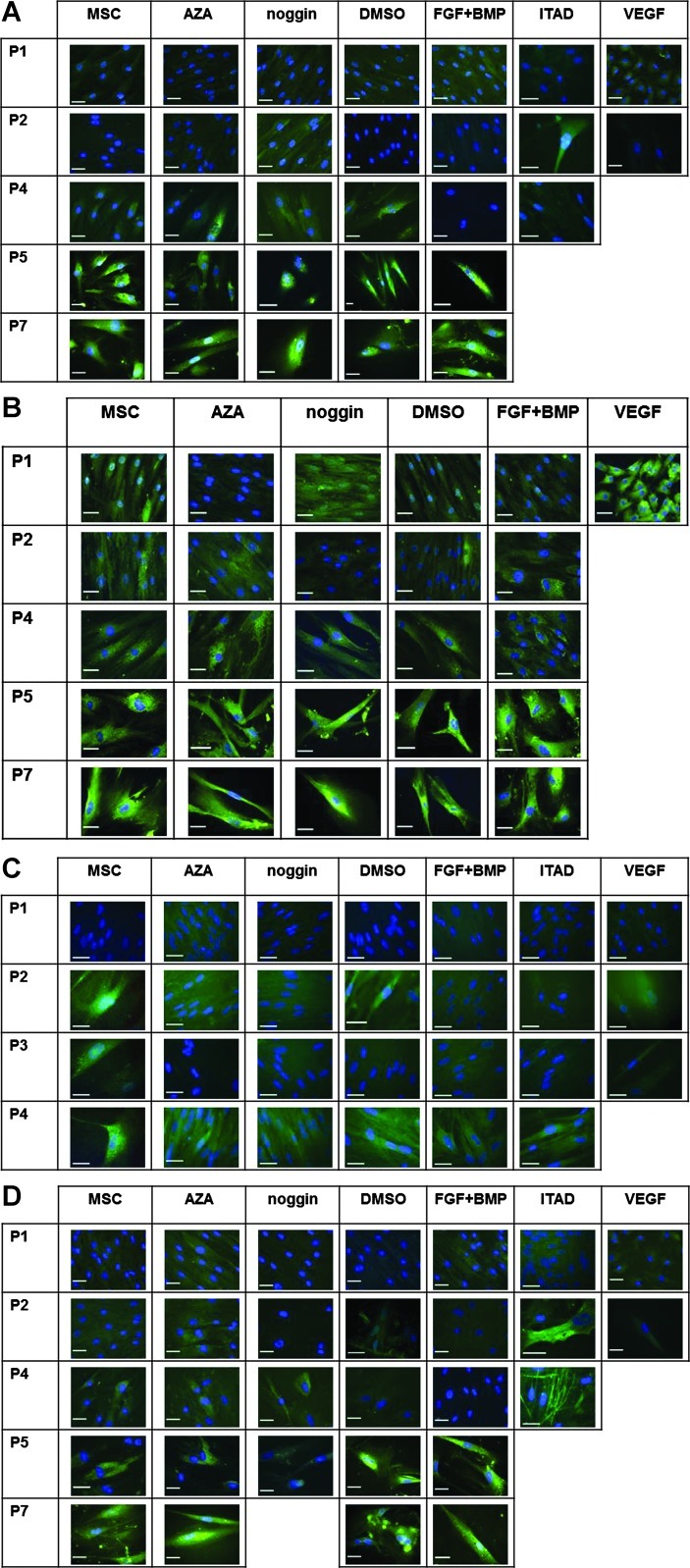
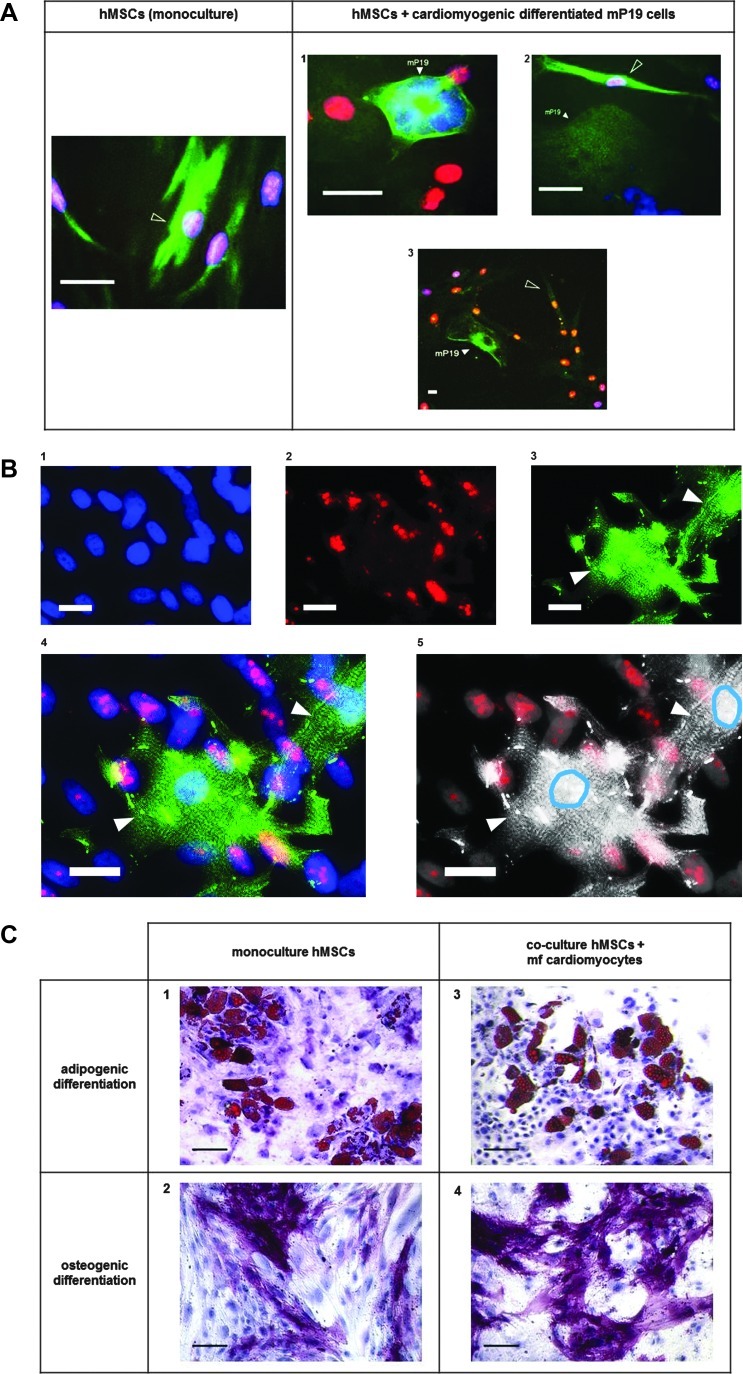
hMSCs do not show functional cardiomyogenic differentiation in the presence of cardiomyocytes in vitro
Co-cultivation of hMSCs with cardiomyocytes derived from murine pluripotent cells (mcP19) or mfCMCs did not increase the number of cardiac actin-positive hMSCs compared to monocultured hMSCs (Fig. 5A). Moreover, direct contact of hMSCs to mcP19 cells did not promote the expression of cardiac actin in hMSCs (Fig. 5A1–3). Co-cultivation of hMSCs with beating mfCMCs did not lead to expression of alpha actinin or to the formation of contractile elements in hMSCs (Fig. 5B1–5).
FIG. 5.
Evaluation of cardiomyogenic and noncardiomyogenic differentiation of hMSCs in the presence of cardiomyocytes in vitro. Co-cultivation of hMSCs with mcP19 cells did not increase the number of cardiac actin-positive hMSCs compared to monocultured hMSCs (A). Moreover, direct contact of hMSCs to mcP19 cells did not promote the expression of cardiac actin in hMSCs (A1–3; cardiac alpha sarcomeric actin: green; human nuclei: red; DAPI: blue; shaped arrowheads: hMSCs expressing alpha sarcomeric actin, filled arrowheads: mcP19 cells; scale bars: 20 μm). Co-cultivation of hMSCs with beating murine fetal cardiomyocytes (mfCMCs) did not lead to expression of alpha actinin by hMSCs (B1–4; alpha actinin: green, human nuclei: red, DAPI: blue; B5: microphotograph shown in B4 in black and white; to emphasize the contrast of mfCMCs to hMSCs, the nuclei of mfCMCs are circled in blue using Adobe Photoshop CS5.1, whereas hMSCs can be identified by red fluorescence of human nuclei; filled arrowheads: mfCMCs; scale bars: 20 μm). hMSCs co-cultivated with mfCMCs could effectively be differentiated into adipogenic and osteogenic lineage, and no difference of the adipogenic and osteogenic differentiation capacity of hMSCs in monoculture or in mfCMC-co-culture could be observed (C1–4; fat droplets for adipogenesis are stained in red, alkaline phosphatase for osteogenesis is stained in pink/violet; scale bars: 100 μm).
hMSCs co-cultivated with beating mfCMCs could effectively be differentiated into adipogenic and osteogenic lineage, and no difference of the adipogenic and osteogenic differentiation capacity of hMSCs in monoculture or in co-culture with beating mfCMCs could be observed (Fig. 5C1–4). Even hMSCs with direct contact to beating mfCMCs showed unimpaired adipogenic differentiation capacity (Supplementary Video S2).
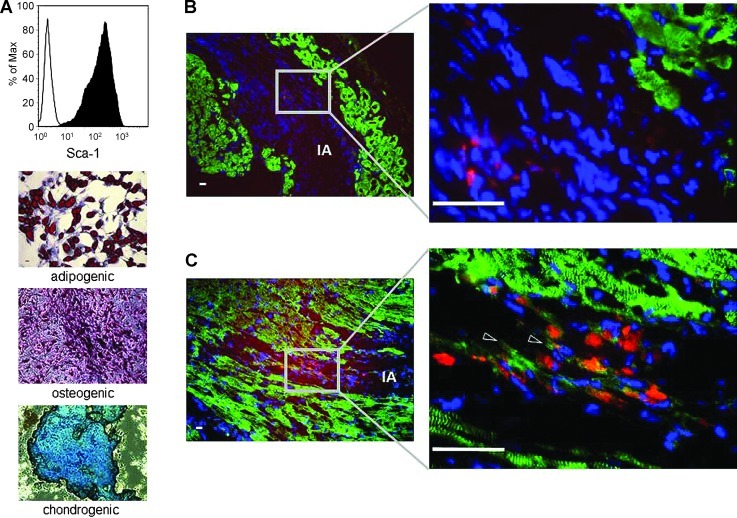
mMSCs inconsistently show functional cardiomyogenic differentiation in the cardiomyogenic environment in vivo
Fourteen days after infarction, the infarcted area (IA) could be identified by the absence of vital alpha actinin-positive cardiomyocytes (Fig. 6B, C). Fourteen days after transplantation of Sca-1-positive PKH-labeled multipotent mMSCs (Fig. 6A) into the infarcted heart, the PKH-positive mMSCs could be detected exclusively in the IA, indicating homing and survival of mMSCs in the IA (Fig. 6B, C). In most of the analyzed sections, the transplanted mMSCs were negative for alpha actinin (Fig. 6B); however, few mMSCs expressed alpha actinin, indicating a possible functional cardiomyogenic differentiation for only a minority of the transplanted mMSCs in vivo (Fig. 6C).
FIG. 6.
Cardiomyogenic differentiation of murine mesenchymal stem cells (mMSCs) in vivo. Flow cytometric analysis showed that mMSCs are highly positive for Sca-1 (A; line histogram shows isotype-matched rat IgG2a antibody control staining, filled histogram shows specific anti-Sca-1 antibody staining). mMSCs could be differentiated into adipocytes (fat droplets stained in red), osteocytes (alkaline phosphatase stained in pink/violet), and chondrocytes (mucopolysaccharides are stained in blue to bluish-green) (A). Fourteen days after infarction, the infarcted area (IA) could be identified by the absence of vital alpha actinin-positive cardiomyocytes (B, C). Fourteen days after transplantation of Sca-1+ multipotent mMSCs into the infarcted heart, the PKH-positive mMSCs could be detected exclusively in the IA indicating homing and survival of mMSCs in the IA (B, C). In most of the analyzed sections the transplanted mMSCs were negative for alpha actinin (B); however, few mMSCs expressed alpha actinin, indicating a possible functional cardiomyogenic differentiation of the minority of the transplanted mMSCs in vivo (C). Alpha actinin: green; PKH26-labeled mMSCs: red; DAPI: blue; shaped arrowheads: mMSCs expressing alpha actinin; scale bars: 20 μm.
Discussion
Given the initial reports of in vitro and in vivo expression by MSCs of proteins normally expressed by cells committed to the cardiomyogenic lineage such as ANP, alpha skeletal actin, beta myosin heavy chain, Nkx2.5, GATA4 [32,33], and functional adrenergic and muscarinic receptors [34], it was reasonable to assume that cardiomyogenic differentiation of MSCs could play a major role in MSC-mediated cardiac regeneration. Several strategies were pursued to enhance the cardiomyogenic differentiation potential of MSCs before transplantation [35]. Although various studies on cardiomyogenic differentiation of non-hMSCs showed that these cells display certain properties of cardiomyocytes in vitro, including typical electrophysiological behavior [32,36], the goal to generate morphological and functional cardiomyocytes from MSCs in high numbers has not been achieved yet. Moreover, a previous study showed no evidence that allogeneic MSCs provide a significant cardiomyogenic differentiation potential after transplantation into a pig model of myocardial infarction [37]. A small percentage of hMSCs engrafted into the myocardium in a mouse model of myocardial infarction showed de novo expression of cardiomyogenic proteins like desmin, β-myosin heavy chain, α-actinin, cardiac troponin T, and phospholamban like the host cardiomyocytes, and sarcomeric organization of the contractile proteins was observed [38]. Recent studies on hBM-MSCs reported on cardiomyogenic differentiation in vitro [39–42]. However, hBM-MSCs co-cultured with neonatal rat cardiomyocytes displayed limited cardiomyogenic plasticity [43], and treatment with 5-azacytidine caused despite the absence of differentiation of hMSCs into cardiomyocytes, profound changes in current density [44]. Finally, a recent study could not provide evidence of functional cardiomyogenic differentiation of murine BM-MSCs [16]. These partially contradictory data raise more questions than eventually answering them with respect to the role of cardiomyogenic differentiation of MSCs in cardiac regeneration. In the first part of our study we systematically evaluated the cardiomyogenic differentiation potential of hMSCs in vitro: we could not detect functional hMSC-derived cardiomyocytes after in vitro treatment of hMSCs with differentiation media or after co-culture with mcP19 cells or mfCMCs. In our study we co-cultured hMSCs by direct cell–cell contact with cardiomyocytes (mcP19, mfCMCs) of developmentally early stages, as co-culturing with adult cardiomyocytes was reported to be ineffective for cardiomyogenic differentiation of MSCs [45]. Treatment with differentiation media led to an enhanced expression of cardiomyogenic mRNA and proteins; however, also untreated hMSCs showed an intrinsic expression of cardiomyogenic mRNA and proteins. This finding is consistent with previously reported data [46] indicating that the expression of cardiomyogenic mRNA and proteins per se might not exclusively be dedicated to cells of the cardiomyogenic lineage. The intrinsic expression of cardiomyogenic genes is obviously regulated as reflected by the dynamic changes of expression during in vitro culture. We hypothesize 2 major mechanisms underlying this observation: prolonged in vitro culturing of hMSCs might randomly induce the expression of cardiomyogenic genes as previously observed for the osteogenic lineage [47]. Alternatively, the in vitro culture conditions might promote the selection for hMSC subpopulations with high intrinsic expression of cardiomyogenic genes. In a recent study investigating BM-MSCs obtained from coronary artery disease patients, a phenotype of cells highly expressing Nkx2.5, Tbx5, Mesp-1, and Mef2C was identified to promote regeneration when transplanted in a mouse model of myocardial infarction [48]. In our study we did not analyze single cells or MSC subpopulations. Therefore, we could not address these questions; however, future investigations using single-cell PCR or flow cytometric sorting for cells expressing cardiomyogenic markers within the hMSC preparation are suggested.
In our study the exposure to cardiomyogenic differentiation media or the co-cultivation with cardiomyocytes did not lead to a functional cardiomyogenic differentiation of hMSCs in vitro, and notably even prolonged direct cell contact with cardiomyocytes did not prevent the hMSCs from noncardiomyogenic differentiation. This observation correlates to the study performed by Yoon et al. reporting on severe unexpected calcifications in the heart after the application of BM-derived multipotent stem cells [49]. However, a limitation in our approach is that we did not investigate on multiple variations of the differentiation protocols, for example, exposing the MSCs at different time points to the same media reflecting the delicate balance of transcription factors as like as brachyury, BMP2, BMP4, and noggin during different time points of cardiomyogenic development [50–53]. In our study we observed the induction of cardiomyogenic genes in MSCs by treatment with noggin at higher passages (>P5). We therefore hypothesize an indirect impact of noggin via (multiple) pathways.
In the second part of our study we evaluated the cardiomyogenic differentiation potential of BM-MSCs in an autologous mouse model of myocardial infarction: a previous study reported on functional cardiomyogenic differentiation of murine c-kit positive BM-derived stem cells in vivo [54]. In this study, the stem cell population was enriched by c-kit sorting, whereas in our study the mMSCs were highly positive for the mMSC marker Sca-1 but only slightly positive for c-kit indicating a different entity of stem cells. Psaltis et al. [55] suggested that the reasons for the modest evidence for in vivo differentiation of MSCs may be (i) the heterogeneity of the MSC populations and (ii) the low retention of multipotent stem cells after ex vivo expansion. It is unavoidable that isolation of MSC by plastic adherence leads to a contamination of the MSC preparation with undesired cell types [56]. The hMSCs and mMSCs used in our study were extensively characterized by flow cytometry and tri-lineage differentiation; however, a clonal expansion of subpopulation(s) under different culture conditions with varying susceptibility to the in vitro differentiation stimuli cannot be excluded. In previous studies, the expression of genes associated with pluripotency as like as Oct4, SOX2, and Nanog in MSCs was reported [57,58]. However, the biological relevance of these findings remains, as like as the plasticity of BM-MSCs, elusive.
In our study we showed that multiple strategies failed to induce functional cardiomyogenic differentiation of hBM-MSCs in vitro. Moreover, even direct co-culture with cardiomyocytes could not prevent hBM-MSCs from noncardiomyogenic differentiation. Our finding that a small minority of the transplanted mMSCs show evidence of functional cardiomyogenic differentiation in vivo may suggest that distinct MSC subpopulations might be more susceptible to in vivo stimuli inducing cardiomyogenic differentiation. An important goal of future MSC-based cardiac therapies will be to avoid undesired noncardiomyogenic differentiation and transformation but retain the paracrine and immunomodulatory capacity of MSCs.
Supplementary Material
Acknowledgments
The technical assistance of Ursula Hermanutz-Klein and Susanne Stachon as well as the article editing by Lorelei Shoemaker, Ph.D., is gratefully acknowledged. This work was partially funded by the German Ministry of Health grant BioProfile 0313619.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Antman EM. Hand M. Armstrong PW. Bates ER. Green LA. Halasyamani LK. Hochman JS. Krumholz HM. Lamas GA, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–247. doi: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Cohen M. Diez JE. Levine GN. Ferguson JJ., 3rd Morrow DA. Rao SV. Zidar JP. Pharmacoinvasive management of acute coronary syndrome: incorporating the 2007 ACC/AHA guidelines: the CATH (cardiac catheterization and antithrombotic therapy in the hospital) Clinical Consensus Panel Report—III. J Invasive Cardiol. 2007;19:525–538. quiz 539–540. [PubMed] [Google Scholar]
- 3.Fitchett D. Rockwood K. Chan BT. Schultz S. Bogaty P. Gillis A. Arnold M. Miller F. Graham MM, et al. Canadian Cardiovascular Society Consensus Conference 2002: management of heart disease in the elderly patient. Can J Cardiol. 2004;20(Suppl. A):7A–16A. [PubMed] [Google Scholar]
- 4.Anversa P. Leri A. Rota M. Hosoda T. Bearzi C. Urbanek K. Kajstura J. Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 5.Anversa P. Kajstura J. Leri A. If I can stop one heart from breaking. Circulation. 2007;115:829–832. doi: 10.1161/CIRCULATIONAHA.106.682195. [DOI] [PubMed] [Google Scholar]
- 6.Laugwitz KL. Moretti A. Lam J. Gruber P. Chen Y. Woodard S. Lin LZ. Cai CL. Lu MM, et al. Postnatal isl1+cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qyang Y. Martin-Puig S. Chiravuri M. Chen S. Xu H. Bu L. Jiang X. Lin L. Granger A, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Bearzi C. Rota M. Hosoda T. Tillmanns J. Nascimbene A. De Angelis A. Yasuzawa-Amano S. Trofimova I. Siggins RW, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillmanns J. Rota M. Hosoda T. Misao Y. Esposito G. Gonzalez A. Vitale S. Parolin C. Yasuzawa-Amano S, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casaclang-Verzosa G. Gersh BJ. Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Schäfer R. Northoff H. Cardioprotection and cardiac regeneration by mesenchymal stem cells. Panminerva Med. 2008;50:31–39. [PubMed] [Google Scholar]
- 12.Williams AR. Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shake JG. Gruber PJ. Baumgartner WA. Senechal G. Meyers J. Redmond JM. Pittenger MF. Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 14.Kawada H. Fujita J. Kinjo K. Matsuzaki Y. Tsuma M. Miyatake H. Muguruma Y. Tsuboi K. Itabashi Y, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 15.Mangi AA. Noiseux N. Kong D. He H. Rezvani M. Ingwall JS. Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 16.Rose RA. Jiang H. Wang X. Helke S. Tsoporis JN. Gong N. Keating SC. Parker TG. Backx PH. Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 17.Rose RA. Keating A. Backx PH. Do mesenchymal stromal cells transdifferentiate into functional cardiomyocytes? Circ Res. 2008;103:e120. doi: 10.1161/CIRCRESAHA.108.186908. [DOI] [PubMed] [Google Scholar]
- 18.Colter DC. Sekiya I. Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Schäfer R. Kehlbach R. Muller M. Bantleon R. Kluba T. Ayturan M. Siegel G. Wolburg H. Northoff H, et al. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to a decrease in migration capacity and colony formation ability. Cytotherapy. 2009;11:68–78. doi: 10.1080/14653240802666043. [DOI] [PubMed] [Google Scholar]
- 21.Wakitani S. Saito T. Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 22.Xaymardan M. Tang L. Zagreda L. Pallante B. Zheng J. Chazen JL. Chin A. Duignan I. Nahirney P, et al. Platelet-derived growth factor-AB promotes the generation of adult bone marrow-derived cardiac myocytes. Circ Res. 2004;94:E39–E45. doi: 10.1161/01.RES.0000122042.51161.B6. [DOI] [PubMed] [Google Scholar]
- 23.Shim WS. Jiang S. Wong P. Tan J. Chua YL. Tan YS. Sin YK. Lim CH. Chua T, et al. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun. 2004;324:481–488. doi: 10.1016/j.bbrc.2004.09.087. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T. Takahashi T. Esaki M. Ushikoshi H. Nagano S. Fujiwara H. Kosai K. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ J. 2004;68:691–702. doi: 10.1253/circj.68.691. [DOI] [PubMed] [Google Scholar]
- 25.Yuasa S. Itabashi Y. Koshimizu U. Tanaka T. Sugimura K. Kinoshita M. Hattori F. Fukami S. Shimazaki T. Ogawa S. Okano H. Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 26.Paquin J. Danalache BA. Jankowski M. McCann SM. Gutkowska J. Oxytocin induces differentiation of P19 embryonic stem cells to cardiomyocytes. Proc Natl Acad Sci U S A. 2002;99:9550–9555. doi: 10.1073/pnas.152302499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Just L. Kursten A. Borth-Bruhns T. Lindenmaier W. Rohde M. Dittmar K. Bader A. Formation of three-dimensional fetal myocardial tissue cultures from rat for long-term cultivation. Dev Dyn. 2006;235:2200–2209. doi: 10.1002/dvdy.20871. [DOI] [PubMed] [Google Scholar]
- 28.Cristofalo VJ. Allen RG. Pignolo RJ. Martin BG. Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckle T. Grenz A. Kohler D. Redel A. Falk M. Rolauffs B. Osswald H. Kehl F. Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–H2540. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- 31.Schafer R. Ayturan M. Bantleon R. Kehlbach R. Siegel G. Pintaske J. Conrad S. Wolburg H. Northoff H. Wiskirchen J. Weissert R. The use of clinically approved small particles of iron oxide (SPIO) for labeling of mesenchymal stem cells aggravates clinical symptoms in experimental autoimmune encephalomyelitis and influences their in vivo distribution. Cell Transplant. 2008;17:923–941. doi: 10.3727/096368908786576480. [DOI] [PubMed] [Google Scholar]
- 32.Hattan N. Kawaguchi H. Ando K. Kuwabara E. Fujita J. Murata M. Suematsu M. Mori H. Fukuda K. Purified cardiomyocytes from bone marrow mesenchymal stem cells produce stable intracardiac grafts in mice. Cardiovasc Res. 2005;65:334–344. doi: 10.1016/j.cardiores.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda K. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif Organs. 2001;25:187–193. doi: 10.1046/j.1525-1594.2001.025003187.x. [DOI] [PubMed] [Google Scholar]
- 34.Hakuno D. Fukuda K. Makino S. Konishi F. Tomita Y. Manabe T. Suzuki Y. Umezawa A. Ogawa S. Bone marrow-derived regenerated cardiomyocytes (CMG cells) express functional adrenergic and muscarinic receptors. Circulation. 2002;105:380–386. doi: 10.1161/hc0302.102593. [DOI] [PubMed] [Google Scholar]
- 35.Heng BC. Haider H. Sim EK. Cao T. Ng SC. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc Res. 2004;62:34–42. doi: 10.1016/j.cardiores.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Makino S. Fukuda K. Miyoshi S. Konishi F. Kodama H. Pan J. Sano M. Takahashi T. Hori S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazhari R. Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl. 1):S21–S26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 38.Toma C. Pittenger MF. Cahill KS. Byrne BJ. Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 39.Mohanty S. Bose S. Jain KG. Bhargava B. Airan B. TGFbeta1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. Int J Cardiol. 2011 doi: 10.1016/j.ijcardiol.2011.08.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Kim MH. Kino-oka M. Maruyama N. Saito A. Sawa Y. Taya M. Cardiomyogenic induction of human mesenchymal stem cells by altered Rho family GTPase expression on dendrimer-immobilized surface with D-glucose display. Biomaterials. 2010;31:7666–7677. doi: 10.1016/j.biomaterials.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 41.Labovsky V. Hofer EL. Feldman L. Fernandez Vallone V. Garcia Rivello H. Bayes-Genis A. Hernando Insua A. Levin MJ. Chasseing NA. Cardiomyogenic differentiation of human bone marrow mesenchymal cells: role of cardiac extract from neonatal rat cardiomyocytes. Differentiation. 2010;79:93–101. doi: 10.1016/j.diff.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Schittini AV. Celedon PF. Stimamiglio MA. Krieger M. Hansen P. da Costa FD. Goldenberg S. Dallagiovanna B. Correa A. Human cardiac explant-conditioned medium: soluble factors and cardiomyogenic effect on mesenchymal stem cells. Exp Biol Med (Maywood) 2010;235:1015–1024. doi: 10.1258/ebm.2010.010003. [DOI] [PubMed] [Google Scholar]
- 43.Koninckx R. Hensen K. Daniels A. Moreels M. Lambrichts I. Jongen H. Clijsters C. Mees U. Steels P. Hendrikx M. Rummens JL. Human bone marrow stem cells co-cultured with neonatal rat cardiomyocytes display limited cardiomyogenic plasticity. Cytotherapy. 2009;11:778–792. doi: 10.3109/14653240902988818. [DOI] [PubMed] [Google Scholar]
- 44.Balana B. Nicoletti C. Zahanich I. Graf EM. Christ T. Boxberger S. Ravens U. 5-Azacytidine induces changes in electrophysiological properties of human mesenchymal stem cells. Cell Res. 2006;16:949–960. doi: 10.1038/sj.cr.7310116. [DOI] [PubMed] [Google Scholar]
- 45.Yoon J. Shim WJ. Ro YM. Lim DS. Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Ann Hematol. 2005;84:715–721. doi: 10.1007/s00277-005-1068-7. [DOI] [PubMed] [Google Scholar]
- 46.Bayes-Genis A. Roura S. Soler-Botija C. Farre J. Hove-Madsen L. Llach A. Cinca J. Identification of cardiomyogenic lineage markers in untreated human bone marrow-derived mesenchymal stem cells. Transplant Proc. 2005;37:4077–4079. doi: 10.1016/j.transproceed.2005.09.103. [DOI] [PubMed] [Google Scholar]
- 47.Seib FP. Franke M. Jing D. Werner C. Bornhauser M. Endogenous bone morphogenetic proteins in human bone marrow-derived multipotent mesenchymal stromal cells. Eur J Cell Biol. 2009;88:257–271. doi: 10.1016/j.ejcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Behfar A. Yamada S. Crespo-Diaz R. Nesbitt JJ. Rowe LA. Perez-Terzic C. Gaussin V. Homsy C. Bartunek J. Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon YS. Park JS. Tkebuchava T. Luedeman C. Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 50.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 51.Ladd AN. Yatskievych TA. Antin PB. Regulation of avian cardiac myogenesis by activin/TGFbeta and bone morphogenetic proteins. Dev Biol. 1998;204:407–419. doi: 10.1006/dbio.1998.9094. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela DM. Economides AN. Rojas E. Lamb TM. Nunez L. Jones P. Lp NY. Espinosa R., 3rd Brannan CI. Gilbert DJ. Identification of mammalian noggin and its expression in the adult nervous system. J Neurosci. 1995;15:6077–6084. doi: 10.1523/JNEUROSCI.15-09-06077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rifas L. The role of noggin in human mesenchymal stem cell differentiation. J Cell Biochem. 2007;100:824–834. doi: 10.1002/jcb.21132. [DOI] [PubMed] [Google Scholar]
- 54.Rota M. Kajstura J. Hosoda T. Bearzi C. Vitale S. Esposito G. Iaffaldano G. Padin-Iruegas ME. Gonzalez A, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Psaltis PJ. Zannettino AC. Worthley SG. Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 56.Schäfer R. Dominici M. Muller I. Dazzi F. Bieback K. Godthardt K. Blanc KL. Meisel R. Pochampally R, et al. Progress in characterization, preparation and clinical applications of non-hematopoietic stem cells, 29–30 September 2006, Tübingen, Germany. Cytotherapy. 2007;9:397–405. doi: 10.1080/14653240701392949. [DOI] [PubMed] [Google Scholar]
- 57.Pittenger MF. Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 58.Ulloa-Montoya F. Kidder BL. Pauwelyn KA. Chase LG. Luttun A. Crabbe A. Geraerts M. Sharov AA. Piao Y. Ko MS. Hu WS. Verfaillie CM. Comparative transcriptome analysis of embryonic and adult stem cells with extended and limited differentiation capacity. Genome Biol. 2007;8:R163. doi: 10.1186/gb-2007-8-8-r163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.