Abstract
A combination of two different methods for the synthesis of oligoribonucleotides, i.e. the two-step phosphotriester method with 2-chlorophenyl phosphate as bifunctional phosphate source and the modified triester method with 2,2,2-trichloroethyl 2-chlorophenyl phosphorochloridate as monofunctional phosphate source, is applied for the synthesis of the fully-protected hexaribonucleotide A-C-C-U-C-C. The two-step method is used for the synthesis of the required dinucleotide monophosphates 9, 10 and 11. Application of the modified triester method for the coupling of the oligonucleotide blocks results in the formation of the fully-protected hexamer 15. Furthermore, attention is paid to 2,4,6-triisopropylbenzenesulphonyl 4-nitroimidazolide as a new condensing agent for the coupling of larger oligonucleotide blocks.
Full text
PDF



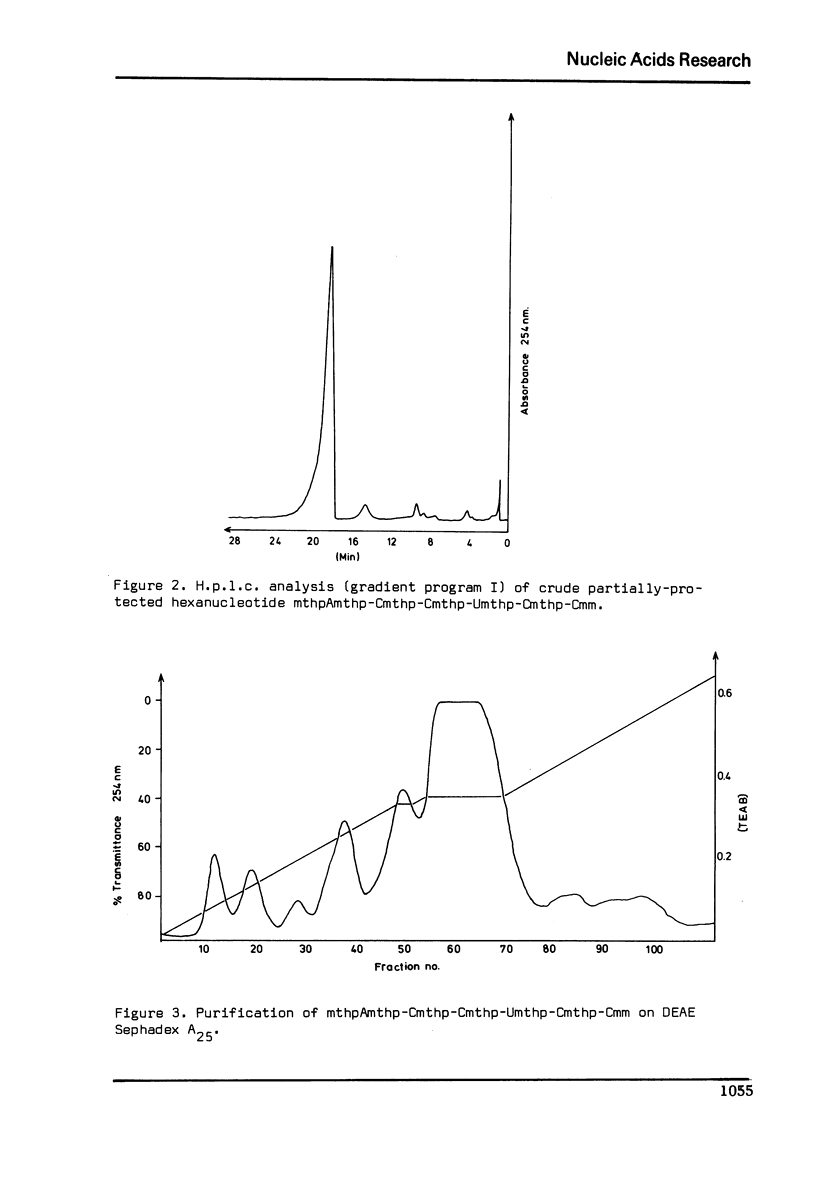








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baan R. A., Duijfjes J. J., van Leerdam E., van Knippenberg P. H., Bosch L. Specific in situ cleavage of 16S ribosomal RNA of Escherichia coli interferes with the function of initiation factor IF-1. Proc Natl Acad Sci U S A. 1976 Mar;73(3):702–706. doi: 10.1073/pnas.73.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Green D. P., Ravindranathan T., Reese C. B., Saffhill R. The synthesis of oligoribonucleotides-8. The preparation of ribonucleoside 2',5'-bisketals. Tetrahedron. 1970 Feb;26(4):1031–1041. doi: 10.1016/s0040-4020(01)98780-0. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B., Sulston J. E. The synthesis of oligoribonucleotides. II. Methoxymethylidene derivatives of ribonucleosides and 5'-ribonucleotides. Tetrahedron. 1967 May;23(5):2301–2313. doi: 10.1016/0040-4020(67)80067-x. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Lohrmann R., Söll D., Hayatsu H., Ohtsuka E., Khorana H. G. Studies on polynucleotides. LI. Syntheses of the 64 possible ribotrinucleotides derived from the four major ribomononucleotides. J Am Chem Soc. 1966 Feb 20;88(4):819–829. doi: 10.1021/ja00956a039. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Stewart J. C., van Boom J. H., de Leeuw H. P., Nagel J., de Rooy J. F. The synthesis of oligoribonucleotides. Part XI. Preparation of ribonucleoside 2'-acetal 3'-esters by selective deacylation. J Chem Soc Perkin 1. 1975;(10):934–942. doi: 10.1039/p19750000934. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., Burgers P. M., Haasnoot C. A. Synthesis of 3'-phosphates of diribonucleoside monophosphates via phosphotriester intermediates. Nucleic Acids Res. 1976 Oct;3(10):2731–2747. doi: 10.1093/nar/3.10.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., de Rooy J. F. Sequence analysis of synthetic oligonucleotides by high-performance liquid anion-exchange chromatography. J Chromatogr. 1977 Jan 21;131:169–177. doi: 10.1016/s0021-9673(00)80930-9. [DOI] [PubMed] [Google Scholar]


