Abstract
CONTEXT:
Matrix metalloproteinases (MMPs) are a family of enzymes that degrade various components of the extracellular matrix and are involved in the development and progression of cancer. Lung cancer is the most commonly diagnosed cancer in Lebanon. MMP1 is responsible for degrading stromal collagens, which enhance the ability of neoplastic cells to cross basal membrane of both the endothelium and the vascular endothelium. A recent meta-analysis has suggested that the MMP1-1607 2G allele may be associated with an increased risk for certain types of cancers.
AIM:
This study was undertaken to investigate the association between guanine insertion polymorphism in the MMP1 promoter and the susceptibility to lung cancer in the Lebanese population.
SETTINGS AND DESIGN:
This case-control study was conducted on 41 patients with lung cancer and 51 age-matched healthy controls, recruited from different regions of Lebanon.
METHODS:
Cases were histologically confirmed lung cancer patients obtained from different hospitals in Lebanon. Controls were healthy unrelated individuals with no history of cancer or genetic diseases. All subjects were genotyped for MMP1 -1607(1G>2G) polymorphism using polymerase chain reaction-restriction fragment length polymorphism method (PCR-RFLP).
RESULTS:
No statistically significant differences were found when genotype and allele distribution of MMP1 -1607(1G>2G) polymorphism were compared between patients with lung cancer and controls [P= 0.6 by chi-squared test on a 3×2 contingency table; allelic P=0.61, OR (95% CI) = 1.18 (0.60-2.31)].
CONCLUSION:
Our data shows that MMP1 promoter polymorphism is not associated with lung cancer susceptibility in the Lebanese population.
Keywords: Lebanon, lung cancer, MMP1, polymorphism
Matrix metalloproteinases (MMPs) are a family of enzymes which are classified according to substrate specificity and structural similarity.[1] Their basic function is to degrade extracellular matrix and basement membrane components.[2] In addition to cancer development, MMPs are also known to be involved in cancer metastasis and invasion.[3] MMP1, also known as interstitial collagenase, is responsible for degrading stromal collagens, which enhance the ability of neoplastic cells to cross basal membrane of both the endothelium and the vascular endothelium.[1] MMP1gene, which is located on chromosome11q, is expressed in normal cells, including macrophages and endothelial cells, as well as in various tumor cells.[4] The level of MMP1 expression can be affected by single nucleotide polymorphism (SNP). An SNP of the MMP1 gene occurs at position 1607 bp upstream of the transcriptional initiation site. An insertion of a guanine base (G) creates the sequence 5’-GGAT-3’, the core binding site for members of the EST family of transcription factors.[5] MMP1 –1607 2G allele has been associated with higher transcriptional activity of the gene.[5]
In Lebanon, lung cancer is among the most commonly diagnosed cancers.[6] The incidence rate for lung cancer in the Lebanese population is about 15.7 per 100,000 for males and 5.9 per 100,000 for females.[7] The exposure to carcinogens is high in Lebanon where smoking is widespread and people are increasingly smoking Narguileh[8,9] which releases smoke rich in carcinogens.[10,11] Therefore, the identification of genetic susceptibility to lung cancer in the Lebanese population is essential for better prevention, screening, and treatment. A case-control study on Caucasian population has demonstrated an association between MMP1 -1607 2G/2G genotype and increased susceptibility to lung cancer.[12] To the best of our knowledge, the association between the MMP1 polymorphism and lung cancer has not yet been assessed in the Lebanese population. This study was undertaken to investigate the association between guanine insertion polymorphism in the MMP1 promoter and the susceptibility to lung cancer in the Lebanese population.
Methods
Study subjects
This case-control study was carried out between October 2008 and October 2009. Samples were consecutively collected from a total of 41 histologically confirmed lung cancer patients admitted to various hospitals in Lebanon. The control group consisted of 51 healthy unrelated individuals with no history of cancer or genetic diseases. Cases and controls were matched by age (± 5). Information about smoking history was obtained from cases only. IRB approval was granted from all the hospitals involved. The consent form was approved and signed by all study subjects.
Determination of the MMP1 -1607(1G>2G) polymorphism
Genomic DNA was extracted from blood samples obtained from the study subjects. DNA isolation was performed from 300 μl whole blood using FlexiGene DNA kit (Qiagen GmBH D-40724 Hilden). The method of detection of the MMP-1 Alu I polymorphism was based on the previously described PCR-RFLP.[13] Briefly, a 269-bp DNA fragment containing a polymorphic site for the Alu I enzyme in the 1607 bp upstream of the transcriptional site of the MMP1 gene was amplified by PCR. An aliquot of 10 μl of the completed PCR reaction was incubated with 20 units of the Alu I enzyme for 16 hours at 37°C. The samples were then visualized on 3% agarose gel. The MMP1 2G allele was represented by a 269-bp band, whereas the 1G allele was represented by 241 and 28 bp bands. Heterozygotes displayed all the three bands.
Statistical analysis
Allele and genotype frequencies were obtained by direct counting. Chi-squared test was used for the statistical analysis to compare allelic and genotypic distributions. We assessed the quality of the genotype data by testing for Hardy–Weinberg equilibrium in the case and control samples, using Chi-squared test. Odds ratio (OR), 95% confidence interval (CI), and corresponding P-value were calculated to describe the strength of any possible association. Associations were considered to be statistically significant at the 0.05 level. All calculations were done using the Statistical Package for Social Sciences (SPSS, version 19).
Results
Characteristics of the study sample
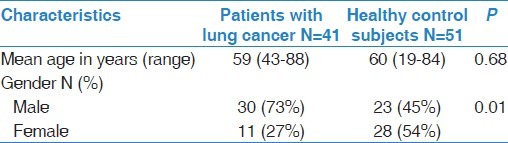
Information about the age and the gender of the subjects included in the study are summarised in Table 1. As expected due to the matching by age, cases and controls had similar age. The majority of the cases were males (73%) and smokers (93%). Indeed, out of the 41 studied cases, only 2 (4.9%) were never smokers, 1 (2.4%) was an ex-smoker, whereas, 38 (92.7%) were current smokers.
Table 1.
Characteristics of the study population
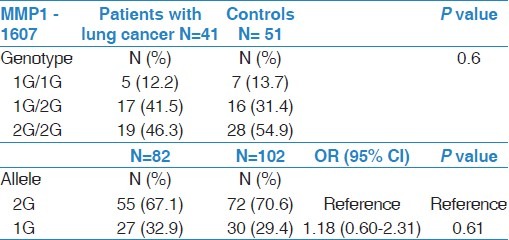
Distribution of MMP1 SNP in patients with lung cancer and controls
The genotype and allele frequencies of the MMP1 gene in lung cancer patients and controls are presented in Table 2. The genotype frequency distribution was in accordance with Hardy-Weinberg equilibrium expectations. There was no statistically significant difference in the genotype frequency distribution between cases and controls (P=0.60). Moreover, the 2G allele was more frequent than 1G allele in both cases and controls. Furthermore, the results showed that the 1G allele is more frequent in patients compared to controls. However, this finding did not reach statistical significance (OR= 1.18; 95% CI = 0.60 to 2.31, P-value = 0.6).
Table 2.
Allele and genotype frequencies of MMP1 -1607(1G>2G) polymorphism in patients with lung cancer and in controls
Discussion
The fact that only a fraction of smokers will eventually suffer from cancer, is most likely explained by life style variations, exposure to other carcinogens, and amount of cigarette consumed. Nonetheless, individual susceptibility remains a major factor. MMP1 has been shown to be correlated with the development of cancer. The present study is the first to examine the correlation between gene variant of MMP1 and lung cancer within the Lebanese population. Our results showed that the variant MMP1 2G allele is not significantly associated with lung cancer in the Lebanese population. This is in agreement with previous studies in China[13] and United Kingdom.[14] However, another study on a Caucasian population reported a significant association between the 2G/2G genotype and lung cancer risk in current smokers.[12]
The contradictory findings in genetic association studies may be explained by the possible variation in the frequency of this polymorphism among different populations. Indeed, a recent meta-analysis has demonstrated that the MMP1-1607 2G allele is associated with an increased risk for certain types of cancers, including colorectal, head and neck and renal cancers but not with lung cancers.[15] Furthermore, the same study has demonstrated the influence of ethnicity on such association studies, where the MMP1 genetic variants influenced susceptibility in the Asian but not the European populations.[15]
A major limitation of the present study was the small sample size of the study subjects. Large-scale studies are required to confirm the present finding. Moreover, a larger study population would allow for result stratification. In the current study, only two of our cases never smoked before; therefore small sample size made data stratification impossible.
In conclusion, this is the first study to investigate the association between the MMP1 polymorphism and lung cancer in the Lebanese population. We found no significant differences in allele or genotype frequencies for the MMP1 gene polymorphism between lung cancer patients and controls. A larger sample size is needed to confirm the current finding.
Footnotes
Source of Support: Lebanese National Council for Scientific Research CNRS
Conflict of Interest: None declared.
References
- 1.Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–30. [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: Prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–66. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 4.Ye S, Dhillon S, Turner SJ, Bateman AC, Theaker JM, Pickering RM, et al. Invasiveness of cutaneous malignant melanoma is influenced by matrix metalloproteinase 1 gene polymorphism. Cancer Res. 2001;61:1296–8. [PubMed] [Google Scholar]
- 5.Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5. [PubMed] [Google Scholar]
- 6.Shamseddine A, Sibai AM, Gehchan N, Rahal B, El-Saghir N, Ghosn M, et al. Cancer incidence in postwar Lebanon: findings from the first national population-based registry, 1998. Ann Epidemiol. 2004;14:663–8. doi: 10.1016/j.annepidem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Shamseddine AI, Musallam KM. Cancer Epidemiology in Lebanon. Middle East J Cancer. 2010;1:41–4. [Google Scholar]
- 8.Waked M, Salameh P, Aoun Z. Water-pipe (narguile) smokers in Lebanon: a pilot study. East Mediterr Health J. 2009;15:432–42. [PubMed] [Google Scholar]
- 9.Saade G, Warren CW, Jones NR, Asma S, Mokdad A. Linking Global youth tobacco survey (GYTS) data to the WHO Framework convention on tobacco control (FCTC): The case for Lebanon. Prev Med. 2008;47(Suppl 1):S15–9. doi: 10.1016/j.ypmed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem Toxicol. 2005;43:655–61. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Monzer B, Sepetdjian E, Saliba N, Shihadeh A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem Toxicol. 2008;46:2991–5. doi: 10.1016/j.fct.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Spitz MR, Lei L, Mills GB, Wu X. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances lung cancer susceptibility. Cancer Res. 2001;61:7825–9. [PubMed] [Google Scholar]
- 13.Fang S, Jin X, Wang R, Li Y, Guo W, Wang N, et al. Polymorphisms in the MMP1 and MMP3 promoter and non-small cell lung carcinoma in North China. Carcinogenesis. 2005;26:481–6. doi: 10.1093/carcin/bgh327. [DOI] [PubMed] [Google Scholar]
- 14.Su L, Zhou W, Park S, Wain JC, Lynch TJ, Liu G, et al. Matrix metalloproteinase-1 promoter polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:567–70. doi: 10.1158/1055-9965.EPI-04-0482. [DOI] [PubMed] [Google Scholar]
- 15.Peng B, Cao L, Wang W, Xian L, Jiang D, Zhao J, et al. Polymorphisms in the promoter regions of matrix metalloproteinases 1 and 3 and cancer risk: A meta-analysis of 50 case-control studies. Mutagenesis. 2010;25:41–8. doi: 10.1093/mutage/gep041. [DOI] [PubMed] [Google Scholar]


