Abstract
INTRODUCTION:
Chronic obstructive pulmonary disease (COPD) is a leading cause of respiratory-related morbidity and mortality. Inhaled steroids are frequently used in patients with moderate to severe disease and may lead to adrenal suppression.
OBJECTIVES:
The aim of this study was to compare the effect of inhaled budesonide/formoterol with inhaled fluticasone/salmeterol in severe COPD.
METHODS:
It was a prospective open-label crossover study of 22 patients. Adrenal suppression was measured by overnight urinary cortisol/creatinine ratio. The measurements were taken while patients were on either combination for at least 4 weeks.
RESULTS:
A total of 12 patients completed the study. The mean age was 64 years (8 males, 4 females). The mean FEV1 was 1 L (range, 0.5-1.8). There was no significant difference in adrenal suppression measured by overnight urinary cortisol/creatinine ratio (budesonide 5.2 ± 4.3, fluticasone 4.7 ± 3.1; 95% CI -2.2 to 1.2; P = 0.52) and urinary cortisol concentration (budesonide 51 ± 53, fluticasone 43 ± 31 [nmol/l]; 95% CI -35 to 20; P = 0.56).
CONCLUSION:
Inhaled budesonide and fluticasone have no significantly different effect on adrenal function in moderate to severe COPD. The adverse event profile of high-dose inhaled steroids should not influence the choice of medication.
Keywords: Adrenal suppression, COPD, cortisol-creatinine ratio, inhaled corticosteroids
Chronic obstructive pulmonary disease (COPD) is the leading cause of respiratory-related morbidity and is one of the leading causes of mortality accounting for 5% of deaths worldwide in 2005.[1] There are limited management options in this group of patients and a significant proportion of this population with moderate disease is prescribed inhaled corticosteroids (ICS) often in combination with long-acting beta agonists (LABA). Current guidelines for management of COPD suggest that ICS LABA combinations should be prescribed for patients with a forced expiratory volume in 1 second (FEV1) of less than 50% predicted who have frequent exacerbations.[2] The aim of this treatment is to reduce the exacerbation rates and slow the decline in health status. The licensed dose of inhaled steroids in combination therapy is high and clinicians need to be aware of the potential risk of systemic side effects. The combination inhalers licensed in COPD include budesonide/formoterol fumarate and fluticasone propionate/salmeterol xinafoate. The recommended daily dose of budesonide is 800 μg and fluticasone is 1 000 μg.
Budesonide/formoterol has been evaluated in randomized controlled trials in patients with COPD and has been found to be beneficial compared with either component alone.[3,4] Similar data are available for fluticasone/salmeterol.[5–7] Although inhaled steroids have a very good safety profile at low doses, the dose used in COPD has a potential for systemic adverse effects such as adrenal suppression. A systematic review and meta-analysis[8] has shown that significant adrenal suppression can occur in asthma with high-dose ICS, especially above 1.5 mg/day (750 μg/day for fluticasone). It is also suggested that fluticasone has greater potency for dose-related adrenal suppression as compared with beclomethasone, budesonide, or triamcinolone.[8,9] However, we cannot extrapolate from this data as the doses used in various studies have been different and these studies have been in patients with asthma, who may have different bioavailability from those with COPD.
As there are no comparative studies of budesonide/fluticasone, on adrenal suppression in COPD, it is difficult to decide which one to choose on initiation of therapy. The aim of this study was to compare the effect of the two combination steroid preparations on adrenal function since this could influence treatment decision in specific patient groups considered more at risk for systemic adverse effects.
The primary objective of the study was to determine the adverse effects of budesonide/formoterol, 400 μg/12 μg twice a day, and fluticasone/salmeterol, 500 μg/50 μg twice a day, with respect to adrenal function as measured by the overnight urinary cortisol/creatinine ratio. The secondary objective of this study was to evaluate local adverse effects with either steroid formulation.
Methods
This was an 8-week open label crossover study. A 4-week treatment period with budesonide or fluticasone, in a dry powder via turbohaler or accuhaler, respectively was followed by 4 weeks of the alternative inhaler.
Patients
Male and female patients with a diagnosis of moderate to severe COPD were invited to participate in the study from outpatient clinics. The patients had to be ≥40 years of age, have a smoking history of ≥10 pack-years, FEV1 of ≤50% of predicted, and their current treatment consisted of a combination of inhaled steroid and LABA at a dose of 800 or 1 000 μg for budesonide or fluticasone, respectively. Treatment requirements had to be stable for at least 4 weeks prior to recruitment. Medications, including short-acting β2-adrenoceptor agonists, anticholinergics, and tiotropium, were permitted during the study.
The patients were excluded if they had an acute exacerbation of COPD requiring oral corticosteroids in the last 8 weeks; had used oral glucocorticosteroids within the last 2 months; had used ocular, intra-articular, rectal, or other glucocorticosteroids during 4 weeks prior to inclusion; had history of asthma; were receiving drugs with endogenous glucocorticoid activity; or had a history of adrenocortical insufficiency or Cushing's syndrome.
The study was approved by Hull and East Riding Ethics Committee (LREC No: 06/Q1104/68) and written consent was obtained from all the participants prior to recruitment into the study.
Study design
The patients were reviewed three times following their recruitment visit. The inhaler technique was checked at each visit and the importance of adherence to the study medication was emphasized to the patients. A bottle for collection of an overnight urine sample was dispensed to the patients at visit 1 and 2. The patients were advised to collect the sample between 20:00 hr and 08:00 hr the night prior to the next study visit.
Urinary cortisol was measured by one-step immunoassay using chemiluminescent microparticle immunoassay technology (Abbott Architect). Urinary creatinine was measured by Jaffe reaction using Beckman Coulter (High Wycombe, UK) Unicel DxC 800 analyzer.
All patients had a baseline spirometry examination and completed the St George's Respiratory Questionnaire (SGRQ) for measuring impaired health and perceived well-being in patients with COPD. The SGRQ was further completed at the end of four weeks on treatment with each inhaled corticosteroid/LABA combination.
A physical examination including oropharyngeal inspection was performed at each visit. The participants were assessed for adverse events at visits 2 and 3 concentrating on local drug-related adverse effects such as oral thrush. They were told to report any adverse effects with either of the steroid formulation immediately. An exacerbation of COPD was classified as an adverse event.
Statistical analysis
The data were analyzed by SPSS (Version 13; Chicago, IL). As the data followed a normal distribution (Kolmogorov-Smirnov Test), the urinary cortisol and cortisol-creatinine ratio were analyzed by paired t-test. A post-hoc power calculation based on effect size showed that the study would have 90% ability to detect a difference in cortisol-creatinine ratio at a- level of 0.05 with 12 paired observations.
Results
Patient characteristics
A total of 22 patients were recruited. Twelve patients completed the study (eight males), with ten exclusions. The reasons for exclusions are shown in Figure 1.
Figure 1.
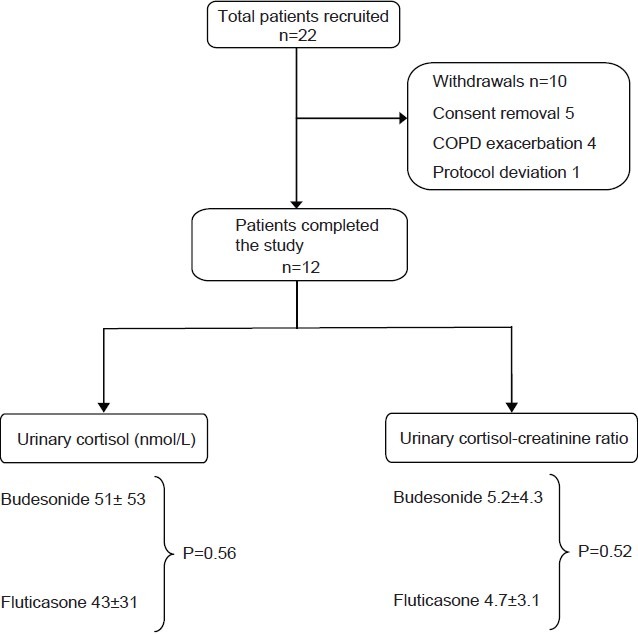
Algorithm for patient recruitment and withdrawals during the study
The baseline characteristics of patients in the study are summarized in Table 1.
Table 1.
Baseline demographics and characteristics of study patients
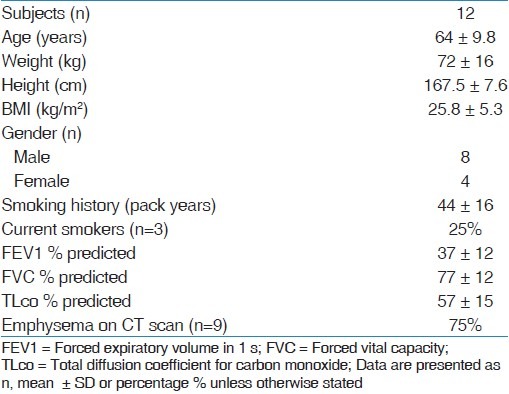
Cortisol measurements and urinary cortisol/creatinine ratio
There was no significant difference in the urinary cortisol-creatinine ratio between fluticasone or budesonide (budesonide 5.2 ± 4.3, fluticasone 4.7 ± 3.1; 95% CI -2.2 to 1.2; P = 0.52; Paired t-test). The change of ratio in 12 patients completing the study is shown in Figure 2. The urinary cortisol concentration did not differ in any group [budesonide 51 ± 53, fluticasone 43 ± 31 (nmol/l); 95% CI -35 to 20; P = 0.56; Paired t-test]. Furthermore, there were no significant drug-related adverse events noted during budesonide or fluticasone treatment periods.
Figure 2.
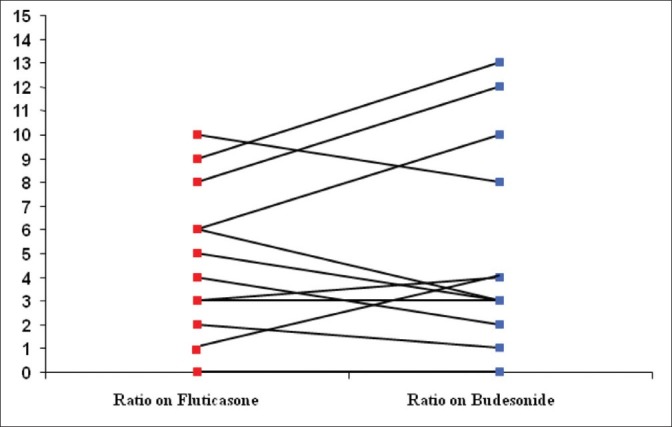
Change of urinary cortisol-creatinine ratio on budesonide (shown in blue) and fluticasone (shown in red)
St George's respiratory questionnaire
There was no significant difference in the SGRQ scores (baseline 27.8 ± 6.7; fluticasone 28.5 ± 5.2; budesonide 26.5 ± 5.1; P = 0.14).
Adverse events and drop-outs
The majority of drop-outs in this study were secondary to either consent withdrawal or exacerbation of COPD requiring oral corticosteroids. There was no difference in drop-out rate with either steroid formulation with exactly same proportion of patients on each drug (n = 5). There were no significant local adverse effects observed with any of the steroid formulation. Only one patient developed headache following crossover from fluticasone to budesonide. However, it did not result in withdrawal from the study.
Discussion
The present study is the first prospective crossover study comparing the effects of high-dose inhaled budesonide with fluticasone on adrenal suppression, in patients with moderate to severe COPD. The results indicate that there is no significantly different effect of either steroid formulation, on adrenal function, when measured by overnight urinary cortisol level and cortisol-creatinine ratio.
Tests for adrenal function include basal tests for adrenocortical activity and dynamic stimulation tests to check adrenocortical reserve. Integrated 24-hour plasma-free cortisol and urinary cortisol excretion is a very sensitive basal test for adrenal function.[10] Measurement of an overnight or early morning urinary cortisol-creatinine excretion is as sensitive as an integrated 24-hour urinary cortisol collection and more sensitive than a 9 am cortisol assay.[11] It is a simple and accurate test for within patient comparison of adverse effects on adrenal function. Hence, it was chosen as primary surrogate marker of adrenal suppression in this study.
The systemic effects of inhaled budesonide and fluticasone, in terms of adrenal suppression, have been investigated in asthmatic patients in a number of studies[12–15] with conflicting results. Ringdal et al.[13] compared the effects of both steroids on hypothalamic-pituitary-adrenal (HPA) axis in a randomized, double blind, multicenter crossover trial. They compared budesonide 1 600 μg daily with fluticasone propionate 1 500 μg daily and found no clinically significant effects on HPA axis in moderate to severe asthma population. In contrast, Clark and Lipworth[14,15] found significantly greater adrenal suppression (measured by both plasma and urinary cortisol) at three different doses, with fluticasone as compared to budesonide in asthmatic children as well as adults. They conclude that fluticasone may exert greater adrenal suppression due to enhanced receptor potency, prolonged tissue retention, and longer half-life. Fluticasone propionate is a trifluorinated glucocorticoid that is 300-fold lipophilic than budesonide, resulting in faster association with glucocorticoid receptor and slower dissociation, resulting in a half-life of >10 hours.[16] The greater lipophilicity of fluticasone results in greater binding in systemic tissues with consequently increased volume of distribution and prolonged airway and systemic retention.[17] Recently, Derom et al.[18] conducted first placebo-controlled, crossover study evaluating the systemic effects of ICS (Ciclesonide and Fluticasone) on cortisol secretion, bronchial hyper-responsiveness, and bone markers in ICS-dependent asthmatic patients. They found a significantly greater suppression of adrenal function and bone formation markers with fluticasone in comparison with ciclesonide. This could largely be explained by the difference in half-life of 3 hours and 7-14 hours in case of ciclesonide and fluticasone, respectively.
In a randomized double blind, crossover study, Dalby et al.[19] evaluated systemic bioavailability and airway clearance of fluticasone and budesonide in combination with LABA in 28 patients with severe COPD (Mean FEV1 37% predicted) and 27 healthy controls. Plasma concentration of ICS over 10 hours post-inhalation and sputum concentration of steroid over 6 hours were measured. The relative systemic bioavailability of the steroid formulation was similar in COPD patients and healthy subjects supporting our finding of the similar adrenal effects of these drugs. In a double blind, randomized crossover study[20] evaluating pharmacokinetics of fluticasone in COPD and healthy controls, fluticasone caused less HPA suppression in COPD patients than healthy subjects. This difference was thought to be likely to be due to the altered pharmacokinetics of fluticasone propionate in COPD with the Cmax and Area under the curve being substantially lower in comparison with controls. This suggests that adrenal response to inhaled steroids is blunted in patients with COPD, making significant adrenal suppression less likely.
The inhaler design may have a differential effect on drug absorption and bioavailability. We compared the two steroid formulations used via turbohaler (budesonide) and accuhaler (fluticasone). It is impossible in our current study to determine the relative contributions of differences in drug deposition or differences in pharmacological properties that contribute to our findings.
The findings of our study are interesting in terms of failure to show any significantly different effect on adrenal function with either formulation, and are in contrast to many studies conducted in patients with asthma. There could be a number of potential explanations for these findings. Patients with COPD, in particular with emphysema, have lung damage secondary to smoking with consequent reduction in steroid absorption in comparison with asthmatics that may have greater tendency to have systemic adverse effects. This mechanism is supported by the reduced gas transfer of 57% of predicted seen in our patients and evidence of extensive emphysema in those in whom thoracic CT scan had been performed. Alternatively, the adrenal response to high-dose ICS in COPD patients may be blunted due to the systemic effects of nicotine on HPA axis as there is evidence of impaired Adrenocorticotropic Hormone (ACTH) and cortisol stimulation in response to insulin-induced hypoglycemia and lysin-8-vasopressin test in smokers when compared with nonsmokers.[21,22] We do not know whether the patients with moderate to severe COPD have significant basal adrenal suppression because of the confounding effect of preceding frequent administration of oral corticosteroids.
In our opinion, the present study has certain limitations. First, we do acknowledge the small number of participants and a high dropout rate in the study. Second, the doses of budesonide and fluticasone studied in this investigation might not be equipotent and may have a differential effect on adrenal function. However, the decision of comparison of 1 000 μg of fluticasone with 800 μg of budesonide per day was based on these doses being the recommended doses in moderate to severe COPD. Evaluation of adrenal suppression at different doses of ICS may enhance our understanding of effects of corticosteroids on HPA axis. Finally, this study was conducted at a single center and multicenter collaboration with a larger sample size may highlight the effects of inhaled steroids on adrenal function in greater detail.
Conclusion
In summary, the results of this prospective crossover study in COPD suggest that there is no significantly different effect on adrenal function with fluticasone in comparison with budesonide. Moreover, there was no evidence of significantly adverse clinical events in any of the group. The decision of commencing high-dose inhaled steroids should be made on an individual basis, taking into account the cost-effectiveness, patient preference, and inhaler technique.
Acknowledgments
We thank Dr. V Allgar and Dr. A Rigby for their help in statistical analysis of the data presented in the study. We are grateful to clinical trials unit staff at Castle Hill Hospital for their help in conducting the study. Clinical Trials Registration. Clinical Trial.Gov Nct01186653.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1. [Last accessed on 2011 Sep 09]. Available from: http://www.who.int/mediacentre/factsheets/fs315/en/index.html .
- 2.National Institute for Clinical Excellence (NICE). www.nice.org.uk. Chronic obstructive pulmonary disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. 2010 [Google Scholar]
- 3.Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 4.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–9. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 5.Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 6.Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124:834–43. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 7.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Trial of Inhaled Steroids And long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 8.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy. A systematic review and meta-analysis. Arch Intern Med. 1999;159:941–55. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 9.Derom E, Van Schoor J, Verhaeghe W, Vincken W, Pauwels R. Systemic effects of inhaled fluticasone propionate and budesonide in adult patients with asthma. Am J Respir Crit Care Med. 1999;160:157–61. doi: 10.1164/ajrccm.160.1.9805106. [DOI] [PubMed] [Google Scholar]
- 10.Lipworth BJ, Seckl JL. Measures for detecting systemic bioactivity with inhaled and intra-nasal corticosteroids. Thorax. 1997;52:476–82. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntyre DH, Mitchell CA, Bowler SD, Armstrong JG, Wooler JA, Cowlie DM. Measuring the systemic effects of inhaled beclomethasone: Timed morning urine collections compared with 24 hour specimens. Thorax. 1995;5:281–84. doi: 10.1136/thx.50.12.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison TW, Wisniewski A, Honour J, Tattersfield AE. Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects. Thorax. 2001;56:186–91. doi: 10.1136/thorax.56.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ringdal N, Lundbäck B, Alton M, Rak S, Eivindson A, Bratten G, et al. Comparable effects of inhaled fluticasone propionate and budesonide on the HPA-axis in adult asthmatic patients. Respir Med. 2000;94:482–9. doi: 10.1053/rmed.1999.0758. [DOI] [PubMed] [Google Scholar]
- 14.Clark DJ, Clark RA, Lipworth BJ. Adrenal suppression with inhaled budesonide and fluticasone propionate given by large volume spacer to asthmatic children. Thorax. 1996;51:941–3. doi: 10.1136/thx.51.9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark DJ, Lipworth BJ. Adrenal suppression with chronic dosing of fluticasone propionate compared with budesonide in adult asthmatic patients. Thorax. 1997;52:55–8. doi: 10.1136/thx.52.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. J Allergy Clin Immunol. 1998;101:S434–9. doi: 10.1016/s0091-6749(98)70155-1. [DOI] [PubMed] [Google Scholar]
- 17.Thorsson L, Dahlstrom K, Edsbacker S, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–61. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derom E, Louis R, Tiesler C, Engelstätter R, Kaufman JM, Joos GF. Effects of ciclesonide and fluticasone on cortisol secretion in patients with persistent asthma. Eur Respir J. 2009;33:1277–86. doi: 10.1183/09031936.00079908. [DOI] [PubMed] [Google Scholar]
- 19.Dalby C, Polanowski T, Larsson T, Borgström L, Edsbäcker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10:104. doi: 10.1186/1465-9921-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SD, Whale C, Houghton N, Daley-Yates P, Kirby SM, Woodcock AA. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2003;55:375–81. doi: 10.1046/j.1365-2125.2003.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellini M, Baccarini S, Dimitriadis E, Sartori MP, Letizia C. Effect of smoking on the hypophyseo-adrenal axis. Medicina. 1989;9:194–6. [PubMed] [Google Scholar]
- 22.Sellini M, Sartori MP, Letizia C, Dimitriadis E, Bassi R, Baccarini S. Changes in the levels of ACTH and cortisol after passive exposure to cigarette smoke in smokers and non-smokers. Boll Soc Ital Biol Sper. 1989;65:365–9. [PubMed] [Google Scholar]


