Abstract
BACKGROUND:
The clinical relevance of bone marrow micrometastasis (BMM) in non–small-cell lung cancer is undetermined, and the value of such analyses in advanced stage patients has not been clearly assessed previously. This study was conducted to estimate the accuracy of both polymerase chain reaction (PCR) and immunohistochemistry (IHC) in micrometastases detection and determine the best site for bone marrow biopsy in order to find micrometastasis.
METHODS:
This prospective cross-sectional study was performed in the Department of Thoracic Surgery, Alzahra University Hospital from September 2008 to June 2009. To evaluate the bone marrow, a 3-cm rib segment and an aspirated specimen from the iliac bone prior to tumor resection were taken. PCR and IHC were performed for each specimen to find micrometastasis.
RESULTS:
Of 41 patients, 14 (34%) were positive for BMM by PCR compared with two positive IHC (4.8%). All BMMs were diagnosed in rib segments, and iliac specimens were all free from metastatic lesion. Our data showed no significant association between variables such as age, sex, histology, tumor location, side of tumor, involved lobe, smoking, or weight loss and presence of BMM.
CONCLUSION:
PCR could use as a promising method for BMM detection. BMM in a sanctuary site (rib) is not associated with advanced stages of lung cancer. In addition, when predictor variables such as age, sex, histology, tumor location, smoking, or weight loss are analyzed, no correlation can be found between micrometastasis prevalence and any of those variables.
Keywords: IHC, lung cancer, micrometastasis, PCR
Precise staging of non–small-cell lung cancer (NSCLC) determines prognosis and promotes important decisions regarding treatment options. Previous staging system such as the tumor-node-metastasis staging system had limitations in the range in survival rates.[1] However, the new staging system has limitations too.[2] For example, after obvious complete resection in patients with stage I disease, the recurrence rates range from 25% to 50% and the overall 5-year survival rate is a dismal 60% to 70%.[3] It would appear from these statistics that a certain number of patients judged to have a localized primary tumor by standard pathologic examination may have more extensive disease with occult locoregional lymph node and/or distant micrometastases.
The two most common approaches used to detect occult tumor cells in lymph nodes and bone marrow are immunohistochemistry (IHC) for epithelial cell-associated proteins and Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) to detect tumor- or epithelial cell-related messenger RNAs. Interestingly, none of the markers described to date are actually cancer-specific markers. Instead, they are typically cell- or tissue type-related markers, such as cytokeratins, epithelial cell surface antigens, mammoglobin (breast cancer), and tyrosinase (melanoma). For IHC studies, this apparent weakness can be overcome because the pathologist can view the stained cells and look for other morphologic characteristics consistent with tumor cells. For RT-PCR studies, however, this can be a serious drawback and is one of the main criticisms of the RT-PCR approach.
If detection of micrometastases in lymph nodes, bone marrow, and/or other sites is conclusively shown to impact staging, this will also influence decisions regarding the use of multimodality treatment strategies. This study was conducted to estimate the accuracy of both RT-PCR and IHC in micrometastasis detection and determine the best site for bone marrow biopsy in order to find micrometastasis.
Methods
This prospective cross-sectional study was conducted from September 2008 to June 2009 in the Department of Thoracic Surgery at Al- Zahra University Hospital (Isfahan University of Medical Sciences). The Ethics committee of Isfahan University of Medical Sciences approved the study protocol.
Forty-one patients with lung cancer (candidates for complete removal of lesion and adjacent lung) were included in the study. Exclusion criteria included patients with history of neoadjuvant therapy, patients with bone metastases in adjacent ribs, and patients with small-cell lung cancer.
After acquiring biographical data and baseline patient characteristics, patients with primary NSCLC were staged according to the TNM classification. The size, histological type, and grade of the primary tumor were assessed by conventional histopathology. After informed consent, in the operation room, a 3-cm segment of rib and an aspirated bone marrow specimen from the iliac region prior to tumor resection were obtained and drawn in fine fix bottle identified with the patient ID, date, and location of specimen. Primary tumors from patients with NSCLC were surgically excised and the rib or iliac bone specimens analyzed by IHC and RT-PCR to detect micrometastases.
Immunohistochemistry
Tissue samples were routinely fixed and dehydrated in a graded series of alcohol and xylene and embedded in paraffin at a temperature not exceeding 60°C. Three to four-micron sections were mounted on Superfrost slides (Shandon) and manually deparaffinized. We used proteinase K antigen retrieval as suggested by the manual of EGFR pharmDx™ kit (DAKO, Glostrup, Denmark). Alternatively, slides were immersed in 0.05 mM citrate buffer (pH = 6), and exposed to 750 W microwave for 3 × 5 minutes (MFX-800-3 automatic microwave, Meditest, Budapest, Hungary). Endogenous peroxidase activity was blocked by 3% H2O2 for 5 minutes at room temperature. The extracellular domain of EGFR was detected by the EGFR pharmDx™ kit using mouse monoclonal antihuman EGFR (EGFR-EC, clone 2-18C9), dextran polymer conjugated with HRP and goat anti-mouse IgG, and diaminobenzidine (DAB) substrate-chromogen, applied rigorously following the manufacturer's instructions. Slides provided by the manufacturer (formalin-fixed and paraffin-embedded pellet of HT29 human colorectal carcinoma cell line) as well as human head and neck carcinoma tissue sample previously diagnosed 3+ in all tumor cells for membrane EGFR by using EGFR pharmDx™ and CONFIRM anti-EGFR (Ventana) were used as positive controls. Rabbit polyclonal antibody—PU355-UP (Biogenex) without dilution were used to detect the cytoplasmic domain of EGFR (EGFR-CY). In case of negative control, slides were exposed to the diluent instead of the primary antibody, but were processed in the same way as other slides. Reactions were developed by an LSAB Kit (DAKO). Immunoreaction was visualized using DAB as chromogen. Nuclei were stained by hematoxylin. Reactions were evaluated by two experts.
Reverse-transcriptase polymerase chain reaction
DNA was extracted from tissue using the MasterPure™ DNA Purification Kit according to the instructions of the manufacturer. Two primer pairs (nested PCR) were used. DNA amplifications were performed with DyNAzyme™ and Mastercycler gradient thermal cycler supplied by Eppendorf. The reaction mixture of reagents for samples was prepared, containing 2.5 μl 10× PCR puffer+Mg2+ (DyNAzyme™), 200 μM/each dNTP, 1.00 pM/reaction of each primer, 0.8 U of DyNAzyme™ polymerase per reaction in the first step, and 0.25 U DyNAzyme™ polymerase per reaction in the nested step. The amplified products were digested with BstNI (New England BioLabs). Enzymatic digestions were performed at 60°C for 3 hours in a total volume of 30 μl. Digested PCR product were separated on 4% agarose gel in TAE buffer and visualized under UV light following ethidium bromide staining.
After data collection, the t test, chi square test, and Fisher's exact test were used for comparison between groups, and P values less than 0.05 were considered significant. Data were analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) software.
Results
Forty-one patients including 15 females and 26 males were enrolled in this cross-sectional study.
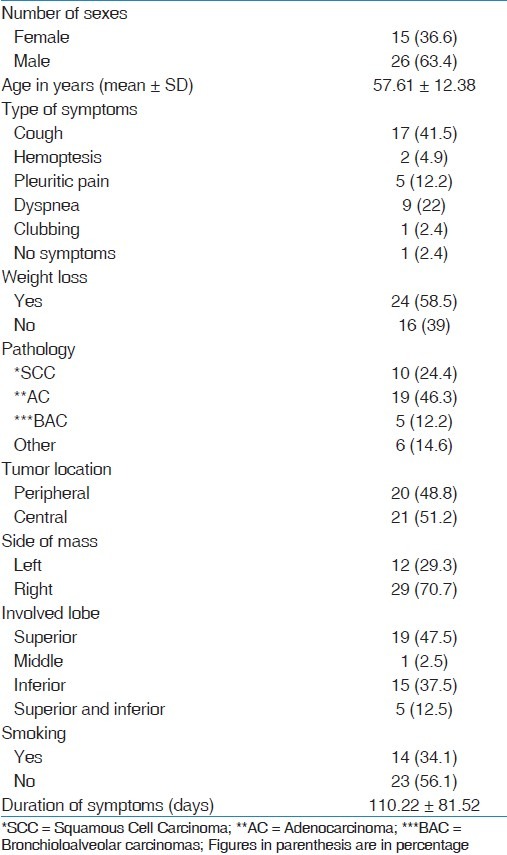
The mean age of patients was 57.61 years. The most common symptom among patients was cough (41.5%). More than 58% of patients mentioned unwanted weight loss in recent 6 months. Majority of tumors in our study were diagnosed as adenocarcinomas (46.3%) and squamous cell carcinomas (24.4%) [Table 1]. Other baseline patient characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics
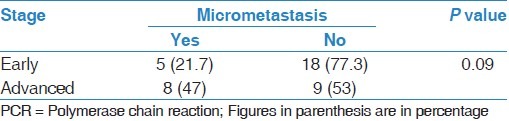
Patients with stage I to IIb were considered as early stage, and patients with stage III (a and b) were considered as advanced stages. Five patients (21.7%) in early stage had positive PCR for micrometastasis and 18 patients (77.3%) had negative results. Eight patients (47%) in advanced stage had positive PCR for micrometastasis and nine patients (53%) had negative results. In order to statistical analysis test, no significant association between pathologic staging and rib micrometastasis was detected (P = 0.09). Table 2 shows and compares pathological cancer staging (early and advanced stage) among patients with and without rib micrometastases diagnosed by PCR. The micrometastases in RT-PCR were positive in 14 (or 13) patients. In contrast, only two patients had positive IHC for micrometastasis (both were stage IIIb, and both were positive for PCR also). Although there were obvious differences in the two groups, statistically no difference existed (P = 0.11). None of the cases were positive for iliac bone micrometastases, either by PCR or IHC.
Table 2.
Frequency of pathological cancer staging (early and advanced) among patients with and without rib micrometastasis diagnosed by PCR
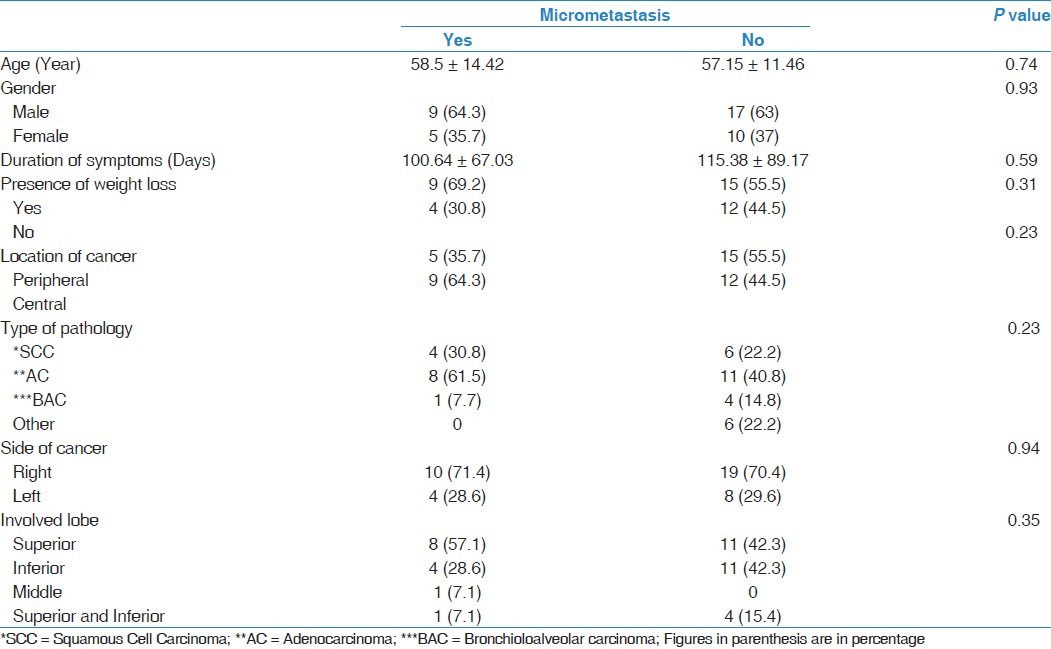
Our data showed that there were no statistical differences between frequency of rib micrometastases and age, gender, duration of symptoms, presence of weight loss, location of cancer, type of pathology, side of cancer, and involved lobe [Table 3].
Table 3.
Correlation between micrometastasis and baseline patient characteristics
Discussion
Bone marrow is among the most frequent site for metastasis of lung cancer, but pathologic studies on bone metastatic disease or on the metastatic tissues are surprisingly rare in the literature. Proper diagnosis of bone marrow involvement should have an influence on decision making as regards to therapeutic strategy and surgical approach in order to achieve better prognosis in these patients with this bad prognostic factor that upgrades the primary lesion to stage IIIA.
The current study indicates that micrometastases detected with IHC are lower than PCR but not statistically significant. Also, we have found that a total of 14 of the 41 (34%) surgically treated patients with NSCLC, mainly Stage IIIa, present with bone marrow micrometastasis (BMM) at the time of surgery. In addition, our data revealed that iliac bone marrow aspiration failed to demonstrate BMM among lung cancer patients. In previous studies, the frequencies of BMM in patients with NSCLC have varied between 22% and 70%.[4–9] This considerable variation could be explained by the use of different assays (immunocytochemistry and flow cytometry) and detection markers with varying specificity and sensitivity, but also the number of bone marrow harvest sites varied between reports. Thus, the two studies reporting the highest frequencies (60% and 70%) both included multiple bone marrow aspiration sites, which have been shown to contribute to increased sensitivity of tumor cell detection.[10]
In the present study, no significant differences in BMM frequency were observed between patients with early (stages I-IIb, n = 23) and advanced stage disease (stages III, n = 17). Interestingly, in this group of mainly early stage disease, the frequency of detected BMM was in the mid-range of previously reported results from almost exclusively early stage patients. Our results are similar to other studies[11,12] revealing that BMM is independent of disease stage. This may support the hypothesis that systemic spread may occur early during tumor development in patients with NSCLC.[11,12]
With respect to the prognostic significance of BMM in NSCLC, no consensus may be derived from the published literature, illustrated by a 2004 review in which four of the seven included studies found that BMM could predict poor prognosis, while the three remaining studies reported the opposite conclusion.[13] Our study may, however, not have been ideally designed to assess the prognostic significance of BMM in NSCLC, since most of the examined patients had failure to follow up. Therefore, we have no data on patient's survival.
These findings suggest that in this selected group of long-term survivors, it is unclear whether the presence of tumor cells in BM could influence patient survival, which could indicate that tumor cells may have entered into a non-proliferative or dormant stage. Metastatic cells can remain dormant indefinitely or may begin to proliferate after variable periods of dormancy due to factors that are not well understood.[14] It has previously been reported that cancer vaccines can induce and maintain dormancy in tumor cells, presumably through immune-mediated mechanisms,[15] which could help explain the observed continued presence of BMM without detectable clinical progression in the study of Brunsvij et al.[16] One study suggests that the presence of micrometastatic cells in a sanctuary site has no major impact on the prognosis and survival of patients with operable NSCLC. Therefore, embarking on adjuvant therapies for early-stage NSCLC-based solely on the finding of bone marrow micrometastatic cells seems not justified at this point (62).[4]
In contrast to our study, other reports confirmed a trend for higher prevalence of BMM with increased cancer staging. However, when predictor variables such as age, sex, histology, tumor location, smoking, or weight loss are analyzed, no correlation can be found between micrometastasis prevalence and any of those variables.
Conclusion
Our study suggests that PCR could use as a promising method for BMM detection. BMM in a sanctuary site (rib) is not associated with advanced stages of lung cancer. In addition, when predictor variables such as age, sex, histology, tumor location, smoking, or weight loss are analyzed, no correlation can be found between micrometastasis prevalence and any of those variables.
Acknowledgment
The authors acknowledge with grateful appreciation, the kind assistance, and financial support provided by the Vice Chancellor for Research at the Isfahan University of Medical Sciences.
Footnotes
Source of Support: Vice Chancellor, Isfahan University of Medical Sciences
Conflict of Interest: None declared.
References
- 1.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–7. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groups in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Devita DV, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 56th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 4.Poncelet AJ, Weynand B, Ferdin F, Robert AR, Noirhomme PH. Bone marrow micrometastasis might not be a short-term predictor of survival in early stages non-small cell lung carcinoma. Eur J Cardiothorac Surg. 2001;20:481–8. doi: 10.1016/s1010-7940(01)00830-2. [DOI] [PubMed] [Google Scholar]
- 5.Cote RJ, Beattie EJ, Chaiwun B, Shi SR, Harvey J, Chen SC, et al. Detection of occult bone marrow micrometastasis in patients with operable lung carcinoma. Ann Surg. 1995;222:415–23. doi: 10.1097/00000658-199522240-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohgami A, Mitsudomi T, Sugio K, Tsuda T, Oyama T, Nishida K, et al. Micrometastatic tumor cells in the bone marrow of patients with non-small cell lung cancer. Ann Thorac Surg. 1997;64:363–7. doi: 10.1016/S0003-4975(97)00543-2. [DOI] [PubMed] [Google Scholar]
- 7.Passlick B, Kubuschok B, Izbicki JR, Thetter O, Pantel K. Isolated tumor cells in bone marrow predict reduced survival in node-negative non-small cell lung cancer. Ann Thorac Surg. 1999;68:2053–8. doi: 10.1016/s0003-4975(99)01125-x. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CP, Shen GH, Ko JL. Matrix metalloproteinase-13 expression is associated with bone marrow microinvolvement and prognosis in non-small cell lung cancer. Lung Cancer. 2006;52:349–57. doi: 10.1016/j.lungcan.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Mattioli S, D’Ovidio F, Tazzari P, Pilotti V, Daddi N, Bandini G, et al. Iliac crest biopsy versus rib segment resection for the detection of bone marrow isolated tumor cells from lung and esophageal cancer. Eur J Cardiothorac Surg. 2001;19:576–9. doi: 10.1016/s1010-7940(01)00652-2. [DOI] [PubMed] [Google Scholar]
- 10.Wiedswang G, Borgen E, Karesen R, Naume B. Detection of isolated tumor cells in BM from breast-cancer patients: Significance of anterior and posterior iliac crest aspirations and the number of mononuclear cells analyzed. Cytotherapy. 2003;5:40–5. doi: 10.1080/14653240310000065. [DOI] [PubMed] [Google Scholar]
- 11.Yasumoto K, Osaki T, Watanabe Y, Kato H, Yoshimura T. Prognostic value of cytokeratin-positive cells in the bone marrow and lymph nodes of patients with resected nonsmall cell lung cancer: A multicenter prospective study. Ann Thorac Surg. 2003;76:194–201. doi: 10.1016/s0003-4975(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 12.Jiao X, Krasna MJ. Clinical significance of micrometastasis in lung and esophageal cancer: A new paradigm in thoracic oncology. Ann Thorac Surg. 2002;74:278–84. doi: 10.1016/s0003-4975(01)03376-8. [DOI] [PubMed] [Google Scholar]
- 13.Coello MC, Luketich JD, Litle VR, Godfrey TE. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–25. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 14.Wieder R. Insurgent micrometastasis: Sleeper cells and harboring the enemy. J Surg Oncol. 2005;89:207–10. doi: 10.1002/jso.20199. [DOI] [PubMed] [Google Scholar]
- 15.Epstein RJ. Maintenance therapy to suppress micrometastasis: the new challenge for adjuvant cancer treatment. Clin Cancer Res. 2005;11:5337–41. doi: 10.1158/1078-0432.CCR-05-0437. [DOI] [PubMed] [Google Scholar]
- 16.Burnsvij PF, Flatmark K, Aamdal S, Hoifodt H, Le H, Jakobsen E, et al. Bone marrow micrometastasis in advanced stage non-small cell lung carcinoma patients. Lung Cancer. 2008;61:170–6. doi: 10.1016/j.lungcan.2007.12.018. [DOI] [PubMed] [Google Scholar]


