Abstract
BACKGROUND:
Right ventricular (RV) dysfunction has been identified as a poor prognostic indicator in sub-massive pulmonary embolism (SPE). We hypothesized that using selective vasodilator agent is beneficial in improving RV function in patients with this condition.
METHODS:
We used inhaled prostacyclin analogue (Iloprost, Ventavis®) in five patients with SPE. Helical computerized tomography angiogram was confirmatory for pulmonary embolism and echocardiography was used to evaluate the RV status. All patients received inhaled Iloprost, 2.5 to 5 μg every 4 hours for 3 weeks.
RESULTS:
Patients were prospectively followed for 3 months. They were assessed at baseline before starting Iloprost treatment and at 3 days, 3 weeks, and 3 months after treatment. All patients showed significant improvement in their functional class, Borg dyspnea score, NT pro-BNP level, and echocardiographic parameters.
CONCLUSION:
In SPE, directing therapy toward decreasing pulmonary vascular resistance improves the associated pulmonary hemodynamic compromise and improves RV function.
Keywords: Iloprost, pulmonary hypertension, pulmonary vascular resistance, right ventricular function, sub-massive pulmonary embolism
Sub-massive pulmonary embolism (SPE) is defined as a pulmonary embolic event that causes an obstructive load in pulmonary vasculatures sufficient enough to cause RV pressure overload, but not systemic hypotension. It tends to have a high mortality (8%).[1,2] Rapid vascular colt lysis by thrombolytic therapy (TT) has gained a lot of interest but, compared with simple anticoagulation, has not shown to consistently improve the outcome.[3,4]
Substantial evidence indicates that the detrimental effect of SPE is related to the bilateral pulmonary vasoconstriction secondary to the neurohormonal inflammatory reaction triggered by the clotting event,[5] leading to acute elevation in the pulmonary vascular resistance (PVR) and RV dysfunction.[6]
We hypothesized that the use of selective pulmonary vasodilator agent that has anti- inflammatory properties might be beneficial in restoring the pathophysiological rise in PVR and consequently may improve RV function and patient outcome.
The current study describes the early initiation of pulmonary vasodilator therapy in a series of patients with acute SPE.
Methods
A prospective follow up for five patients, who presented with SPE to Riyadh Military Hospital (RMH), Kingdom of Saudi Arabia and treated with inhaled Iloprost (Ventavis®) during the period between September 2009 and April 2010, is described.
All patients had stable hemodynamics (systolic blood pressure, >90 mmHg and adequate urine output). The diagnosis of pulmonary embolism (PE) was confirmed by Helical computerized tomography (CT) angiogram. Evaluation was done at baseline, and on day 3, 21, and 90 after starting Iloprost treatment, and includes WHO functional class, NT-pro BNP, 6 minutes walk test (6MWT), Borg dyspnea score, and echocardiography.
Pulmonary hypertension was diagnosed by echocardiography done on day 1 of presentation and defined as systolic pulmonary artery pressure (sPAP) >45 mmHg, which was calculated by the modified Bernoulli equation based on tricuspid regurge (TR) jet velocity plus the estimated RA pressure based on inferior vena cava (IVC) measurement. RV dysfunction was assessed by both quantitative and qualitative criteria [Table 1].
Table 1.
Criteria for RV dysfunction

All patients received anticoagulation initiated with intravenous unfractionated heparin (as per hospital protocol) or low molecular weight heparin (LMWH), Enoxaparin, 1 mg/kg twice daily. Warfarin was started on day 1, aiming for therapeutic INR ratio between 2 to 3.
All five patients refused TT, but agreed to receive inhaled Iloprost. After IRB approval at RMH, Iloprost was delivered through an ultrasonic nebulizer (OMRON MicroAIR U22 Nebulizer, OMRON healthcare Co. Ltd., Japan) with dose escalation from 2.5 to 5 mg (highest tolerated dose), every 4 hours for a total of 3 weeks.
Results and Cases Description
All patients were symptomatic (WHO class III-IV) at presentation with abnormal baseline evaluation [Table 2]. After treatment with Iloprost, all five patients showed significant improvement in their symptoms, 6MWT, NT pro BNP level, and physiological parameters [Table 2 and Figure 1].
Table 2.
Clinical and physiological parameters of the patients before and after Iloprost (3 days, 3 weeks, and 3 months)
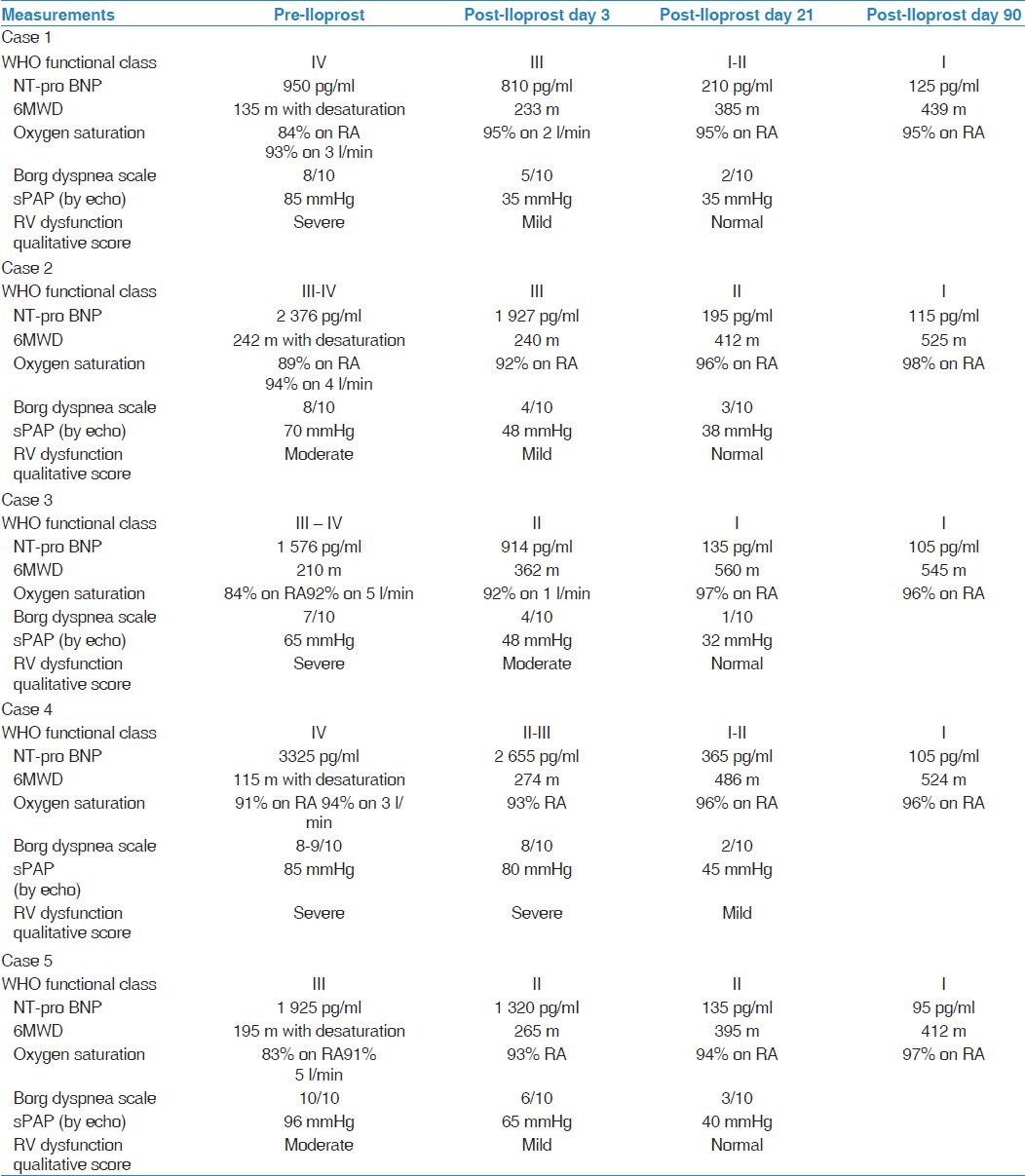
Figure 1.
The mean of sPAP by Echo, NT-pro BNP, 6 MWT, and WHO FC (pre and post Iloprost)
No patients reported any significant adverse events or discontinued therapy during the treatment period.
The following is a brief description of the five cases.
Case 1
A 74-year-old man presented with severe dyspnea (WHO functional class IV) over 10-day period. He had one attack of syncope 2 days earlier. Blood pressure was 145/82.
Hospital course
Helical CT angiography showed extensive bilateral PE and small bilateral pleural effusion. Electrocardiogram revealed sinus tachycardia. The baseline evaluation is shown in Table 2.
The patient was started on anticoagulation. Seventy-two hours after admission, he showed no improvement. TT was suggested but refused by the patient and his family. Inhaled Iloprost was then offered and started at 2.5 mg every 4 hours and up titrated to 5 mg, 4 hourly for 3 weeks. The follow-up results are shown in Table 2.
Case 2
A 34-year-old man presented with 5-day history of dyspnea and few attacks of hemoptysis. His blood pressure was 135/85.
Hospital course
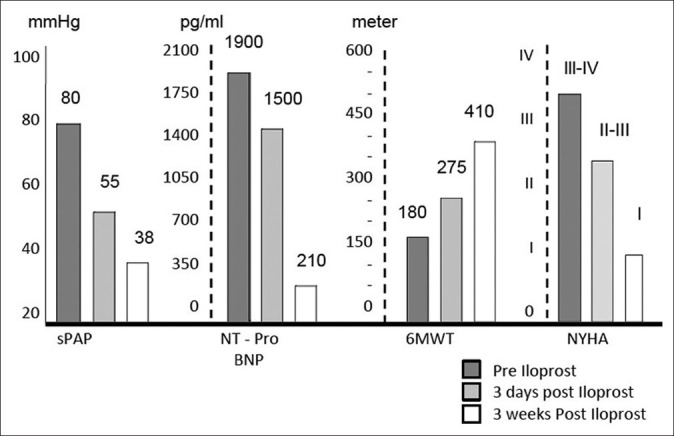
Helical CT showed extensive filling defects seen at the main pulmonary trunk, right and left pulmonary arteries, extending distally to lobar and segmental branches [Figure 2]. There was a small right-sided pleural effusion and segmental consolidations involving the right lower lobe, suggestive of pulmonary infarct. The initial evaluation is shown in Table 2.
Figure 2.
Helical CT for Case 2
The patient was treated with LMWH and warfarin. TT was suggested but refused by the patient. On day 3 of admission, the patient showed no improvement. NT-pro BNP level was repeated and found to be rising. Repeated echo revealed no improvement in RV function.
Nebulized Iloprost was offered and started at 5 mg every, 4 hours for 3 weeks. The follow-up results are shown in Table 2.
Case 3
A 46-year-old woman on contraceptive pills presented with 1-day history of pleuritic chest pain and severe dyspnea. She was in respiratory distress. Blood pressure was maintained at 115/65.
Hospital course
The patient was started on LMWH. Helical CT angiography confirmed the presence of bilateral PE. The initial assessment results are described in Table 2.
Thirty -six hours after admission, the patient had one attack of near syncope. She refused TT. On day 3 of admission, the patient was shifted to step-down unit, and started on inhaled Iloprost 2.5 mg 4 hourly, which increased to 5 mg, 4 hourly within 24 hours and continued for 3 weeks. The follow-up results are shown in Table 2.
Case 4
A 32-year-old Saudi soldier presented with severe dyspnea for 3 days. He had history of unprovoked deep vein thrombosis treated for 6 months. Clinical examination revealed a very distressed patient. Blood pressure was 115/76.
Hospital course
Helical CT angiography confirmed the presence of bilateral PE and Doppler ultrasound of lower limbs showed extensive bilateral deep venous thrombosis (DVT). Antiphospholipid screen was positive. The initial evaluation is described in Table 2.
The patient was started on anticoagulation. Twenty-four hours after admission, his oxygen requirement increased to 6 l/min. TT was offered but refused. IVC filter was inserted without complications. The patient was started on inhaled Iloprost 4 hourly, which was increased to 5 mg, 4 hourly within 24 hours and continued for 3 weeks. The follow-up results are shown in Table 2.
Case 5
A 22-year-old Saudi lady presented with severe dyspnea for 4 hours. Clinically, she was in significant respiratory distress. Blood pressure was 105/68.
Hospital course
Helical CT angiography confirmed the presence of a large filling defect occluding the left main and the left lower lobe pulmonary artery. Initial assessment results are shown in Table 2.
The patient was started on anticoagulation. On day 2 of admission, she had an episode of hemoptysis and severe left-sided pleuritic chest pain. She was transferred to intensive care unit (ICU) for observation. TT was offered but refused. The patient was started on inhaled Iloprost 5 mg, 4 hourly for 3 weeks. The follow-up results are shown in Table 2.
Discussion
RV pressure overload and RV dysfunction were found to be the most powerful predictor of in-hospital death among patients with SPE.[7] However, the best therapeutic intervention for SPE is still controversial. Recent studies were directed toward colt lysis by using TT in order to rapidly minimize the obstructive load and improve RV function. In a randomized study of 101 patients who received r-TPA or heparin,[8] greater improvement on echocardiography was observed in patients receiving r-TPA at 3 and 24 hours, confirming the efficacy of TT in rapid clot lysis. However, there were no clear clinical advantages. Further studies that looked at different clinical endpoints showed conflicting results.[3,4,9]
Animal models and clinical investigations demonstrate that the impact of embolic material in the pulmonary vascular outflow tract precipitates an increase in RV impedance, which is predominantly related to the interaction of the mechanical obstruction with the underlying cardiopulmonary status.[10,11] Although the embolic material size is certainly the main cause for pulmonary hemodynamic compromise following massive PE, such obstructive load cannot explain the whole picture in SPE, and other factors are also important for abnormal pulmonary physiology seen in this condition.
Substantial evidence indicates that a clot-driven neurohormonal inflammatory mediators’ release[12,13] and systemic hypoxia[14] are essential for the development of bilateral pulmonary vasoconstriction and worsen the V/Q mismatch leading to acute rise in PVR. In an animal study, PE was associated with an early influx of inflammatory cells, and monocyte chemo-attractant protein-1 elevation within the pulmonary artery wall.[15] Another animal study of hypertensive PE was also associated with a 50- to 100-fold increase in the CXC- chemokines cytokine-induced neutrophil chemo-attractant and macrophage-inflammatory protein in bronchoalveolar fluid.[16] These results indicate that SPE may induce expression of proinflammatory mediators and establish a proinflammatory environment in the affected patients leading to bilateral vasoconstriction and subsequent acute rise in PVR and RV failure.
Based on this, we hypothesized that treatment of SPE should be directed toward decreasing PVR and probably not rapid clot lysis. Such effect on PVR should lead to rapid improvement of RV function and hopefully the patients’ outcome.
Figure 3 shows our recommend treatment algorithm for patients who presented with SPE.
Figure 3.
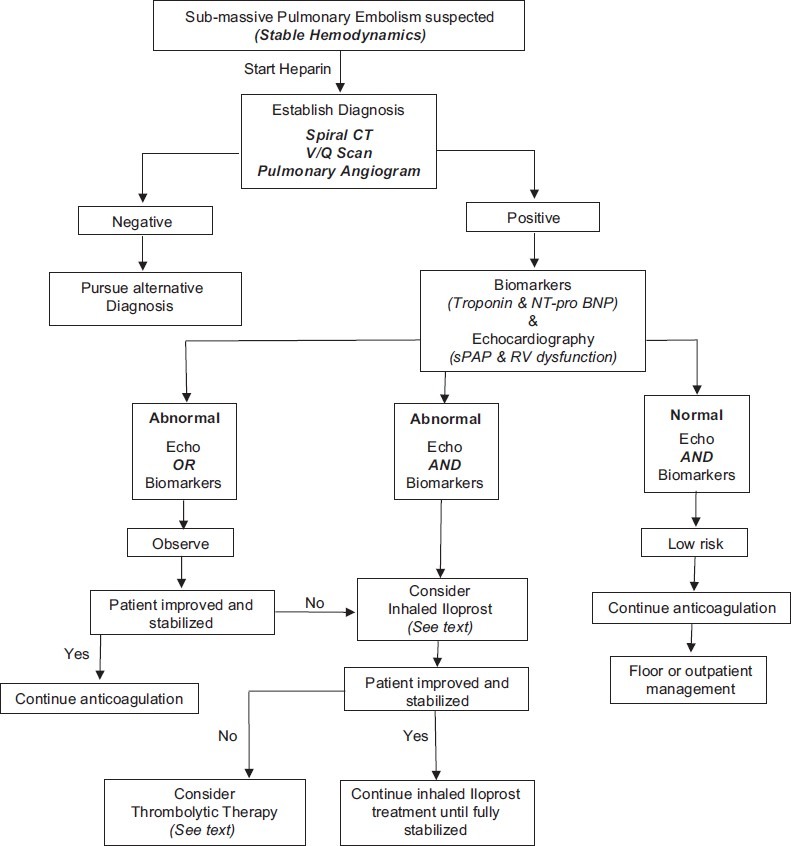
Algorithm for management of sub-massive PE
We realize that this report has some limitations. First, it is a small case series and lacks a control group. However, to our knowledge, this is the first attempt directed at modifying other underlying pathophysiological derangements in SPE and we believe is worth presenting. Second, the absence of invasive hemodynamic measurements is another weakness in this study, as echocardiography is not the ideal test for accurate measurement of pulmonary hemodynamics. Nevertheless, the improvement in echocardiographic parameters early after treatment with Iloprost in combination with the improvement in clinical and physiological measures may support and enhance the accuracy of echocardiography.
In this small series, we were able to demonstrate for the first time that targeting pulmonary vasculature with a selective pulmonary vasodilator in SPE is safe and potentially beneficial.
We are in the process of starting a randomized controlled study to further study this interesting hypothesis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser K, Rauber K, et al. Association between thrombolytic treatment and the prognosis of hemodynamically stable patients with major pulmonary embolism: results of a multicenter registry. Circulation. 1997;96:882–8. doi: 10.1161/01.cir.96.3.882. [DOI] [PubMed] [Google Scholar]
- 2.Dalen JE. The uncertain role of thrombolytic therapy in the treatment of pulmonary embolism. Arch Intern Med. 2002;162:2521–3. doi: 10.1001/archinte.162.22.2521. [DOI] [PubMed] [Google Scholar]
- 3.Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: Results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–71. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 4.Hamel E, Pacouret G, Vincentelli D, Forissier JF, Peycher P, Pottier JM, et al. Thrombolysis or heparin therapy in massive pulmonary embolism with right ventricular dilation: Results from a 128-patient monocenter registry. Chest. 2001;120:120–5. doi: 10.1378/chest.120.1.120. [DOI] [PubMed] [Google Scholar]
- 5.Smulders YM. Pathophysiologu and treatment of hemodynamic instability in acute pulmonary embolism: The pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48:23–33. doi: 10.1016/s0008-6363(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 6.Kreit JW. The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest. 2004;125:1539–45. doi: 10.1378/chest.125.4.1539. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: Right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479–87. doi: 10.1016/s0002-8703(97)70085-1. [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Goldhaber SZ. Cardiovascular risk stratification of pulmonary embolism. Am J Cardiol. 1996;78:1149–51. [PubMed] [Google Scholar]
- 9.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143–50. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre KM, Sasahara AA. Hemodynamic and ventricular responses to pulmonary embolism. Prog Cardiovasc Dis. 1974;17:175–90. doi: 10.1016/0033-0620(74)90042-5. [DOI] [PubMed] [Google Scholar]
- 11.Parker BM, Smith JR. Pulmonary embolism and infarction: A review of the physiologic consequences of pulmonary artery obstruction. Am J Med. 1958;24:402–27. doi: 10.1016/0002-9343(58)90326-7. [DOI] [PubMed] [Google Scholar]
- 12.Stein M, Levy SE. Reflex and humoral responses to pulmonary embolism. Prog Cardiovasc Dis. 1974;17:167–74. doi: 10.1016/0033-0620(74)90041-3. [DOI] [PubMed] [Google Scholar]
- 13.Malik AB. Pulmonary microembolism. Physiol Rev. 1983;63:1114–207. doi: 10.1152/physrev.1983.63.3.1114. [DOI] [PubMed] [Google Scholar]
- 14.Alpert JS, Godtfredsen J, Ockene IS, Anas J, Dalen JE. Pulmonary hypertension secondary to minor pulmonary embolism. Chest. 1978;73:795–7. doi: 10.1378/chest.73.6.795. [DOI] [PubMed] [Google Scholar]
- 15.Eagleton MJ, Henke PK, Luke CE, Hawley AE, Bedi A, Knipp BS, et al. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg. 2002;36:581–8. doi: 10.1067/mva.2002.126556. [DOI] [PubMed] [Google Scholar]
- 16.Zagorski J, Debelak J, Gellar M, Watts JA, Kline JA. Chemokines Accumulate in the lungs of tats with severe pulmonary embolism induced by polystyrene microspheres. J Immunol. 2003;171:5529–36. doi: 10.4049/jimmunol.171.10.5529. [DOI] [PubMed] [Google Scholar]


