Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a fatal cancer-related pulmonary complication with rapidly progressing dyspnea, and occasionally induces sudden death. Here, we describe a postmortem-diagnosed PTTM case caused by gastric cancer, with the complaint of progressing dyspnea for 5 days.He did not have any abdominal symptoms or cancer history. PTTM should be considered in patients with rapidly worsening respiratory conditions, even if there is no cancer history.
Keywords: Dyspnea, gastric cancer, PTTM
Pulmonary tumor thrombotic microangiopathy (PTTM), which was first reported by Von Herbay et al.,[1] causes narrowing of the pulmonary artery and induces a respiratory disturbance with pulmonary hypertension, right-side heart failure, and sudden death. The most frequent cause of PTTM is gastric cancer.[1] PTTM is histologically characterized by widespread tumor emboli together with fibrocellular intimal proliferation and thrombus formation in small pulmonary arteries and arterioles in patients with metastatic carcinomas. The clinical course is very rapid, and antemortem diagnosis is usually difficult, particularly in patients without a cancer history.[2] PTTM induces occasional sudden death following rapidly developing dyspnea, and awareness of PTTM is important for respiratory physicians.
Case Report
A 43-year-old Japanese man visited a neighboring hospital complaining of dyspnea on effort, dry cough, and rhinorrhea for 2 weeks. He did not show any abdominal symptoms and had no cancer history. A chest computed tomography scan revealed diffuse centrolobular tiny nodules and thickening of both bronchovascular bundles and interlobular septa in lungs, and bronchiolitis and lymphangiosis carcinomatosa were suspected [Figure 1]. He was admitted to our hospital for further examination and treatment. Laboratory tests showed the following findings: white blood cells, 13 990/μl; platelets, 229 × 103/μl; plasma D-dimer, 7.0 μg/ml (normal <2.0); carcinoembryonic antigen, 4.0 ng/ml (<5.0); CA19-9, 95.7 U/ml (<37); and vascular endothelial growth factor (VEGF), 832 pg/ ml (<120). His oxygen saturation was 90% when breathing room air. Antibiotic administration and oxygen supplementation therapy were performed, and a whole-body screening was planned. However, his respiratory condition deteriorated and, on the fifth day after admission, he suddenly complained of severe dyspnea and died.
Figure 1.
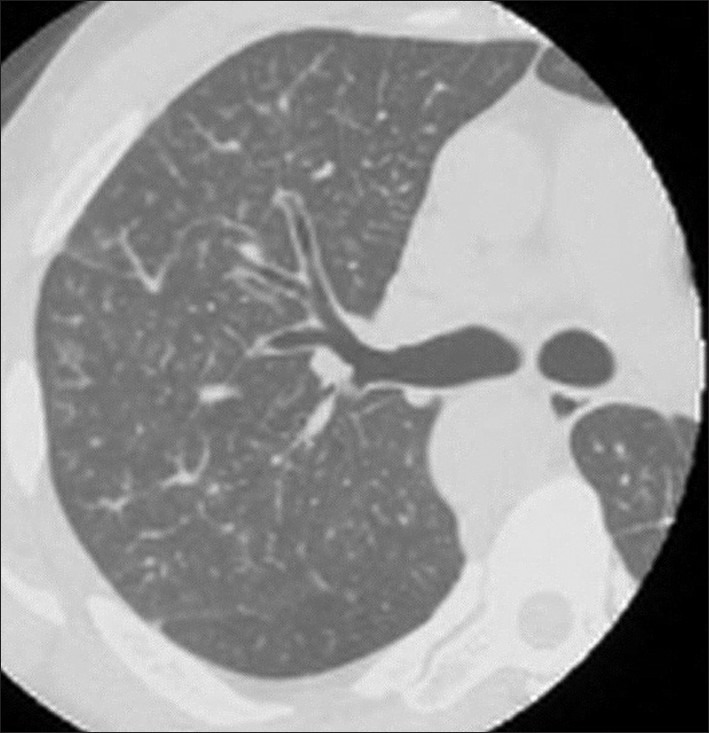
Chest computed tomography scan showing diffuse tiny nodules and the swelling of bronchovascular bundles
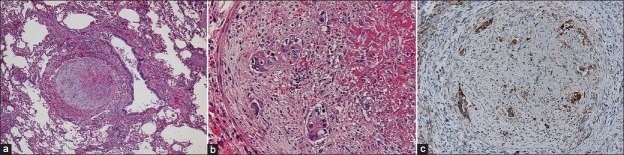
A postmortem examination revealed an ulcerated tumor, measuring 12.5 × 9 cm, in the stomach angle. Histologically, the tumor was a poorly differentiated adenocarcinoma with remarkable lymphatic permeation that had invaded into the serosa. Multiple abdominal lymph nodes were involved. Ascites was 200 ml and peritoneal dissemination was absent. The left and right lungs weighed 620 and 740 g, respectively. Hemorrhagic infarctions were present in the right lower lobe, and macroscopic thrombi were absent in the pulmonary arteries. Microscopic examination revealed widespread fibrin thrombi and fibrocellular and fibromuscular intimal proliferation with or without gastric cancer cells, resulting in luminal stenosis and occlusion in the small pulmonary arteries [Figure 2a, b]. These findings were compatible with PTTM. Lymphangiosis carcinomatosa and mediastinal lymph node involvement were also seen. Carcinoma cells in the stomach and lungs were immunohistochemically positive for VEGF [Figure 2c]. The heart weighed 385 g and showed dilatation of the right ventricle. The liver weighed 1 645 g and showed congestion, probably owing to congestive heart failure. Every organ except for the stomach lacked a space-occupying tumor mass.
Figure 2.
(a) Obstruction of a pulmonary artery (H and E, ×40), (b) High-power view of an artery (H and E, ×400), (c) Immunopositivity for vascular endothelial growth factor among the tumor cells (×200)
Discussion
The pathogenesis of PTTM is generally explained as follows. Tumor cells metastasize to the pulmonary vascular system, activate the coagulation cascade, and release inflammatory mediators and growth factors, resulting in deposition of platelets and fibrin, fibrocellular subintimal proliferation, and smooth muscle colonization.[3] The components of the tissue factor—VEGF system, especially VEGF, are proposed to be the mediators that induce PTTM.[2] In fact, our case had carcinoma cells that were positively stained for VEGF and elevated serum VEGF. Clinically, PTTM-related manifestations are rapid, and an antemortem diagnosis is usually difficult, as seen in our case. Previously, a gastric cancer patient with an antemortem diagnosis of PTTM by serum VEGF and D-dimer elevation, probably owing to the activated coagulation cascade, and specimens from a transbronchial lung biopsy and video-assisted thoracoscopic surgery, was cured by corticosteroid, anticoagulants, and oral anticancer drug therapies.[4] For early antemortem diagnosis of PTTM, serum VEGF and D-dimer measurements may be effective for screening. In addition, PTTM is frequently associated with lymphangiosis carcinomatosa,[1] and the latter may be a predisposing factor to PTTM.
In summary, it is important to suspect PTTM as a differential diagnosis for rapidly worsening respiratory conditions, not only in patients with cancer, but also in patients without a cancer history. The early diagnosis may cure the PTTM patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Von Herbay A, Illes A, Waldherr R, Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587–92. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Chinen K, Tokuda Y, Fujiwara M, Fujioka Y. Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: An analysis of 6 autopsy cases and review of the literature. Pathol Res Pract. 2010;206:682–9. doi: 10.1016/j.prp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Pinckard JK, Wick MR. Tumor-related thrombotic pulmonary microangiopathy: Review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol. 2000;4:154–7. doi: 10.1016/s1092-9134(00)90038-8. [DOI] [PubMed] [Google Scholar]
- 4.Miyano S, Izumi S, Takeda Y, Tokuhara M, Mochizuki M, Matsubara O, et al. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol. 2007;25:597–9. doi: 10.1200/JCO.2006.09.0670. [DOI] [PubMed] [Google Scholar]