Abstract
Oroxylum indicum Vent. (O. indicum) is a tree commonly called Indian trumpet tree found in tropical countries, such as India, Japan, China, Sri Lanka, Malaysia. The chemical constituents obtained from different parts of plant include baicalein-7-O-diglucoside (Oroxylin B), baicalein-7-O-glucoside, chrysin, apegenin, prunetin, sitosterol, oroxindin, biochanin-A, ellagic acid, baicalein and its 6- and 7-glucuronides, scutellarein, tetuin, antraquinone and aloe-emodin. Various parts of the plant are used in Ayurveda and folk medicine for the treatment of different ailments such as cancer, diarrhea, fever, ulcer and jaundice. Recent in vivo and in vitro studies have indicated its antiinflammatory, antiulcer, hepatoprotective, anticancer, antioxidant, photocytotoxic, antiproliferative, antiarthritic, antimicrobial, antimutagenic and immunostimulant properties. Exhaustive literature survey reveals that there are some activities which are still not proven scientifically. This article is an attempt to compile an up-to-date and comprehensive review on O. indicum covering its traditional and folk medicinal uses, phytochemistry and pharmacology.
Keywords: Ethnomedicinal, ethnopharmacology, O. indicum, phytochemical
Presently there is an increasing interest worldwide in herbal medicines accompanied by increased laboratory investigations into the pharmacological properties of the bioactive ingredients and their ability to treat various diseases. Various drugs have entered the international market through exploration of ethnopharmacology and traditional medicine. Although scientific studies are carried out on a large number of plants but smaller numbers of marketable drugs or phytochemical entities have entered the evidence-based therapeutics. Efforts are therefore needed to establish and validate evidence regarding safety and practice of Ayurvedic medicines. Oroxylon refers to the tree O. indicum; commonly called the tree of Damocles, Indian caper, Indian trumpet flower, Indian trumpet tree. The tree is named as “Tree of Damocles” after an incident depicted in an ancient story by Cicero. O. indicum Vent. a member of the bignoniaceae family is a small or medium sized deciduous tree growing throughout Asia continent[1]. It has been used traditionally for a wide diversity of ailments such as cancer, diarrhea, fever, ulcer, jaundice and arthritis.
TAXONOMY
Scientific name:
O. indicum Vent. (L) (Syn. name- Bignonia indica, Spathodea indica, Calosanthes indica, Hippoxylon indica, Bignonia quadripinnata)
Vernacular names:
Shayonak Kul (Ayurvedic); Hanyu pinyin, Mu hudie, Butterfly tree (Chinese); Broken bones plant, Indian calosanthes, Indian trumpet, Indian trumpet flower, Mid night horror, Tree of Damocles (English); Bonglai (Malaysia); Tentu (Gujrati); Patrorna, Putiveriksha, Shallaka, Shuran, Son, Vatuk, Arlu, Urru, Sauna (Hindi); Ane-mungu (Karnatka); Devamadak (Konkani); Tatelo (Nepalese); Palakappayyani, Vella, Pathiri (Malaylam); Aralu, Shyonaka (Sanskrit); Totila, Thotila (Singhala-Sri-Lanka); Cari-konnai, Kalai-y-utaicci, Puta-puspam, Achi, Pana, Pei-maram, Venga maram, Peruvaagai (Tamil); Manduka-paramu, Pampena, Suka-nasamu, Dundilamu, Pampini, Nemali, Chettu (Telugu); Other common names Kampong, Sonapatta, Sonaapaathaa, Urru, Saona, Tatpalenga, Kinnauri phool, Shoshana, Tuntuka, Kutunata, Mandukparna, Bhalluka, Prthushimba, Katvan[2–4].
Botanical classification:
Kingdom–Plantae, Class–Magnoliophyta, Order–Lamiales, Family–Bignoniaceae, Genus–Oroxylum, Species–Indicum.
Distribution and relationships:
O. indicum is a deciduous tree growing throughout India, South Asia, South East Asia, Sri Lanka, Philippines, Indonesia, China, Bhutan, Malaysia and Mallaca. It is found up to an altitude of 1200 m mainly in ravines, in damp region and moist places in the forests[2]. In India, it is distributed in Himalayan foothills, Eastern and Western Ghats and North East India[5]. It is mostly sighted along the river banks or slopes of the hills. O. indicum lives in relationship with the actinomycete Pseudonocardia oroxyli present in the soil surrounding the roots[6].
Description:
O. indicum is a small or medium sized deciduous tree up to 12 m in height with soft light brown or grayish brown bark with corky lenticels[7] (fig. 1a). The leaves are very large, 90-180 cm long 2-3 pinnate with 5 or more pairs of primary pinnae, rachis very fast, cylindrical, swollen at the junction of branches, leaflets 2-4 pairs ovate or elliptic, acuminate, glabrous (fig. 1b). The large leaf stalks wither and fall off the tree and collect near the base of the trunk, appearing to look like a pile of broken limb bones. The flowers are reddish purple outside and pale, pinkish-yellow within, numerous, in large erect racemes (fig. 1c)[4]. The flowers bloom at night and emit a strong, stinky odor which attracts bats. The tree has long fruit pods that curved downward, hang down from the branches, looking like the wings of a large bird or dangling sickles or sword in the night. Fruits are flat capsules, 0.33-1 m long and 5-10 cm broad and sword shaped. When the pod bursts open the seeds flutter to the ground, often traveling some distance, looking like butterflies[3]. The seeds are numerous, flat and winged all around like papery wings, except at the base. The plant flowers in June-July and bears fruits in November. The fresh root bark is soft and juicy; it is sweet, becoming bitter later. On drying, the bark shrinks, adhere closely to the wood and becomes faintly fissured (fig. 1d).
Fig. 1.

Oroxylum indicum: (a) tree (b) leaves (c) flowers (d) wood bark
ETHNOBOTANY
The tree is often grown as an ornamental tree for its strange appearance. The sword like fruit or a branch of the plant is used by the farmers to kill crabs in wet paddy fields. A paste made of the bark is applied to wounds of animals to kill maggots[3]. In Thailand, the fruits and flowers of the plant are consumed as a vegetable[8].
Traditional uses:
Roots are sweet, astringent, bitter, acrid, refrigerant[9], antiinflammatory, anodyne, aphrodisiac, expectorant, appetizer, carminative, digestive, anthelmintic, constipating, diaphoretic, diuretic, antiarthritic, antidiabetic and febrifuges. Tonic is useful in dropsy, cough, sprains neuralgia, hiccough, asthma, bronchitis, anorexia, dyspepsia, flatulence, colic, diarrhea, dysentery, strangury, gout, vomiting, leucoderma, wounds, rheumatoid arthritis and fever. Root bark is used in stomatitis, nasopharyngeal cancer and tuberculosis[4,10]. Leaves are used as stomachic, carminative and flatulent. Leaf decoction is given in treating rheumatic pain, enlarged spleen[4], ulcer, cough, and bronchitis. Mature Fruits are acrid, sweet, anthelmintic, and stomachic. They are useful in pharyngodynia, cardiac disorders, gastropathy, bronchitis, haemarrhoids, cough, piles, jaundice, dyspepsia, smallpox, leucoderma and cholera[3]. Seeds are used as purgative. Dried seed powder is used by women to induce conception. Seeds yield non-drying oil used in perfume industry. The seeds are ground with fire soot and the paste is applied to the neck for quick relief of tonsil pain. The seeds are used in traditional Indian Ayurvedic medicine, included in famous tonic formulations such as Chyawanprash. Bark decoction is taken for curing gastric ulcer and a paste made of the bark powder is applied for mouth cancer, scabies and other skin diseases. The medicated oil of O. indicum in sesame oil base instilled into ears mitigates the pain in otitis[2,3].
Ayurvedic preparations:
O. indicum is used as one of the important ingredient in most commonly used Ayurvedic preparations such as Dasamularistha, Syonaka putapaka, Syonaka sidda ghrta, Brhatpancamulyadi kvatha, Amartarista, Dantyadyarista, Narayana Taila, Dhanawantara Ghrita, Brahma Rasayana and Chyavanaprasa[11,12].
CHEMISTRY
O. indicum leaves are known to contain flavones and their glycosides, baicalein (5, 67-trihydroxy flavone) and its 6 and 7-glucuronides, chrysin (5,7-dihydroxy flavone)[13,14], scutellarein and its 7-glucuronides, anthraquinone and aloe-emodin[5,7,15], chrysin-7-O-glucuronide, chrysin-diglucoside and irridoids[16]. Ethyl acetate extract of leaves of O. indicum was separated using high speed counter-current chromatography to get chrysin (160.9 mg, 97.3% purity), baicalein (130.4 mg, 97.6% purity), baicalein 7-O-glucoside (314.0 mg, 98.3% purity), baicalein-7-O-diglucoside (179.1 mg, 99.2% purity)[17]. From methanol extract of the leaves of O. indicum, chrysin-7-O-glucuronide, chrysin-diglucoside and baicalein were separated. Structure of the chrysin-diglucoside has yet to be obtained[16]. Chloroform extraction of defatted leaves gives gummy solid yielding anthraquinone and aloe-emodine[15]. Stem bark contain flavones oroxylin A (5,7-dihydroxy-6-methoxy flavone), chrysin, baicalein and its 6 and 7-glucuronide, scutellarin-7-rutinoside, traces of alkaloid[5,18], tannic acid, sitosterol and galactose, baicalein, biochanin-A, ellagic acid[7]. Ethyl acetate extract of root of O. indicum is reported to contain two flavonoids- i) 2,5-dihydroxy-6,7-dimethoxy flavone and ii) 3,7,3’,5’,-tetramethoxy-2-hydroxy flavone. Flavonoid (i) has Rf value 0.621 in solvent system (petroleum ether: ethyl acetate, 3:1). This flavonoid was separated as fine crystals with M.P. 195-198°. Flavonoid (ii) has Rf value 0.721 in solvent system (petroleum ether:ethyl acetate, 3:1) and this flavonoid was separated as needle shaped crystals with M.P. 210-211°[19]. Root bark contain chrysin, scutellarin-7-rutinoside, weak acids, traces of alkaloids[18], sitosterol, galactose, baicalein, biochanin-A, ellagic acid, oroxylin-A[7] and a yellow crystalline coloring matter 5,7-dihydroxy-6-methoxy flavone[11]. Heartwood contains prunetin, sitosterol. Methanol extract of the fruits pods is reported to contain oroxylin A, chrysin, baicalein, a triterpene carboxylic acid and ursolic acid[20]. Seeds contain oils and flavonoids such as chrysin, oroxylin A, baicalein, baicalein-7-O-diglucoside (Oroxylin B), baicalein-7-O-glucoside, apigenin[21], terpenes, alkaloids, saponins[22], tetuin, the 6-glucoside of baicalein, benzoic acid and fatty acids[13,14]. A new flavone glucuronide-oroxindin and chrysin-7-O-diglucoside were also isolated. The seed oil contains caprylic, lauric, myristic, palmitic, palmotoleic, stearic, oleic and linoleic acids. Seeds also contain twenty percent shiny oil. Ether fraction of O. indicum gave scutellarein[23]. Aqueous mother liquor gave scutellarein and baicalein[23,24]. Baicalein was found to be major flavonoid present in petroleum ether extract[11] (fig. 2).
Fig. 2.
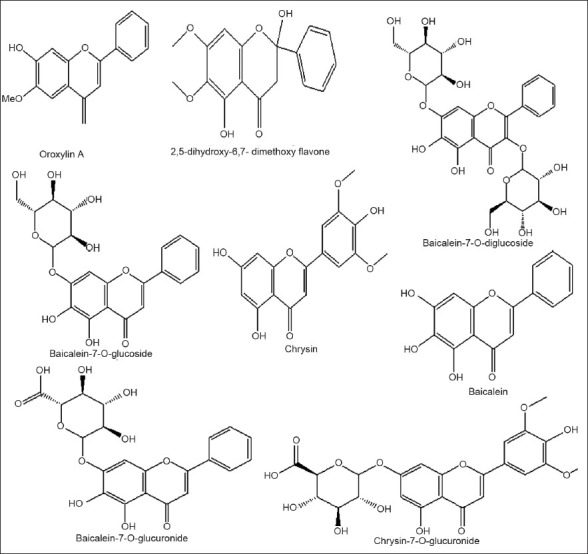
Chemical structure of the biologically active compounds isolated from Oroxylum indicum.
TLC of alcoholic extract of O. indicum was carried out on Silica gel G plate using solvent system, n-butanol: acetic acid: water, 4:1:5. TLC plate showed under UV (366 nm) a fluorescent zone at Rf 0.10 (blue). On exposure to iodine vapour, 6 spots appeared having Rf values 0.10, 0.30, 0.58, 0.70, 0.85, 0.95 and all were yellow in color. On spraying with 5% methnolic sulphuric acid and heating the plate for 10 min at 105°, 5 spots appeared having Rf values 0.25, 0.58, 0.70, 0.85, 0.95 and all grey in color[23]. Yield of aqueous and ethanol extract of stem bark of O. indicum was reported to be 7.03% w/w and 6.14% w/w respectively[25]. Yields of petroleum ether, chloroform, ethyl acetate and n-butanol extract of root bark of O. indicum were reported to be 0.32, 0.78, 1.52 and 1.68% w/w respectively[11]. Yield of methanol extract of leaves of O. indicum was found to be 11.1% w/w[16].
PHARMACOLOGY
In crude plant extracts, flavonoids are often present as O- or C- glycosides. Flavonoids have various biological activities such as apoptosis induction, cell cycle arrest, antiproliferation, antiangiogenesis and antioxidation or a combination of these activities[26,27]. Flavonoids are known for their antiinflammatory and antiallergic effect[28]. Chrysin is a flavone which have many biological activities i.e. antibacterial[29], antioxidant[30], antiinflammatory[31], antiallergic[32], anticancer[33], antiestrogenic[28] and anxiolytic activity[34]. Chrysin also have tyrosine inhibitory[35] and moderate aromatase inhibitory activity[36]. It can also inhibit the metabolism of the carcinogen benzo[α]pyrene by hamster embryo cells in tissue culture[37]. Chrysin has antibacterial activity and to increase it chrysin derivative was also prepared, in which chrysin ring system linked to the alkyl amines by different spacers at C-7 position with a view to enhance their lipophilicity. A series of chrysin derivatives were prepared containing 3, 4 and 6-carbon spacers, in between heterocyclic ring and chrysin and were evaluated for antibacterial activity. Among them compounds which contain 4-carbon spacer in between chrysin and heterocyclic ring, displayed good anti-bacterial activity[38]. Oroxylin-A, a naturally occurring monoflavonoid exhibit many biological activities such as COX-2 inhibition[39,40], cytotoxic[41], and anti-microbial[42,43]. It also demonstrated anti-HIV[44] and lipid per-oxidation inhibition activities[45]. Oroxylin-A has antibacterial activity, introduction of acyl group at C-7 position of oroxylin-A has enhanced the inhibitory potential to great extent[46]. No detailed toxicity study of O. indicum has yet been published but available information shows that maximum tolerated dose is about 100 mg/kg.
PHARMACOLOGICAL PROPERTIES
Several workers have reported different biochemical activities of O. indicum in various in vivo and in vitro test models. Different part of this plant have been found to exhibit antiinflammatory, antimicrobial, antioxidant, anticancer, antimutagenic, photocytotoxic, antiarthritic, immunostimulant, hepatoprotective, antiproliferative and hepatoprotective activities.
Antiinflammatory activity:
The antiinflammatory activity was evaluated by carageenan induced rat paw edema model in rats using diclofenac sodium as standard drug. Two doses 150 mg/kg and 300 mg/kg of aqueous extract of O. indicum were used. Result showed that paw volume was significantly reduced in dose dependent manner as compared to control. Extract at a dose of 300 mg/kg showed maximum antiinflammatory activity. However, the activity produced by both the doses was less than the reference standard. Extract at both doses showed significant (P<0.05) anti-inflammatory activity at 5 h suggesting that the extract predominantly inhibit the release of prostaglandin like substances[47].
Antiulcer activity:
The 50% alcohol extract of root bark of O. indicum and its petroleum ether, chloroform, ethyl acetate and n-butanol fractions were studied against ethanol-induced gastric mucosal damage. The alcohol extract (300 mg/kg, p.o.) and its different fractions (100 and 300 mg/kg, p.o.) showed reduction in gastric ulceration. The petroleum ether and n-butanol fractions showed maximum inhibition of gastric lesions against ethanol-induced gastric mucosal damage. The results were comparable with omeprazole (reference standard). In the ethanol-induced gastric ulcer model, treatment with both the active fractions and omeprazole showed significant antioxidant activity as evident from the reduction in the extent of lipid peroxidation. The effect of active fraction of root bark on the ulcer index, total acidity, total acid output, pepsin activity, pepsin output and total carbohydrate to protein ratio in pyloric-ligated rat was studied. The active fraction of root bark at a dose level of 100 mg/kg p.o. showed significant reduction (P<0.05) in the ulcer index, total acidity, total acid output, pepsin activity and pepsin output along with a significant rise in total carbohydrate to protein ratio. The mechanism of antiulcer activity could be attributed to a decrease in gastric acid secretory and antioxidant activities leading to gastric cytoprotection. This activity could be linked to the presence of baicalein in the root bark of the plant[48].
Antimicrobial activity:
The antimicrobial activity of crude extract of O. indicum (petroleum ether, ethyl acetate and methanol), compound 1 (2,5-dihydroxy-6,7-dimethoxy flavone) and compound 2 (3,7,3’,5’-tetramethoxy-2-hydroxy flavone) was tested against fourteen pathogenic bacteria (5 Gram-positive and 9 Gram-negative) and seven pathogenic fungi. Nutrient agar and nutrient broth were used as bacteriological media and potato dextrose agar (PDA) was used for fungal growth. In antibacterial screening, each sample was dissolved in methanol at a concentration of 200 μg/10 μl. The activity of these samples was compared with standard kanamycin disc (K-30 μg/disc) using the standard disc diffusion method. Similarly antifungal screening was done at a concentration of 300 μg/disc for each sample and the activity was compared with the standard clotrimazole disc (K-30 μg/disc). From the antibacterial and antifungal experimental results, it was evident that the crude extracts (petroleum ether, ethyl acetate) and the compound 1 and 2 showed significant antibacterial and antifungal activity but were less potent than that of standard kanamycin and clotrimazole, where as methanol extract showed little activity. The findings support the use of O. indicum in traditional medicine for the treatment of bacterial and fungal infection[19,49].
Antioxidant activity:
The antioxidant activity of ethanol and aqueous extract of O. indicum leaves was studied in two in vitro models viz. radical scavenging activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH) reduction and nitric oxide radical scavenging activity in Griess reagent system. Ethanol extract possessed significant antioxidant activity in both the models. In scavenging DPPH radical, extracts activity was IC50=24.22 μg/ ml while in scavenging nitric oxide (NO) radical, the activity was IC50=129.81 μg/ml. The result showed that ethanol extract of O. indicum leaves possesses free radical scavenging activity[50].
Anticancer activity:
Methanol extract of the fruits of O. indicum inhibited in vitro proliferation of HL-60 cells. The flavonoid baicalein was found as an active component in the extract. The in vitro effects of baicalein on the viability and induction of apoptosis in the HL-60 cell line was further investigated. The cell viability after treating with baicalein for 24 h was quantified by counting viable cells using tryptan blue staining. The result showed that baicalein caused a 50% inhibition of HL-60 cells at concentration of 25-30 μM. The inhibition of proliferation of HL-60 cells due to 36-48 h exposure with 10 or 20 μM baicalein was associated with the accumulation of cells at S or G2M phases. The results indicated that baicalein has antitumors effects on human cancer cells[20].
Antimutagenic activity:
Methanol extract of O. indicum strongly inhibited the mutagenicity of Trp-P-1 in an Ames pre-incubation method in the presence of S9 mix using Salmonella typhimurium. Only 5 μl of the crude extract inhibited 91±5% of the mutagenesis induced by 50 ng of Trp-P-1. The major antimutagenic constituent was identified as baicalein with an IC50 value of 2.78±0.15 μM. The potent antimutagenicity of the extract was correlated with the high content (3.95±0.43%, dry weight) of baicalein. Baicalein act as desmutagen since it inhibits N-hydroxylation of Trp-P-2. The antimutagenic effect of baicalein was found mainly due to the inhibition of N-hydroxylation catalyzed by P450 monooxygenases in S-9[45].
Photocyotoxic activity:
The photocytotoxic activity of methanol extract of leaves of O. indicum was studied against promyelocytic leukemia cell line, HL60. The HL60 was incubated with 21 μg/ml of crude extracts for 2 h and irradiated with 9.6 J/cm2 of a broad spectrum light source in four replicates. Survival of cells was assessed 24 h later following the colorimetric MTT protocol. Pheophorbide-a, a commercially available and well-characterized photosensitizer was used as the positive control. To determine samples that have general cytotoxicity, a parallel assay without irradiation was also carried out. The result showed that methanol extract of leaves of O. indicum have photocytotoxic activity at concentration 21 μg/ml[51].
Antiarthritic activity:
Aqueous and ethanol extract of O. indicum were tested for in vitro release of myeloperoxidase (MPO) from rat peritoneal leukocytes. The results indicated that aqueous extract had a significant effect i.e. 64% inhibition of release of MPO[25].
Immunostimulant activity:
n-Butanol extract of root bark of O. indicum (100 mg/kg, once daily for 22 days) was studied for immunomodulatory activity in rats using measures of immune responses to sheep red blood cells (SRBC haemagglutinating antibody [HA] titer) and delayed-type hypersensitivity (DTH) reactions. In response to SRBC, treatment with the n-butanol fraction caused a significant rise in circulating HA titers during secondary antibody responses, indicating a potentiation of certain aspects of the humoral response. The treatment also resulted in a significant rise in paw edema formation, indicating increased host DTH response. Histopathologic analysis of lymphoid tissues in the treatment group showed an increase in cellularity, e.g., T-lymphocytes and sinusoids. In contrast, dexamethasone treatment caused significant reduction in the HA titer, DTH responses, and antioxidant activity. In a triple antigen-mediated immunological edema model, the extent of edema raised in drug-treated rats was greater compared to that in control rats, thus confirming enhanced DTH reactions in response to the drug treatment. Activity of the O. indicum might be attributed to its ability to enhance specific immune response (both humoral and cell-mediated)[52].
Antiproliferative activity:
The antiprolifirative activity of O. indicum was studied on human breast tumor cell lines. Results indicated that O. indicum have antiproliferative activity against MCF7 and MDA-MB-231 breast cancer cell lines[53].
Hepatoprotective activity:
The hepatoprotective activity of O. indicum was studied against carbon tetrachloride (CCl4)-induced hepatotoxicity in mice and rats. Biochemical study indicated that alcoholic (300 mg/kg), petroleum ether (300 mg/kg) and n-butanol (100 and 300 mg/kg) extracts significantly (P<0.05) lowered the elevated serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvate transaminase (SGPT), alkaline phosphatase (ALP) and total bilirubin (TB) levels as compared to the control group. The increased lipid peroxide (LPO) formation, reduced glutathione (GSH) and decreased antioxidant enzyme activities of superoxide dismutase (SOD), catalase (CAT) in the tissues of CCl4-treated animlas were significantly normalized by O. indicum treatment. Histopathological study also revealed that pretreatment with O. indicum restored CCl4-induced alteration in antioxidant status of the tissues. It is suggested that root bark showed significant antioxidant activity, which might be in turn responsible for its hepatoprotective activity[54].
CONCLUSIONS
O. indicum is a well known plant used in the Indian system of medicine, besides folklore medicine also claims its use in cancer, ulcer, diarrhea, dysentery, wound healing, inflammation, cough, enlarged spleen, jaundice, scabies and other skin diseases. Research carried out using different in vivo and in vitro techniques of biological evaluation support most of these claims. Recent studies have focused mainly on its antiinflammatory, antiulcer, antimicrobial, antiarthritic, hepatoprotective, immunostimulant, photocytotoxicity, antimutagenic, antiproliferative and antioxidant activities. Literature survey reveals that despite the enormous work done on this plant some of the pharmacological activities are still not proven scientifically. Some of the compounds present in O. indicum (chrysin, oroxylin A, baicalein, baicalin, irridoid, baichanin-A, 2,5-dihydroxy-6,7-dimethoxy flavone and 3,7,3’,5’-tetramethoxy-2-hydroxy flavone) are pharmacologically well known and provide additional supporting evidence for possible mechanism of action. O. indicum is used in the manufacture of various Ayurvedic preparations for a range of ailments and its non-drying oil used in perfume industry. This review was an attempt to compile an up-to-date and comprehensive review of O. indicum that covered its distribution, description, traditional and folk medicinal uses, phytochemistry and pharmacology.
ACKNOWLEDGMENTS
Authors would like to thank Prof. (Dr.) Ranjit Singh of School of Pharmaceutical Sciences, Shobhit University for their technical assistance.
Footnotes
Harminder, et al.: Phytopharmacological Study of Oroxylum indicum
REFERENCES
- 1.Nakahara K, Roy MK, Alzoreky NS, Thalang V, Trakoontivakorn G. Inventory of indigenous plants and minor crops in Thailand based on bioactivities. 9th JIRCAS International Symposium- Value addition to Agricultural Product. 2002:135–9. [Google Scholar]
- 2.Chauhan NS. Medicinal and Aromatic plants of Himachal Pradesh. 1st ed. New Delhi: Indus Publishing; 1999. Oroxylum indicum; pp. 96–298. [Google Scholar]
- 3.Warrier PK, Nambiar VP, Ramankutty C, Vasudevan R, editors. Indian Medicinal Plants: A compendium of 500 species. 1st ed. Chennai: Orient Longmam Private Ltd; 1995. Oroxylum indicum; pp. 186–90. [Google Scholar]
- 4.Khare CP. Oroxylum indicum. In: Khare CP, editor. Indian Herbal Remedies: Rational Western Therapy, Ayurvedic and other traditional usage, Botany. 4th ed. New York: Springer-Verlag Berlin Heidelberg; 2004. pp. 340–1. [Google Scholar]
- 5.Jayaram K, Prasad MN. Genetic diversity in Oroxylum indicum (L.) Vent. (Bignoniaceae), a vulnerable medicinal plant by random amplified polymorphic DNA marker. Afr J Biotech. 2008;7:254–62. [Google Scholar]
- 6.Qiang G, Luo H, Zheng W, Liu Z, Huang Y. Pseudonocardia oroxyli sp. nov., a novel actinomycete isolated from surface-sterilized Oroxylum indicum root. Int J Syst Evol Microbiol. 2006;56:2193–7. doi: 10.1099/ijs.0.64385-0. [DOI] [PubMed] [Google Scholar]
- 7.Dalal NV, Rai VR. In vitro propagation of Oroxylum indicum Vent. a medicinally important forest tree. J For Res. 2004;9:61–5. [Google Scholar]
- 8.Nakahara K, Trakoontivakorn G, Alzoreky NS, Ono H, Onishi-Kameyama M, Yoshida M. Anti-mutagenicity activity of some edible Thai plants, and a bioactive carbazole alkaloid, mahanine, isolated from Micromelum minutun. J Agri Food Chem. 2002;50:4796–802. doi: 10.1021/jf025564w. [DOI] [PubMed] [Google Scholar]
- 9.Yoganarasimhan SN. Vol. 1. Bangalore: Interline Publishing; 1996. Medicinal plants of India: Karnataka; pp. 366–7. [Google Scholar]
- 10.Bhattacharje SK. Use of flavours and fragrances. In: Bhattacharje SK, editor. Handbook of aromatic plants. 2nd ed. Jaipur: Pointer Publishers; 2005. [Google Scholar]
- 11.Zaveri M, Khandhar A, Jain S. Quantification of Baicalein, Chrysin, Biochanin-A and Ellagic acid in root bark of Oroxylum indicum by RP-HPLC with UV detection. Eurasian J Anal Chem. 2008;3:245–57. [Google Scholar]
- 12.Jabbar S, Khan MT, Choudhuri MS, Sil BK. Bioactivity studies of the individual ingredients of the Dashamularishta. Pak J Pharm Sci. 2004;17:9–17. [PubMed] [Google Scholar]
- 13.Chen LJ, Games DE, Jones J. Isolation and identification of four flavonoids constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. J Chromatogr A. 2003;988:95–105. doi: 10.1016/s0021-9673(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen LJ, Song H, Lan XQ, Games DE, Sutherland IA. Comparisons of high speed counter-current chromatography instruments for the separation of the extracts of the seeds of Oroxylum indicum. J Chromatogr A. 2005;1063:241–5. doi: 10.1016/j.chroma.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 15.Dey AK, Mukherjee A, Das PC, Chatterjee A. Occurrence of aloe-emodin in the leaves of Oroxylum indicum Vent. Indian J Chem. 1978;16B:1042. [Google Scholar]
- 16.Yuan Y, Hou W, Tang M, Luo H, Chen LJ, Guan YH, et al. Separation of flavonoids from the leaves of Oroxylum indicum by HSCCC. Chromatographia. 2008;68:885–92. [Google Scholar]
- 17.Yuan Y, Houding L, Lijuan C. Linear scale-up of the separation of active components from Oroxylum indicum using high speed counter current chromatography. Chinese J Chromatogr. 2008;26:489–93. doi: 10.1016/s1872-2059(08)60024-3. [DOI] [PubMed] [Google Scholar]
- 18.Subramaniam SS, Nair AG. Flavonoids of the stem bark of Oroxylum indicum. Curr Sci. 1972;41:62–3. [Google Scholar]
- 19.Kawsar U, Sayeed A, Islam A, Rahman AA, Ali A, Khan AM, et al. Biological activities of extracts and two flavanoids from Oroxylum indicum Vent. J Biol Sci. 2003;3:371–5. [Google Scholar]
- 20.Roy MK, Nakahara K, Trakoontivakorn G, Takenaka M, Isobe S, Tsushida T. Baicalein, a flavanoid extracts from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie. 2007;62:149–53. [PubMed] [Google Scholar]
- 21.Tomimori T, Imoto Y, Ishida M, Kizu H, Namba T. Studies on the Nepalese crude drug.VIII. On the flavonoid constituents of the seed of Oroxylum indicum. Shoyakugaku Zasshi. 1988;42:98–101. [Google Scholar]
- 22.Bhattacharje AK, Das AK. Phytochemical screening of some Indian plants. QJ Crude Drug Res. 1969;9:1408. [Google Scholar]
- 23.Kapoor LD. 1st ed. Florida: CRC press Inc; 2001. Handbook of Ayurvedic Medicinal Plants: Herbal reference library; p. 252. [Google Scholar]
- 24.Subramaniam SS, Nair AG. Flavonoids of leaves of Oroxylum indicum and Pajanelia longifolia. Phytochem. 1972;11:439–70. [Google Scholar]
- 25.Laupattarakasem P, Houghton PJ, Hoult JR, Itharat A. An evaluation of the activity related to inflammation of four plants used in Thailand to treat arthritis. J Ethnopharmacol. 2003;85:207–15. doi: 10.1016/s0378-8741(02)00367-7. [DOI] [PubMed] [Google Scholar]
- 26.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, et al. The antitumor activities of flavonoids. In vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 27.Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–34. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 28.Kao YC, Zhou C, Sherman M, Laughton CA, Chen S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A Site-directed Mutagenesis Study. Environ Health Perspect. 1998;106:85–92. doi: 10.1289/ehp.9810685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qais N, Rahman MM, Rashid MA, Koshino H, Nagasawa K, Nakata T. Antibacterial flavonoids from Desmos chinensis. Fitoterapia. 1996;67:554–5. [Google Scholar]
- 30.Hecker M, Preiss C, Klemm P, Busse R. Inhibition by antioxidants of nitric oxide synthase expression in murine. Br J Pharmacol. 1996;118:2178. doi: 10.1111/j.1476-5381.1996.tb15660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishkin RJ, Winslow JT. Endotoxin-induced reduction of social investigation by mice: interaction with amphetamine and antiinflammatory drugs. Psychopharmacology (Berl) 1997;132:335–41. doi: 10.1007/s002130050353. [DOI] [PubMed] [Google Scholar]
- 32.Pearce FL, Befs AD, Bienenstock J. Mucosal mast cells. III. Effect of quercitin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. J Allergy Clin Immunol. 1984;73:819–23. doi: 10.1016/0091-6749(84)90453-6. [DOI] [PubMed] [Google Scholar]
- 33.Habtemariam S. Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-alpha in L-929 tumor cells. J Nat Prod. 1997;60:775–8. doi: 10.1021/np960581z. [DOI] [PubMed] [Google Scholar]
- 34.Wolfman C, Viola H, Paladini A, Dajas F, Medina JH. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passifloara coerulea. Pharmacol Biochem Behav. 1994;47:1–4. doi: 10.1016/0091-3057(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 35.Kubo I, Kinst-Hori I, Choudhuri SK, Kubo Y, Sanchez Y, Ogura T. Flovonols from Heterothesca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg Med Chem Lett. 2000;8:1749–55. doi: 10.1016/s0968-0896(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 36.Suresh CT, Leena S, Sari M, Risto S. Inhibition of 17β-estradiol formation by isoflavonoids and flavonoids in cultured JEG-3 cells: Search for aromatase-targeting dietary compounds. J Med Food. 1999;2:235–8. doi: 10.1089/jmf.1999.2.235. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Ho DK, Cassady JM, Cook VM, Baird WM. Isolation of potential anticancer chemopreventive agents from Eriodictyon californicum. J Nat Prod. 1992;55:357–63. doi: 10.1021/np50081a012. [DOI] [PubMed] [Google Scholar]
- 38.Babu KS, Babu TH, Srinivas PV, Kishore KH, Murthy US, Rao JM. Synthesis and biological evaluation of novel C (7) modified chrysin analogues as antibacterial agents. Bioorg Med Chem Lett. 2006;16:221–4. doi: 10.1016/j.bmcl.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Chen YC, Yang LL, Lee TJ. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and cox-2 gene expression via suppression of nuclear factor-kb activation. Biochem Pharmacol. 2000;59:1445–57. doi: 10.1016/s0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 40.You KM, Jong HG, Kim HP. Inhibition of cyclooxygenase/lipooxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants. Arch Pharmacol Res. 1999;22:18–24. doi: 10.1007/BF02976430. [DOI] [PubMed] [Google Scholar]
- 41.Ryu SH, Ahn BZ, Pack MY. The cytotoxic principle of Scutellariae radix against L1210 cell. Planta Med. 1995;4:335. doi: 10.1055/s-2007-969517. [DOI] [PubMed] [Google Scholar]
- 42.Ali RM, Houghton PJ, Hoult JRS. Antimicrobial and antiinflammatory activities of extracts and constituents of Oroxylum indicum (L.) Vent Phytomed. 1998;5:375–81. doi: 10.1016/S0944-7113(98)80020-2. [DOI] [PubMed] [Google Scholar]
- 43.Rasadah MA, Houghton PJ. Anti-microbial activity of some species of Bignoniaceae. ASEAN Review of Biodiversity and Environmental Conservation (ARBEC) 1998;3:1–3. [Google Scholar]
- 44.Kimura Y, Okuda Y, Taira Z, Shoji N, Takemoto T, Arichi S. Studies on Scutellariae radix. IX. New component inhibiting lipid peroxidation in rat liver. Planta Med. 1984;50:290–5. doi: 10.1055/s-2007-969712. [DOI] [PubMed] [Google Scholar]
- 45.Nakahara K, Onishi-Kameyama M, Ona H, Yoshida M, Trakoontivakorn G. Antimutagenic activity against Trp-P-1 of the edible Thai plant, Oroxylum indicum Vent. Biosci Biotechnol Biochem. 2001;65:2358–60. doi: 10.1271/bbb.65.2358. [DOI] [PubMed] [Google Scholar]
- 46.Babu KS, Babu TH, Srinivas PV, Sastry BS, Kishore KH, Murty US, et al. Synthesis and in vitro study of novel 7-O-acyl derivatives of Oroxylin A as antibacterial agents. Bioorg Med Chem Lett. 2005;15:3953–6. doi: 10.1016/j.bmcl.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 47.Upaganlawar AB, Tende CR, Yeole PG. Antiinflammatory activity of aqueous extract of Oroxylum indicum Vent.leaves extract-preliminary study. Pharmacology online. 2009;1:22–6. [Google Scholar]
- 48.Khandhar M, Shah M, Santani D, Jain S. Antiulcer activity of the root bark of Oroxylum indicum against experimental gastric ulcers. Pharm Bio. 2006;44:363–70. [Google Scholar]
- 49.Chopade VV, Upaganlawar AB, Yeole PG. Antimicrobial activity of Oroxylum indicum. Antiseptic. 2008;105:146–7. [Google Scholar]
- 50.Upaganlawar AB, Tende CR. In vitro antioxidant activity of leaves of Oroxylum indicum Vent. Biomed. 2007;2:300. [Google Scholar]
- 51.Ong CY, Ling SK, Ali RM, Chee CF, Samah ZA, Ho AS, et al. Systemic analysis of in vitro photo-cytotoxic activity in extracts from terrestrial plants in Peninsula Malaysia for photodynamic therapy. J Photochem Photobiol B. 2009;96:216–22. doi: 10.1016/j.jphotobiol.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Zaveri M, Gohil P, Jain S. Immunostimulant activity of n-butanol fraction of root bark of Oroxylum indicum Vent. J Immunotoxicol. 2006;3:83–99. doi: 10.1080/15476910600725942. [DOI] [PubMed] [Google Scholar]
- 53.Lambertini E, Piva R, Khan MT, Lampronti I, Bianchi N, Borgatti M, et al. Effects of extracts from Bangladeshi medicinal plants on in vitro proliferation of human breast cancer cell lines and expression of estrogen receptor alpha gene. Int J Oncol. 2004;24:419–23. [PubMed] [Google Scholar]
- 54.Zaveri M, Jain S. Hepatoprotective effect of root bark of Oroxylum indicum on carbon tetrachloride (CCl4)-induced hepatotoxicity in experimental animals. [Last updated on 2009 Aug 08]. Available from: http://www.biology-online.org/articles/hepatoprotective-effect-root-bark-oroxylum.html .


