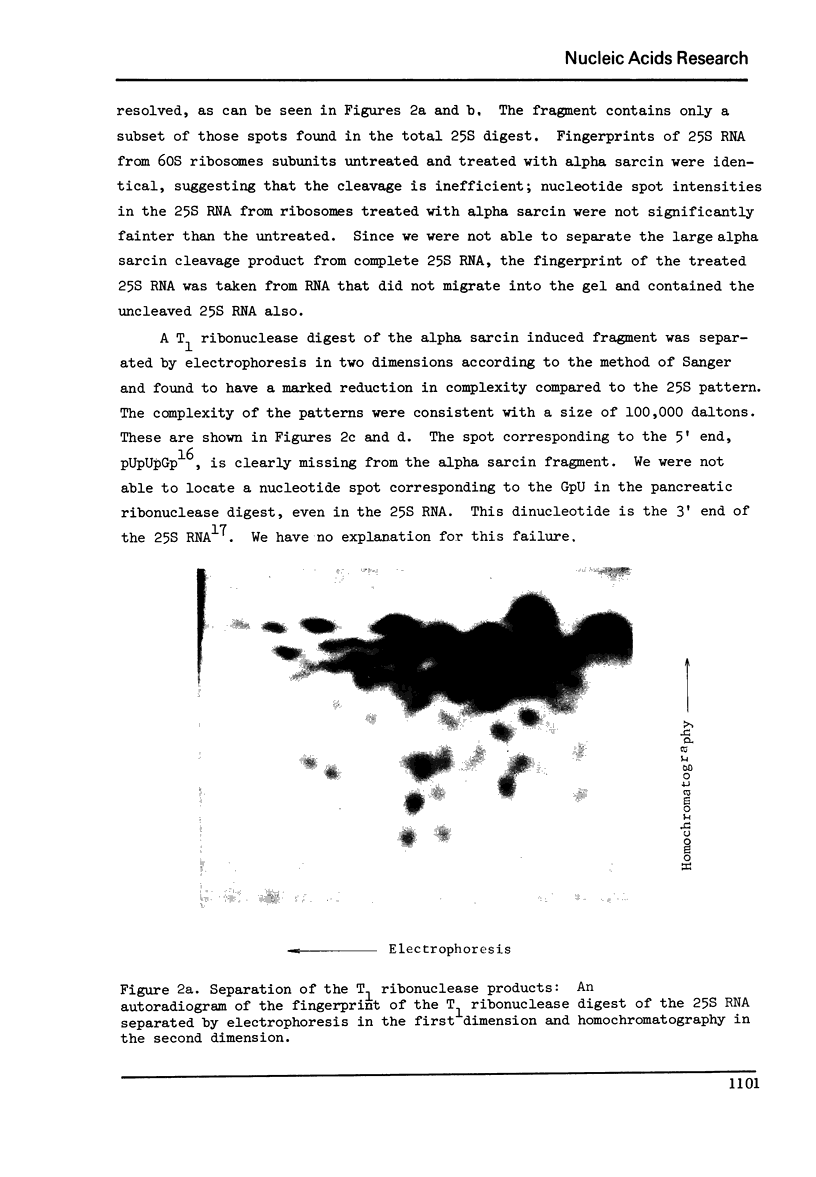
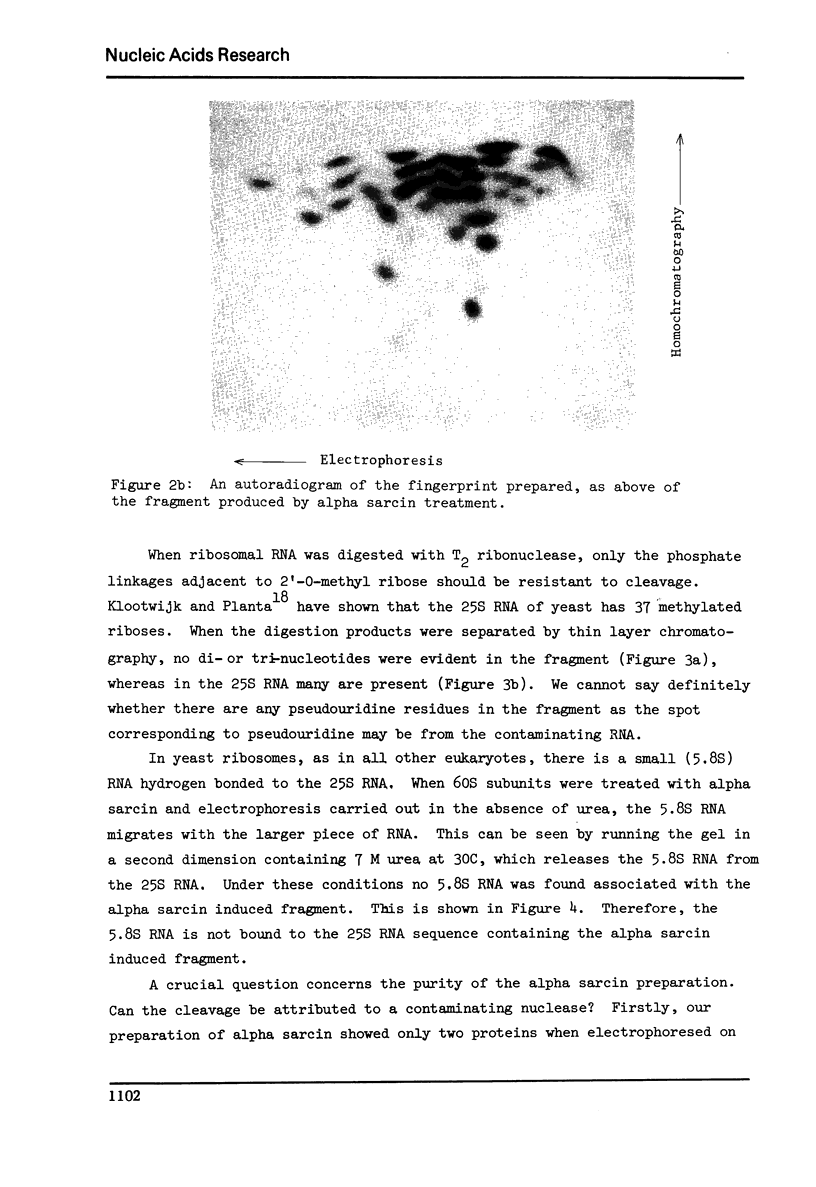
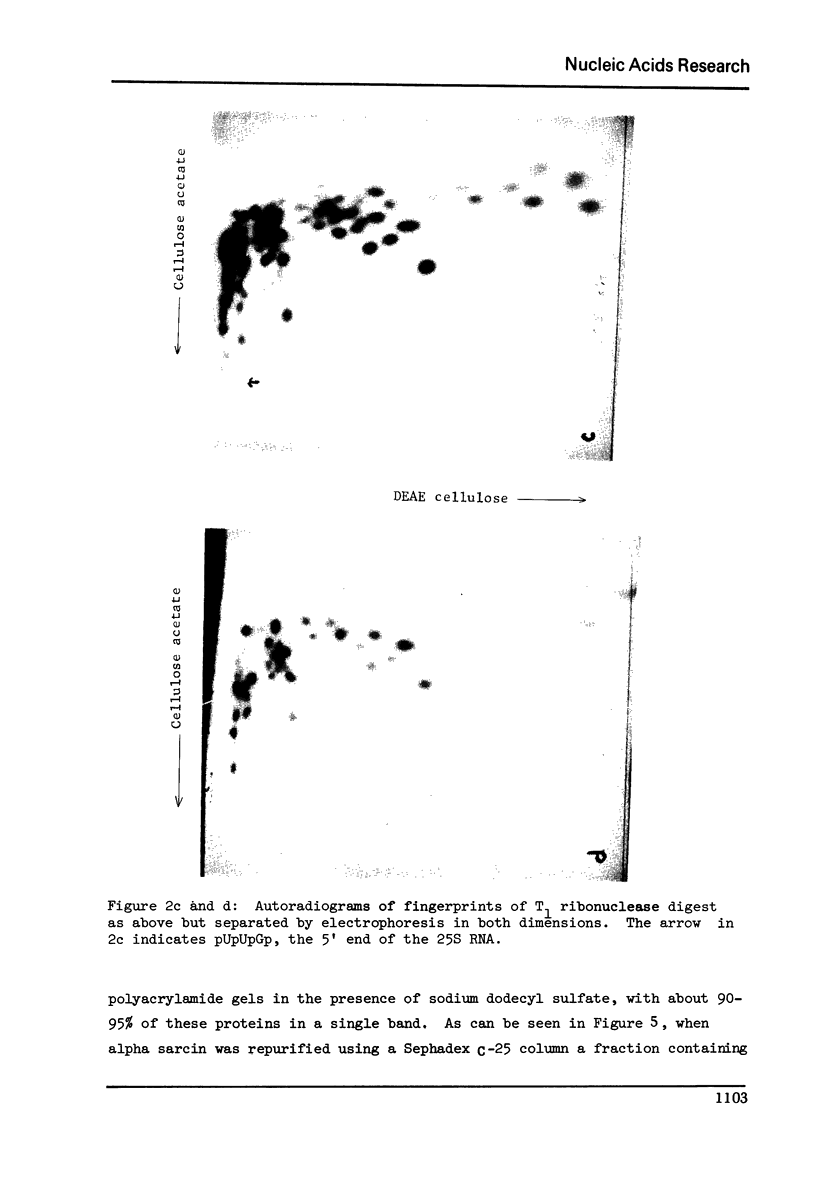
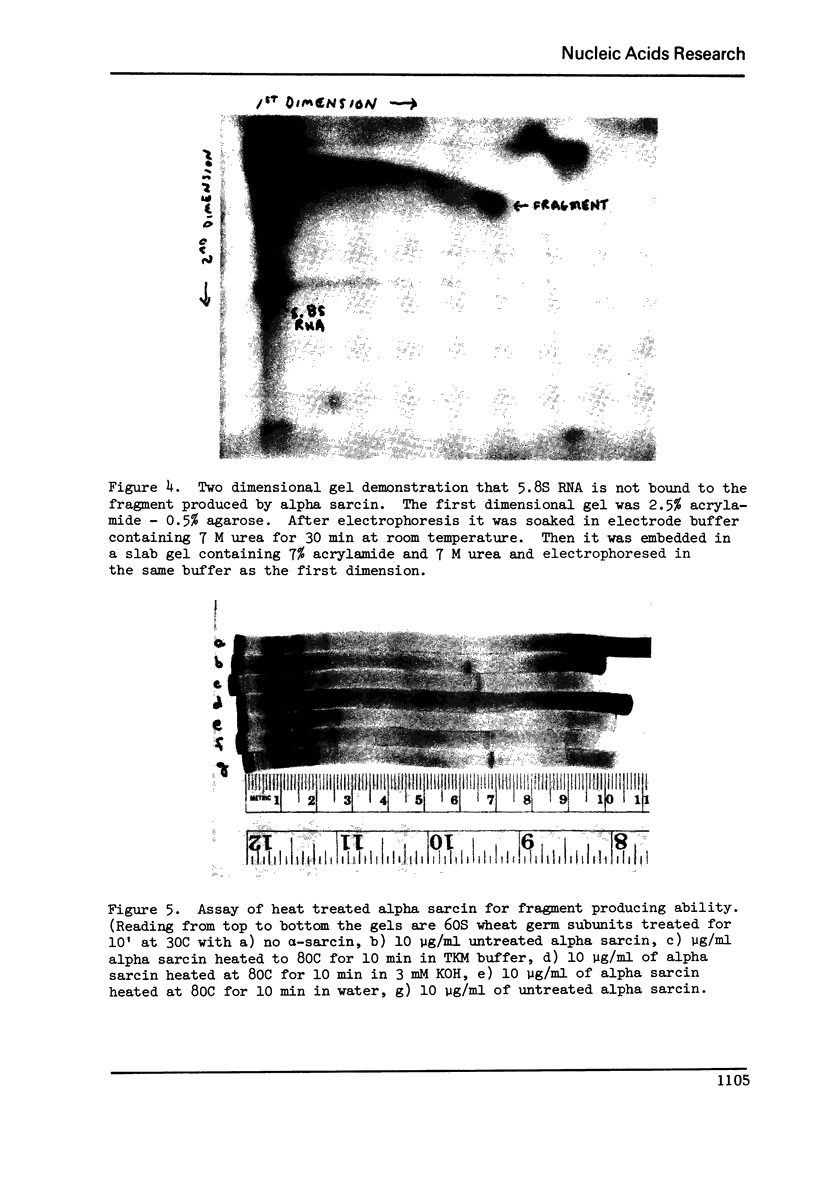
Abstract
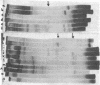
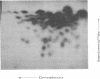
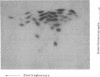
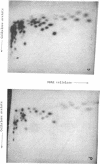
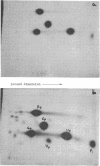
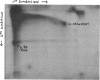
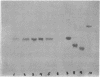
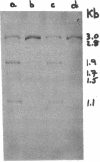
Alpha sarcin causes the specific cleavage of RNA from 80S ribosomes and 60S subunits of yeast, but not from the 40S subunits to produce a small RNA fragment. The fragment was also produced on treatment of the 60S subunits of wheat germ ribosomes. The fragment has a molecular weight of 100,000 and is a cleavage product of the large RNA species in the 60S subunits. The fragment is not derived from the 5'end of the yeast 25S RNA nor does it bind to 5.8S RNA and we propose that the fragment represents the 3' terminal 320 nucleotides of 25S rRNA. The ability to produce fragment could not be separated from the ability of alpha sarcin to inhibit protein synthesis. Alpha sarcin also causes the specific cleavage of the 23S RNA of the E. coli subunit to produce a smaller fragment of RNA than that produced from eukaryote ribosomes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Spahr P. F. Binding sites of ribosomal proteins on two specific fragments derived from Escherichia coli 50 S ribosomes. Eur J Biochem. 1971 Mar 11;19(2):250–255. doi: 10.1111/j.1432-1033.1971.tb01311.x. [DOI] [PubMed] [Google Scholar]
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 and its mechanism of action. Proc Natl Acad Sci U S A. 1972 Mar;69(3):549–552. doi: 10.1073/pnas.69.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Nucleotide sequences from the low molecular weight ribosomal RNA of Escherichia coli. J Mol Biol. 1967 Feb 14;23(3):337–353. doi: 10.1016/s0022-2836(67)80109-8. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol. 1967 Apr 14;25(1):83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Muramatsu M. Differences in nucleotide sequences of ribosomal RNA between the liver and a hepatoma of C3H-He mice. Eur J Biochem. 1973 Mar 15;33(3):446–458. doi: 10.1111/j.1432-1033.1973.tb02702.x. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., Planta R. J. Analysis of the methylation sites in yeast ribosomal RNA. Eur J Biochem. 1973 Nov 15;39(2):325–333. doi: 10.1111/j.1432-1033.1973.tb03130.x. [DOI] [PubMed] [Google Scholar]
- OLSON B. H., GOERNER G. L. ALPHA SARCIN, A NEW ANTITUMOR AGENT. I. ISOLATION, PURIFICATION, CHEMICAL COMPOSITION, AND THE IDENTITY OF A NEW AMINO ACID. Appl Microbiol. 1965 May;13:314–321. doi: 10.1128/am.13.3.314-321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Fernandez-Puentes C., Carrasco L., Vazquez D. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur J Biochem. 1975 Dec 1;60(1):281–288. doi: 10.1111/j.1432-1033.1975.tb21001.x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Preparation of RNA and ribosomes from yeast. Methods Cell Biol. 1975;12:45–64. doi: 10.1016/s0091-679x(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Schindler D., Grant P., Davies J. Trichodermin resistance--mutation affecting eukaryotic ribosomes. Nature. 1974 Apr 5;248(448):535–536. doi: 10.1038/248535a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Hunt J. A., Dalgarno L. Studies on the 3'-terminal sequences of the large ribosomal ribonucleic acid of different eukaryotes and those associated with "hidden" breaks in heart-dissociable insects 26S ribonucleic acid. Biochem J. 1974 Sep;141(3):617–625. doi: 10.1042/bj1410617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Takanami M. Analysis of the 5'-terminal nucleotide sequences of ribonucleic acids. II. Comparison of the 5'-terminal nucleotide sequences of ribosomal RNA's from different organisms. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1595–1602. doi: 10.1073/pnas.58.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnowsky F., Drews J., Eich F., Högenauer G. In vitro inactivation of ascites ribosomes by colicin E 3. Biochem Biophys Res Commun. 1973 May 1;52(1):327–334. doi: 10.1016/0006-291x(73)90991-1. [DOI] [PubMed] [Google Scholar]