Abstract
In benign prostatic hyperplasia (BPH) there will be a sudden impact on overall quality of life of patient. This disease occurs normally at the age of 40 or above and also is associated with sexual dysfunction. Thus, there is a need of update on current medications of this disease. The presented review provides information on medications available for BPH. Phytotherapies with some improvements in BPH are also included. Relevant articles were identified through a search of the English-language literature indexed on MEDLINE, PUBMED, Sciencedirect and the proceedings of scientific meetings. The search terms were BPH, medications for BPH, drugs for BPH, combination therapies for BPH, Phytotherapies for BPH, Ayurveda and BPH, BPH treatments in Ayurveda. Medications including watchful waitings, Alpha one adrenoreceptor blockers, 5-alpha reductase inhibitors, combination therapies including tamsulosin-dutasteride, doxazosin-finasteride, terazosin-finasteride, tolterodine-tamsulosin and rofecoxib-finasteride were found. Herbal remedies such as Cernilton, Saxifraga stolonifera, Zi-Shen Pill (ZSP), Orbignya speciosa, Phellodendron amurense, Ganoderma lucidum, Serenoa Repens, pumpkin extract and Lepidium meyenii (Red Maca) have some improvements on BPH are included. Other than these discussions on Ayurvedic medications, TURP and minimally invasive therapies (MITs) are also included. Recent advancements in terms of newly synthesized molecules are also discussed. Specific alpha one adrenoreceptor blockers such as tamsulosin and alfuzosin will remain preferred choice of urologists for symptom relief. Medications with combination therapies are still needs more investigation to establish as preference in initial stage for fast symptom relief reduced prostate growth and obviously reduce need for BPH-related surgery. Due to lack of proper evidence Phytotherapies are not gaining much advantage. MITs and TURP are expensive and are rarely supported by healthcare systems.
Keywords: 5 Alpha one reductase inhibitors, alpha one adrenoreceptor blockers, benign prostatic hyperplasia, therapies for BPH, treatment for BPH
INTRODUCTION
At the start of a new millennium, prostatic diseases continue to affect and account for a substantial number of lives in the USA. An estimated one in 11 men will develop a malignant neoplasm of the prostate and approximately 37,000 men will die each year from prostate cancer.[1,2] For European Community countries, more than 35,000 annual deaths are forecast due to prostate cancer.[1,3] Prostate cancer mortality results from metastases to the bone and lymph nodes together with progression from androgen-dependent to androgen independent disease.[1,4] The high mortality associated with these tumors is due to the fact that more than 50% of newly diagnosed patients present with advanced, metastatic disease.[5,6] Radical prostatectomy, androgen-ablation mono-therapy and radiotherapy are considered to be curative for localized disease,[7–9] but there is no effective treatment for metastatic prostate cancer that increases patient survival.
In humans, the prostate lies immediately below the base of the bladder surrounding the proximal portion of the urethra and consists of canals and follicles lined with columnar epithelial cells and surrounded by a fibromuscular stroma consisting of connective tissue and smooth muscle. The prostate contributes to seminal fluid, where its secretions are important in optimizing conditions for fertilization by enhancing the viability of sperm in both male and female reproductive tracts. In all mammals, the prostatic secretions are stored in the acini and released into the urethra, at ejaculation, by contraction of the prostatic (stromal) smooth muscle.[10]
BPH is a progressive disease that is commonly associated with bothersome lower urinary tract symptoms (LUTS) such as frequent urination, urgency, nocturia, decreased and intermittent force of stream, and the sensation of incomplete bladder emptying. The term BPH actually refers to a histologic condition, namely the presence of stromal glandular hyperplasia within the prostate gland.[11]
BPH is a common and progressive clinical disease of ageing men, which may be associated with enlargement of the prostate, bothersome LUTS and bladder outlet obstruction (BOO). In the large scale Multinational Survey of the Aging Male, = 34% of men in the USA and 29% of European men aged 50–80 years reported moderate to severe LUTS. Sexual dysfunction is another common condition in ageing men; the results of the Multinational Survey of the Aging Male also showed that LUTS is an independent risk factor for sexual dysfunction in ageing men. Both of these age-dependent conditions have a measurable effect on overall quality of life (QOL). Thus, LUTS and sexual dysfunction are common and important health concerns of men aged ≥50 years.[12]
At least 300,000 patients with LUTSs are treated annually by physicians in Japan, and this figure is expected to increase in the coming years.[13]
BPH is defined as a disease that manifests as a lower urinary tract dysfunction due to benign hyperplasia of the prostate, usually associated with enlargement of the prostate and LUTS suggestive of lower urinary tract obstruction.[14] Although BPH is generally not a life threatening condition, it can have a marked effect on a patient's QOL.[15] The cost of managing BPH is > $4 billion per year.[16]
DIAGNOSIS
When assessing men presenting with LUTS, all patients should undergo a physical examination including a careful digital rectal examination (DRE). DRE is unreliable in assessing the size of the prostate and has been found to underestimate; the larger the prostate the more its size is underestimated. However, it is important to assess the prostate, because some men are still found to have prostate cancer on the basis of DRE. In addition, urine analysis and a serum PSA assay are recommended as part of the diagnosis and as a marker to help differentiate men with BPH from those with prostate cancer, when in the opinion of the physician, the PSA is abnormal. PSA levels rise with age, so during assessment, to achieve a specificity of 70% maintaining a sensitivity between 65% and 70%, approximate age-specific figures for detecting men with prostate glands of >40 mL are PSA levels of >1.6 ng/mL, >2.0 ng/mL, and >2.3 ng/mL for men with BPH aged 50–59 years, 60–69 years and 70–79 years, respectively.[17]
TREATMENT OPTIONS FOR BPH
The predominant treatment of BPH over the last 60 yrs has been based on an ablative surgical approach. Recently, acquired knowledge on epidemiology and pathophysiology (of the prostate and bladder) as well as information acquired from endocrinologic and urodynamic investigation shave caused urologists to reevaluate the conventional guidelines on which both diagnosis and treatment have been based.[18]
The increasing elderly population within society has caused healthcare givers and the pharmaceutical industry to spend more effort on age related diseases such as BPH. The prostate is a male sex auxiliary gland situated just below the bladder and surrounding the urethra. Excessive growth of the prostate with age will result in BPH, which causes obstruction of the bladder outlet and eventually leads to LUTS.[19] LUTS and BPH are progressive conditions in many men, and such progression is characterized by increased prostate size, worsening of symptoms, bother, QOL, deterioration of flow rate and urodynamics, and finally development of outcomes such as acute urinary retention AUR) and surgical interventions.[20]
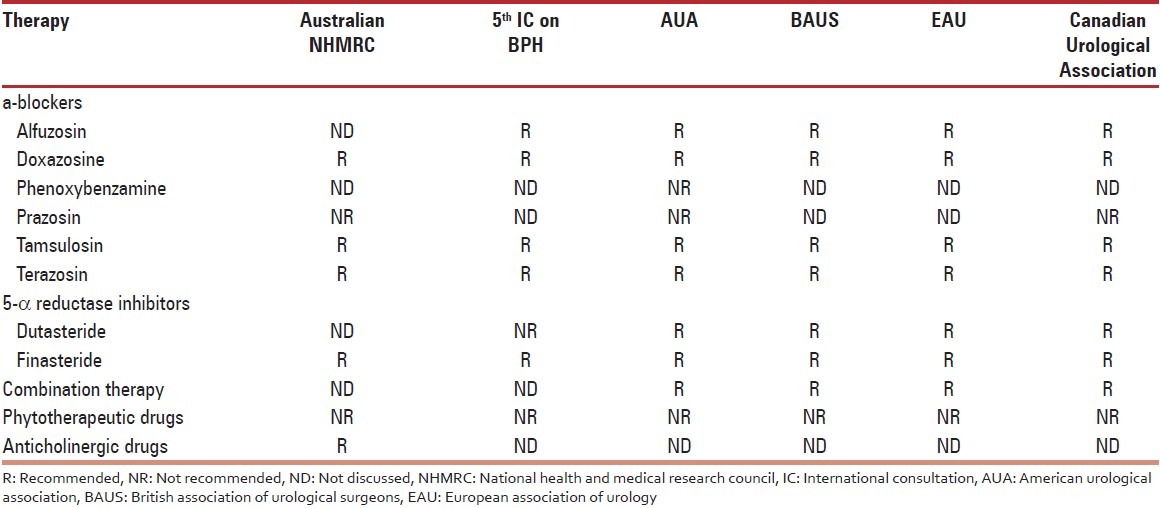
Over the last decade, multiple new treatment modalities for symptomatic BPH have arisen. New minimally invasive surgical therapies (MIST), new medications, and novel combinations of medical therapies have expanded the number of treatment options—still ranging from watchful waiting to open surgery. The range of treatment options is as broad as the BPH spectrum of symptoms. BPH is rarely lethal, most agree that management should safely improve QOL.[21] The aim of therapy for BPH is to improve QOL by providing symptom relief and increasing maximum flow rate as well as reducing disease progression and the development of new morbidities.[22] Pharmacologic therapies recommended by the various guidelines for male LUTS are presented under Table 1.
Table 1.
Pharmacologic therapies recommended by the analysed guidelines for male lower urinary tract symptoms[23]
Watchful waiting
Watchful waiting WW is a management strategy in which the patient is monitored by his physician without receiving any active intervention for LUTS.[23] (WW) refers to active monitoring of patients with BPH symptoms. Deciding on absolute indications for surgery is more straightforward than deciding on which men are the best candidates for WW.[21] In men in whom surgery is not indicated, WW has been shown to be safe over a five-year period. In a study by Ball et al., 107 men with LUTS not requiring surgery were followed for 5 years.[21,24] Only ten patients underwent surgery in that time: two for acute retention and eight for worsening symptoms. Of the 97 untreated patients, 31 reported subjective improvement, 50 unchanged, and 16 felt worse.
Drug mono-therapy
Alpha one adrenoreceptor blockers
α1-Adrenergic receptors (AR) mediate many of the physiological functions of the endogenous catecholamine's noradrenaline and adrenaline such as smooth muscle contraction or cellular hypertrophy. Moreover, they are the molecular target for clinically used drugs for the treatment of e.g. arterial hypertension or BPH.[25] The predominance of α1-AR in the bladder neck or prostate (40 times the bladder concentration) helped focus interest on α1-adrenergic blocking agents in the treatment of symptomatic BPH. Presently, α1-adrenoreceptor antagonists (α1-blockers that include doxazosin, terazosin, tamsulosin, and alfuzosin) are common for treating BPH related LUTS. They treat the dynamic component of BPH by blocking α1-receptor-mediated sympathetic stimulation to relax the smooth muscle in the prostate. All these agents produce their effects on voiding within hours of administration, regardless of prostate size, without altering serum prostate specific antigen or volume.[26]
The α1-blockers reduce smooth muscle tone in the prostate and result in rapid improvements in urinary symptoms and flow. Currently available α1-blockers include the nonselective α1-blockers, terazosin, doxazosin, and alfuzosin, and the highly selective α1A-blocker, tamsulosin. These agents have comparable efficacy; the main difference among these agents relates to their tolerability profiles.[27]
Silodosin is a new agent with high selectivity for α1A-receptors, which predominate in the male bladder outflow tract relative to a1B-receptors. It has been demonstrated in vitro that silodosin's α1A -to- α1B binding ratio is extremely high (162:1), suggesting the potential to markedly reduce dynamic neutrally mediated smooth muscle relaxation in the lower urinary tract while minimizing undesirable effects on blood pressure regulation. Both preclinical and clinical studies support the contention that silodosin has high uroselectivity and a positive cardiovascular safety profile, likely related to its selectivity for the α1A-AR subtype. Silodosin has a rapid onset of action and a sustained efficacy on LUTS due to BPH.[28]
Naftopidil is an alpha1D-selective blocker, which has been recently reported to less likely induce ejaculatory disorders. Efficacies on LUTS of the two alpha-1 blockers, silodosin and naftopidil are almost equivalent, with a small advantage of silodosin on voiding symptoms. The alpha1D-selective blocker, naftopidil may possess superior property of preserving sexual function (especially for ejaculation), compared with the alpha1A-selective blocker, silodosin.[29]
The greatest safety concern associated with the use of these agents is the occurrence of vasodilatory symptoms such as dizziness and orthostatic hypotension resulting from inhibition of α1-ARs in the systemic vasculature; this effect is minimized by use of agents that selectively antagonize the α1A-AR.[30]
α1-AR antagonists are a reasonably well-tolerated drug class, but cardiovascular side-effects can occur, and these can lead to serious morbidity such as falls and fractures. Although the available data are not conclusive, it appears that patients with cardiovascular comorbidities and those concomitantly using anti-hypertensive and/or PDE-5 inhibitors might be particularly at risk. The safety of tamsulosin in such risk groups is better documented than that of other α1-AR antagonists, and this should affect drug choice in patients with LUTS/BPH belonging to any of these risk groups.[31]
5-alpha reductase inhibitors
5 ARIs inhibit the conversion of testosterone to dihydrotestosterone (DHT), the primary androgen involved in both normal and abnormal prostate growth. There are currently two 5 ARIs licensed for the management of BPH, finasteride and dutasteride. Dutasteride, the only 5 ARI to inhibit both type 1 and type II 5 a reductase, induces a more profound reduction of serum DHT in the range of 90–95% compared with 70–75% for finasteride.[32]
Finasteride was the first steroidal 5 a-reductase inhibitor approved by U.S. Food and Drug Administration (USFDA). In human it decreases the prostatic DHT level by 70–90% and reduces the prostatic size. Dutasteride another related analogue has been approved in 2002. Unlike Finasteride, Dutasteride is a competitive inhibitor of both 5 a-reductase type I and type II isozymes, reduced DHT levels > 90% following 1 year of oral administration. Finasteride and Dutasteride are the only two steroidal clinically used drugs that have evolved from nearly 40 years of research on steroids as 5 a-reductase inhibitors but many compounds have shown promising results such as Epristeride which is in clinical trials.[33]
Epristeride, a novel 5 a-reductase inhibitor, is an interesting drug in the treatment of BPH. It belongs to class of carboxy steroid. It has been shown to be an uncompetitive inhibitor against both testosterone and NADPH. Its inhibitory action results from a preferential association to an enzyme binary complex containing NADP and hence, increases in testosterone concentration does not overcome its inhibition. It is a specific inhibitor of type II 5 a-reductase isoenzyme. It also attenuates the growth rate of some androgen responsive prostate cancers.[34]
Both finasteride and dutasteride have also shown promise in preventing prostate cancer in men at risk for developing the disease.[35]
Side effects of 5 ARI treatments mainly relate to sexual function, with a decreased libido in 6%, erectile dysfunction in 8% and decreased ejaculation in 4% (compared with 3%, 4% and 1%, respectively, for placebo). Current guidelines recommend that patients initiated on a 5 ARI are reviewed (IPSS, uroflowmetry and post-void residual volume assessment) after 12 weeks and at 6 months to determine response and then annually, provided there is no deterioration of symptoms.[32]
FK-143, 4-[3-[3-[bis (4-isobutylphenyl) methyl amino] benzoyl]-lH-indol-l-yl] butyric acid is a potent dual inhibitor of both human 5 a-reductase isozymes. It inhibited in vitro human and rat prostatic 5 a-reductase in a dose-dependent manner with an IC50 of 1.9 and 4.2 nM, respectively, in a non-competitive fashion while in vivo showed potent inhibitory activity against castrated young rat model. This compound can be a potential drug for the treatment of BPH.[36–38]
Combination therapies
In the study, Symptom Management After Reducing Therapy (SMART-1), 327 patients with BPH were treated with the combination of an a1-blocker (tamsulosin, 0.4 mg/d) plus a 5 a-reductase inhibitor (dutasteride, 0.5 mg/d). One arm of the study received combination therapy for 36 wk, and the other arm combination therapy for 24 wk, after which placebo was substituted for tamsulosin for the remaining 12 wk of the study. Overall, 91% of patients in the arm in which tamsulosin therapy was continued for 36 wk felt the same or better at week 30 as they did at week 24 compared with 77% of patients who had tamsulosin withdrawn at week 24. In patients with moderate symptoms (IPSS < 20), the relative percentages were 93% and 84% and in patients with severe symptoms (IPSS3 20), the percentages were 86% and 58%. These data suggest that tamsulosin can be withdrawn from a tamsulosin-dutasteride combination after 24 wk of therapy, but that men with severe symptoms may need a longer period of combination treatment.
It has been suggested that this might reflect the possibility that short-term benefits from α1-blockers, namely, the relaxation of the smooth muscle to relieve urinary tension, are negated by continued prostate growth, which itself can be halted and reversed by 5 a-reductase inhibition.
Combination therapy with doxazosin and finasteride has been shown to provide fast symptom relief, reduced prostate growth, reduced risk of AUR, and the need for BPH-related surgery.[39]
All the guidelines published after the Medical Therapy of Prostatic Symptoms (MTOPS) trial discussed the option to offer 5-a-reductase inhibitors or combination therapy of 5-a-reductase inhibitors and a-blockers as appropriate treatment for patients with LUTS with demonstrated prostatic enlargement.[23] By combining the beneficial effects of each class, the use of α1ARA/5 ARI therapy has the potential to address concerns associated with mono-therapy.[40] The 4-yr CombAT data provide support for the long-term use of dutasteride and tamsulosin combination therapy in men with moderate-to-severe LUTS due to BPH and prostatic enlargement.[41]
One more study[42] concluded that the combination of terazosin and finasteride was no more effective than the alpha-blocker terazosin used alone. As described by the authors, a limitation of this study was the failure to include men with prostate volumes similar to those in other trials in which finasteride was shown to improve AUA symptom scores and peak urinary flow rates over placebo.
COX-2 was found to be expressed in basal epithelial cells with 60% BPH and 94% peripheral zone of the prostate. There are multiple mechanisms through which COX-2 may play a role in prostate growth. The advantage of the combination therapy compared to finasteride alone is significant in a short-term interval (4 weeks). It can be hypothesized that the association of rofecoxib with finasteride induces a more rapid improvement in clinical results until the effect of finasteride becomes predominant.[43]
Kaplan et al.[44] conducted a randomized, double blind, placebo controlled trial reported that the man who received a combination of tolterodine (antimuscarinic agent) and tamsulosin had better symptom control and QOL than men treated with either of medications or the placebo.
Desmopressin
Noctoria is one of the bothersome LUTS and also most difficult to eliminate in aging men. Nocturnal polyurea associated with circadian change of arginine vasopressine and atrial natriuretic peptide in the elderly has been suggested as the most dominant type of nocturia. Desmopressin is effective in treating nocturia to improve the patients QOL, although a few adverse events such as hypernatremia might occur.[45]
Minimally invasive therapies
Minimally invasive therapies (MITs) usually involve heating the prostate gland by various means (electrical, microwave, laser). Insertion can be directly into the prostate via a needle or into the urethra via a catheter, probe or endoscope.
Heating can be relatively low energy (e.g. microwave, laser or electrical methods) when the effects are thought to be due to aadrenoceptor blockade or damage and the net effect akin to ablockers.
It can be high energy, usually requiring anesthesia, when there is a more direct thermal coagulating or vaporizing effect on prostatic tissue with the intention of destroying or removing obstructing prostatic tissue, but with less bleeding than conventional surgery (e.g. KTP or Holmium laser vaporization).
Long-term data and large-scale studies are rare; they are expensive and are rarely supported by healthcare systems. Retreatment rates appear higher than conventional surgery, thereby reducing their cost effectiveness.[46] Microwave treatment offers greater versatility than drug therapy, allowing patients with severe baseline symptoms and small prostates to be treated successfully. Medical management improves symptoms to a more modest extent than does microwave treatment.[47]
According to different clinical studies TUMT (Transurethral microwave thermotherapy) proved to be an effective, safe, and durable therapy for the treatment of LUTS secondary to BPH. However, TURP still holds the steadier long-term results and is more effective to reduce obstruction as well as other LUTS. Other treatment options are transurethral needle ablation of the prostate (TUNA), high-intensity focused ultrasound (HIFU), interstitial laser thermotherapy (ILTT), water-induced thermotherapy (WITT), intra prostatic injection therapy with ethanol or hyperosmolar sodium chloride, and transurethral enzyme ablation of the prostate.[48]
Phytotherapy
Cernilton, prepared from the rye-grass pollen Secale cereale, is one of several phytotherapeutic agents available for the treatment of BPH. It is used by millions of men worldwide and is a registered pharmaceutical product throughout Western Europe, Japan, Korea and Argentina. The Cernilton trials analysed were limited by their short duration, limited number of enrolees, omissions in reported outcomes, and the unknown quality of the preparations used. The comparative trials had no confirmed active control. The available evidence suggests that Cernilton is well tolerated and modestly improves overall urological symptoms, including nocturia.[49]
Study of Shao et al. herbal Saxifrage tablet prepared with extraction from the Chinese herb Saxifraga stolonifera Meerb. It mainly contains bergenin, quercitrin, quercetin, protocatechic acid, gallic acid, succinic acid and mesoconic acid. TCM (HST) is a potentially effective treatment in improving the QOL, prostate volumes and maximum UFR for patients with BPH, though it is less effective in ameliorating the IPSS score when compared with Terazosin Hydrochloride (Hytrin).[50]
The Zi-Shen Pill (ZSP) was originally reported in the Secret Record of the Chamber of Orchids, which was written by Li Gao (1279-1368 AD, Yuan Dynasty of China). It consisted of three kinds of medicinal plants: Anemarrhena asphodeloides Bge (Liliaceae, rhizome), Phellodendron amurense Rup. (Rutaceae, bark) and Cinnamomum cassia Presl (camphoraceae, bark) with a ratio of 10:10:1 in weight. Investigation demonstrates that ZSPE can inhibit BPH in experimentally induced rats In addition, treatment with ZSPE can enhance the expression of TGF-ß1 in prostate, which can induce cell apoptosis.[51]
Babassu is the common name of a Brazilian native palm tree called Orbignya speciosa, whose kernels are commonly used (eaten entirely or as a grounded powder), in parts of Brazil for the treatment of urinary disorders. Orbignya speciosa nanoparticle (NanoOse) extract shows no toxicity in animals and acts incisively by promoting morphological cell changes, reducing cell proliferation as well as inducing necrosis/apoptosis on BPH cells and tissues.[52]
Phellodendron or cork tree is a genus of deciduous trees in the family Rutaceae. The bark of the plant is used in Traditional Chinese Medical to clear heat, purge fire and moisten dryness. Studies suggested Phellodendron amurense is able to inhibit prostatic contractility suggesting that it may be useful in the treatment of urological disorders caused by prostatic urethral obstruction such as in the case of BPH.[53]
The extract of Ganoderma lucidum Fr. Krast (Ganodermataceae) showed the strongest 5 a-reductase inhibitory activity. The treatment of the fruit body of Ganoderma lucidum or the extract prepared from it significantly inhibited the testosterone-induced growth of the ventral prostate in castrated rats. These results showed that Ganoderma lucidum might be a useful ingredient for the treatment of BPH.[54]
Sexual function is one of the aspects in the treatment of LUTS associated with BPH that has gained increasing attention and Permixon, a lipido-sterolic extract of Serenoa Repens has no negative impact on male sexual function.[55] The magnitude of the actual clinical benefit, the identification of the active compound, and the mechanism of action of Serenoa repens products all have yet to be determined.[56]
Bach et al.[57] randomized 476 men 1:1 to placebo or a pumpkin extract. Two extracts have been tested against standard therapy: saw palmetto against tamsulosin and a combination product (saw palmetto/stinging nettle root) against finasteride both trials revealed similar outcomes regarding symptoms, Qmax, and PVR between the plant extract and standard therapy.
Freeze-dried aqueous extract of the red variety of Lepidium meyenii (Red Maca) on testosterone-induced BPH in adult rats of the Holtzman strain was investigated and compared with finasteride. Study suggested that Finasteride was able to reduce both prostate and seminal vesicles weight but Red Maca was specific to prostate weight. Authors suggested this may be an interesting alternative in the treatment of prostatic diseases.[58]
BPH and ayurveda
Ayurveda describes two conditions known as mootrakruchra and mootraaghaata, which coincide with the symptoms of prostatism. Mootrakruchra or strangury is characterized by severe pain in passing urine whereas in mootraaghaata, there is total suppression or intermittent flow of urine during urination.
Natural therapies have a long history of use in our country to support optimal prostate health. Gokshura (gokhru), whose botanical name is Tribulus terrestris, has been traditionally used in treating urogenital conditions. Take two teaspoons of the fruit, grind coarsely, and bring to a boil in two cups of water until about half the water remains. Take a cup of this. You can also take it along with sugar and milk if you prefer. Gokshura may also be brought to a boil in milk. Similarly, two other botanicals deserve mention here. Both varuna (Crataeva religiosa) and punarnava (Boerhaavia diffusa) have been shown to be effective for symptoms of BPH. In different clinical trials, both these have shown significant anti-inflammatory effect, especially pertaining to genito-urinary tract.
Shilajit, a herbo-mineral compound ejected out of rocks during hot weather in the lower Himalayas is specially used in genito-urinary disease.
Kshaaras are the alkaline salts obtained from the ash of medicinal plants. Yava-kshaara is one such substance obtained from dried wheat plant, before blooming. This contains altered form of potassium carbonate, which is indicated in enlargement of the glands with special concern to prostate.
Long-term insufficient zinc intake is also linked to BPH. Good dietary sources of zinc include meat, eggs, and seafood. Yassada bhasma, obtained by calcination of zinc is the specific medicine for this purpose. A daily dose of 125 to 250 mg with honey will give relief from the problem.[59]
Ushira (Vetiveria zizanioides)
Ushira is popularly known as Khas, Khas or Khus grass in India. It is a densely tufted grass, found throughout the plains and lower hills of India, particularly on the riverbanks. Different parts of this grass is used for many diseases such as mouth ulcer, fever, boil, epilepsy, burn, headache, and enlarge prostate etc.
Swet Chandan (Santalum album)
Indigenous to southern part of India. Useful in the state of anxiety, mental tension, headaches, enlarge prostate, anger negativity and depression.
Khadir
Areca catechu has traditionally been used as an aphrodisiac and to reduce an enlarged prostate.
Shatavari
Asparagus racemosus helps relieve inflammation and improves urination – including urine retention.
Punarnava
Hog Weed (Boerhaavia diffusa) it is prescribed in case of all urinary problems that are caused due to prostate ailments.
Gorakhmundi
Globe thistle (Sphaeranthus hirtus) is very useful in enlargement of prostate.
Salam Mishri
Salep Orchid (Orchis mascula) the salep orchid is known as salam mishri in Ayurveda. It is prescribed in case of prostate problems brought on by vata vitiation.
Lata Karanj
Caesalpinia bonducella has also been found to exert a soothing, anti-inflammatory action, which makes it particularly beneficial for improving an enlarged prostate.[60]
Chandraprabha vati
This is a mixture of Purified Shilajit (Black bitumen), Purified Guggulu (Balsamodendron mukul), Karpoor (Camphora), Musta (Cyperus rotundus), Kiratatikta (Swertia chirata), Guduchi (Tinospora cordifolia), Daruharidra (Berberis species), Pippalimool (Piper longum), Chitraka (Plumbago zeylanica), Dhanyaka (Coriandrum sativum), Triphala, Vidanga (Embelia ribes), Trikatu, Saindhava (Rock salt), Twak (Cinnamon), Ela (Elettaria cardamomum), Vansalochan (Bambusa arundinacia).[61]
Varunadi Vati
Crataeva nurvala is the tree known as Varuna in Ayurveda. The herbal tablets made from the bark of this tree are recommended in Ayurveda for prostate enlargement or BPH. Ayurveda is an age old traditional medical system still in practice in India and recognized by the WHO. Herbal non-hormonal ayurvedic medicine that treats BPH by reducing prostate weight. It improves the urinary flow rate as reducing post-void residual urine.
Kachnaar Guggul
This is another effective herbal remedy for Enlarged prostate gland. Traditionally in Ayurvedic medicine, this herbal supplement is used for all types of excessive growth of various tissues including prostate gland. Kachnaar Guggul gives results with other supplements like Varunadi vati, Shilajit Capsules within few days of using them.
Tribulus Power
Tribulus is an herb very popular for its use as a male sex power enhancement herb. The herb Tribulus helps to maintain regular urine flow, stops dribbling after urination, helps to control urgency of urine as well as gives strength to genitor-urinary system. Tribulus power capsules are used along with other herbal supplements as effective herbal remedy for enlarged prostate gland.[62]
‘Prostalyn’, introduced by EIPWL (East India Pharmaceutical Works Limited) in the therapeutic category of urology contains two herbs namely, Surabhinimba or Murraya koenigii and Gokantaka or Tribulus terrestris. (EIPWL) claims that it relieves urinary symptoms associated with prostate enlargement, decreases the size of the gland, improves urinary flow and helps in near complete emptying of the bladder thus decreasing urine retention. This helps to relieve symptoms of prostate enlargement.[63]
Himplasia (Himalaya) possess both alpha one adrenoreceptor blocker and 5 alpha one reductase inhibitor activities. Company also claims to inhibit stromal cells proliferation. Himplasia is introduced in tablet form and contains Gokshura (Tribulus Terrestris), Putikaranja (Casalpinia bonducella), Puga (Arega Catechu), Shatavari (Asparagus Racemosus), Varuna (Crataeva nurvala) and Akika Pisti.[64]
Bangshil is described to have antiseptic and antibacterial properties; it increases body resistance. Fortege is described to tone up genito-urinary and neuro-glandular systems. The combined therapy of Bangshil and For-tege is described to act synergistically and relieves prostatic congestion and associated urinary symptoms and particularly symptoms like burning micturition, frequent micturition, difficult micturition, etc. Tablets of Bangshil contains Shilajit (Asphaltum), Guggul (Balsamodendron Mukul), Svarnamakshika Bhasma (Ferri sulphuratum), Kasis (Ferr: sulphas), Vanslochan (Ba.mbusa arundinaecia), Bang Bhasma (Tin Bhasma), Sandalwood oil Chandraprabha Co. Tablet of Fortege contains Kamboji (Breynia patens), Kuuncha beej (Mucuna pruriens), Suddha Kachura (Strychmos nuxvomica), Samudra Sesh Beej (Argyria speciosa), Vardhara beej (Rourea s.antaloides seeds), Asan (Withania Somnifera), Vardha.ra mool (Rourea santaloids root), Laving (Myrtus caryophyllus), Piper (Piper longum), Vacha (Acorus calamus), Mari (Piper nigrum), Sunth (Zingiber ofl’tcinale), Chini Kabab (Cubebs officinalis), Akalkara (Anocyclus pyrethrun), Sukhad Ver (Santalum album), Jaiphal (Myristica fragrans), Javantri (Arillus of Myristica fragrans), Jeevanti (Leptadenia reticulata).
Clinical studies in 16 cases where prostatic enlargement was present along with prostatic congestion, the congestion disappeared completely.[65]
TURP
In the case of TURP, short-term (mainly perioperative) complications include death, bleeding, clot retention, transurethral resection (TUR) syndrome (hypernatremia resulting in mental confusion, nausea, vomiting, and raised blood pressure), urinary tract infection, and inability to void, among which, bleeding is the most common. Some of these complications (e.g. bleeding and TUR syndrome) may be serious and life-threatening. Short-term complications of TURP include death, bleeding, clot retention, TUR, urinary tract infection, and inability to void. Long-term complications of TURP include failure to void, retrograde ejaculation, impotence, partial or complete incontinence, and retreatment.[27]
Miscellaneous
One study suggested protective effect of carotene on the risk of BPH. The risk tended to decrease also with the intake of vitamin C and iron and tended to increase with the intake of sodium and zinc. Study further concluded that other antioxidants, including folic acid, lycopene, lutein/zeaxanthin, and vitamins D and E, and retinol were not related to the risk for this disease.[66]
A series of phenoxyisoquinolines, N-phenoxyethyl-1-(2-nitrophenyl)-1,2,3,4- trihydroisoquinolines, N-phenoxyethyl-1-benzyl-1,2,3,4- trihydroisoquinolines, N-phenoxyethyl-1-(2-aminophenyl)-1,2,3,4- trihydroisoquinolines, N-phenoxyethyl-1-(2-phenoxyethylaminophenyl)-1,2,3,4- trihydroisoquinolines, have been synthesized and tested in isolated rat vas deferens a-adrenoreceptors.[67] Authors recommended alpha one blocker property in these compounds. Another report describes an improved synthesis of enantiomerically pure (S)-2-[4-(Dimethylamino)phenyl]-2,3-dihydro-N-[2-hydroxy-3-[4-[2-(1-methylethoxy)-phenyl]-1-piperazinyl]propyl]-1,3-dioxo-1 H-isoindole-5-carboxamide (RWJ 69442), a potent and selective αla-adrenergic receptor antagonist for the treatment of BPH.[68]
Botulinum neurotoxins (BoNTs) are well known for their ability to potently and selectively disrupt and modulate neurotransmission. BoNT is currently undergoing regulatory evaluation for urological disorders in the United States and the European Union and is not FDA approved for urologic use. Several case studies (level III evidence) have looked at specific BPH patient sub-populations to determine if BoNTA treatment was also effective.[69] Kuo (2005)[70] [cross reference] treated 10 patients who were either in frank urinary retention or carried a large PVR, who had already failed combination medical therapy (finasteride and alpha-blockers), and who had morbid medical conditions that prohibited them from having conventional TURP surgery.
LASSBio-772, a 1,3-benzodioxole N-phenylpiperazine derivative a novel potent and selective alpha 1A/1D adrenoceptor (AR) antagonist selected after screening of functionalized N-phenylpiperazine derivatives in phenylephrine-induced vasoconstriction of rabbit aorta rings. The affinity of LASSBio-772 for alpha 1A and alpha 1BAR subtypes was determined through displacement of [3H]prazosin binding. This compound presents pharmacological features higher affinity for the alpha 1A/1D than alpha 1B-AR, being therefore putatively useful for the treatment of the LUTS, including the BPH in mammals.[71]
A case report suggests that carvedilol may be considered for the management of HF with systolic dysfunction in patients with concomitant BPH thus eliminating the need for an α1-adrenergic blockers.[72]
CONCLUSION
The condition known as BPH may be defined as a benign enlargement of the prostate gland resulting from a proliferation of both benign epithelial and stromal elements. It might also be defined clinically as a constellation of LUTSs in aging men.[73] a-Adrenoreceptor antagonists are frequently used to treat patients with LUTS and benign prostatic enlargement because of their significant effect on storage and voiding symptoms, QOL, flow rate, and post void residual urine volume.[74] Silidosin is recently approved for Marketing in India (23/6/2011). Alpha one blockers are the first line treatment for symptomatic relief. Development of more and more specific α1A adrenoreceptor antagonist is under development for improving the prostate selectivity of α1 blockers. Other popular medications 5-a reductase inhibitors, combination therapies and phytotherapies (in some respect) are also popular among the medical practitioners. The presented review is helpful for researchers involve in development of new formulations in the field of BPH and to aware practitioners about recent improvements.
ACKNOWLEDGEMENT
Authors acknowledge the support given by Indian Institute of Technology, Roorkee for permission grant for literature survey through this study becomes possible. We also acknowledge support given by Mr. Santosh Prajapati, Assistant Librarian (IIT, Roorkee) for assist in collection of articles. This review is part of research work for the grant of Doctoral in Philosophy in Pharmaceutical Science from Jodhpur National University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Anglin IE, Glassman DT, Kyprianou N. Induction of prostate apoptosis by α1 -adrenoceptor antagonists: Mechanistic significance of the quinazoline component. Prostate Cancer Prostatic Dis. 2002;5:88–95. doi: 10.1038/sj.pcan.4500561. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Boyle P. Prostate Cancer 2000; evolution of an epidemic of unknown origin. In: Dennis L, editor. Prostate Cancer. Heidelberg: Springer; 2000. pp. 5–11. [Google Scholar]
- 4.Isaacs JT. Role of androgens in prostatic cancer. Vitam Horm. 1994;49:433–502. doi: 10.1016/s0083-6729(08)61152-8. [DOI] [PubMed] [Google Scholar]
- 5.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ confined prostate cancer is associated through prostate-specific antigen-based screening. JAMA. 1993;270:948–54. [PubMed] [Google Scholar]
- 6.Richie JP, Catalona WJ, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Effect of patient age on early detection of prostate cancer with prostate specific antigen and digital rectal examination. Urology. 1993;42:365–74. doi: 10.1016/0090-4295(93)90359-i. [DOI] [PubMed] [Google Scholar]
- 7.Raghavan D. Non-hormone chemotherapy for prostate cancer: Principles of treatment and application to the testing of new drugs. Semin Oncol. 1988;15:371–89. [PubMed] [Google Scholar]
- 8.Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. Affiliations department of medical physics, memorial sloan-kettering cancer center, 1275 York Ave., New York, NY 10021, USA. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 9.Zelefsky MJ, Leibel SA, Burman CM, Kutcher GJ, Harrison A, Happersett L, et al. Neoadjuvant hormonal therapy improves the therapeutic ration in patients with bulky prostatic disease treated with three dimensional conformal therapy. Int J Radiat Oncol Biol Phys. 1994;29:755–61. doi: 10.1016/0360-3016(94)90563-0. [DOI] [PubMed] [Google Scholar]
- 10.Haynes JM, Ventura S. Current models of human prostate contractility. Clin Exp Pharmacol Physiol. 2005;32:797–804. doi: 10.1111/j.1440-1681.2005.04268.x. [DOI] [PubMed] [Google Scholar]
- 11.Alan C, Kırılmaz B, Koçoğlu H, Ersay AR, Ertung Y, Eren AE. Comparison of effects of alpha receptor blockers on endothelial functions and coagulation parameters in patients with benign prostatic hyperplasia. Prostatic diseases and male voiding dysfunction. Urology. 2011;77:1439–43. doi: 10.1016/j.urology.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Nordling J. Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int. 2005;95:1006–12. doi: 10.1111/j.1464-410X.2005.05456.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsukamoto T, Masumori N. Epidemiology and natural history of benign prostatic hyperplasia. Int J Urol. 1997;4:233–46. doi: 10.1111/j.1442-2042.1997.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 14.Homma Y, Gotoh M, Yokoyama O, Masumori N, Kawauchi A, Yamanishi T, et al. JUA clinical guidelines for benign prostatic hyperplasia. Int J Urol. 2011;18:e1–33. [Google Scholar]
- 15.de Reijke TM, Klarskov P. Comparative efficacy of two a1-adrenoreceptor antagonists, doxazosin and alfuzosin, in patients with lower urinary tract symptoms from benign prostatic enlargement. BJU Int. 2004;93:757–62. doi: 10.1111/j.1464-410X.2003.04720.x. [DOI] [PubMed] [Google Scholar]
- 16.Kortt MA, Bootman JL. The economics of benign prostatic hyperplasia: A literature review. Clin Ther. 1996;18:1227–41. doi: 10.1016/s0149-2918(96)80078-6. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan SA. Current role of a-blockers in the treatment of benign prostatic hyperplasia. BJU Int. 2008;102(Suppl 2):3–7. doi: 10.1111/j.1464-410X.2008.08086.x. [DOI] [PubMed] [Google Scholar]
- 18.Djavan B. The correlation between inflammation, BPH and prostate cancer. Eur Urol Suppl. 2009;8:863–4. [Google Scholar]
- 19.Chiu G, Shengjian Li, Connolly PJ, Pulito V, Jingchun L, Middleton SA. (Arylpiperazinyl) cyclohexylsufonamides: Discovery of α1a/1d -selective adrenergic receptor antagonists for the treatment of Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms (BPH/LUTS) Bioorg Med Chem Lett. 2007;17:3292–7. doi: 10.1016/j.bmcl.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Roehrborn CG. Drug treatment for LUTS and BPH: New is not always better. Eur Urol. 2006;49:5–7. doi: 10.1016/j.eururo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez RR, Kaplan SA. First-line treatment for symptomatic benign prostatic hyperplasia: Is there a particular patient profile for a particular treatment? World J Urol. 2006;24:360–6. doi: 10.1007/s00345-006-0092-0. [DOI] [PubMed] [Google Scholar]
- 22.Rigatti P. Medical therapy for BPH: What factors should we consider? Eur Urol Suppl. 2006;5:989–90. [Google Scholar]
- 23.Novara G, Galfano A, Gardi M, Ficarra V, Boccon-Gibod L, Artibani W. Critical review of guidelines for BPH diagnosis and treatment strategy. Eur Urol Suppl. 2006;5:418–29. [Google Scholar]
- 24.Ball AJ, Feneley RC, Abrahams PH. The natural history of untreated ‘prostatism’. Br J Urol. 1981;53:613–6. doi: 10.1111/j.1464-410x.1981.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 25.Hein P, Michel CM. Signal transduction and regulation: Are all a1-adrenergic receptor subtypes created equal? Biochem Pharmacol. 2007;73:1097–106. doi: 10.1016/j.bcp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Irani J. Are all alpha-blockers created the same? Eur Urol. 2006;49:420–2. doi: 10.1016/j.eururo.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Montorsi F, Moncada I. Safety and tolerability of treatment for BPH. Eur Urol Suppl. 2006;5:1004–12. [Google Scholar]
- 28.Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernández; Fernández E European Silodosin Study Group. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: Results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol. 2011;59:342–52. doi: 10.1016/j.eururo.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S, Yamaguchi K Sakura Clinical Study Group. Treatment of benign prostatic hyperplasia and aging: Impacts of alpha-1 blockers on sexual function. J Mens Health. 2011;8:S25–8. [Google Scholar]
- 30.Schilit S, Benzeroual KE. Silodosin: A selective α1A-adrenergic receptor antagonist for the treatment of benign prostatic hyperplasia. Clin Ther. 2009;31:2489–502. doi: 10.1016/j.clinthera.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 31.Barendrecht MM, Koopmans RP, De La Rosette JJ, Michel MC. Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: The cardiovascular system. BJU Int. 2005;95:19–28. doi: 10.1111/j.1464-410X.2005.05487.x. [DOI] [PubMed] [Google Scholar]
- 32.Marberger M. Managing benign prostatic hyperplasia and prostate cancer – the challenges today. J Mens Health. 2010;7:113–24. [Google Scholar]
- 33.Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. An overview on 5 a -reductase inhibitors. Steroids. 2010;75:109–53. doi: 10.1016/j.steroids.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Thareja S, Aggarwal S, Bhardwaj TR, Kumar M. Self organizing molecular field analysis on a series of human 5 a-reductase inhibitors: Unsaturated 3-carboxysteroid. Eur J Med Chem. 2009;44:4920–5. doi: 10.1016/j.ejmech.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt LJ, Tindall DJ. Steroid 5 a-reductase inhibitors targeting BPH and prostate cancer. J Steroid Biochem Mol Biol. 2011;125:32–8. doi: 10.1016/j.jsbmb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Sawada K, Okada S, Golden P, Kayakiri N, Sawada Y, Hashimoto M, et al. 4-(1-Benzoylindol-3-yl) butyric acids and FK-143: Novel nonsteroidal inhibitors of steroid 5 a -reductase (II) Chem Pharm Bull. 1999;47:481–91. doi: 10.1248/cpb.47.481. [DOI] [PubMed] [Google Scholar]
- 37.Hirosumi J, Nakayama O, Fagan T, Sawada K, Chida N, Inami M, et al. FK143, a novel, nonsteroidal inhibitor of steroid 5 a -reductase.(1) In vitro effects on human and animal prostatic enzymes. J Steroid Biochem Mol Biol. 1995;52:357–63. doi: 10.1016/0960-0760(94)00187-q. [DOI] [PubMed] [Google Scholar]
- 38.Hirosumi J, Nakayama O, Chida N, Inami M, Fagan T, Sawada K, et al. FK143, a novel nonsteroidal inhibitor of steroid 5 a-reductase. 2. In vivo effects on rat and dog prostates. J Steroid Biochem Mol Biol. 1995;52:365–73. doi: 10.1016/0960-0760(94)00188-r. [DOI] [PubMed] [Google Scholar]
- 39.Marberger M. The MTOPS Study: New findings, new insights, and clinical implications for the management of BPH. Eur Urol Suppl. 2006;5:628–33. [Google Scholar]
- 40.McVary KT. A review of combination Therapy in patients with benign prostatic hyperplasia. Clin Ther. 2007;29:387–98. doi: 10.1016/s0149-2918(07)80077-4. [DOI] [PubMed] [Google Scholar]
- 41.Roehrborn CG, Siami P, Barkin J, Damião R, Major-Walker K, Nandy I, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol. 2010;57:123–31. doi: 10.1016/j.eururo.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533–9. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 43.Di Silverio F, Bosman C, Salvatori M, Albanesi L, Proietti Pannunzi L, Ciccariello M, et al. Combination therapy with rofecoxib and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) Eur Urol. 2005;47:72–9. doi: 10.1016/j.eururo.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolteradine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: A randomized control trial. JAMA. 2006;296:2319–28. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 45.Shiu-Dong C, Hong-Jeng Y. Impact of treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging men. Incont Pelvic Floor Dysfunct. 2007;1(Suppl 1):7–10. [Google Scholar]
- 46.McNicholas T, Swallow D. Benign prostatic hyperplasia. Surgery (Oxford) 2011;29:282–6. [Google Scholar]
- 47.Djavan B, Seitz C, Marberger M. Heat versus drugs in the treatment of benign prostatic hyperplasia 2003. BJU Int . 2003;91:131–7. doi: 10.1046/j.1464-410x.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann TR, Gross AJ, Schultheiss D, Kaufmann PM, Jonas U, Burchardt M. Transurethral microwave thermotherapy for the treatment of BPH: Still a challenger? World J Urol. 2006;24:389–96. doi: 10.1007/s00345-006-0098-7. [DOI] [PubMed] [Google Scholar]
- 49.MacDonald R, Ishani A, Rutks I, Wilt TJ. A systematic review of Cernilton for the treatment of benign prostatic hyperplasia. BJU Int. 1999;85:836–41. doi: 10.1046/j.1464-410x.2000.00365.x. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Lu A, Wang Y. Symptomatic comparison in efficacy on patients with benign prostatic hyperplasia treated with two therapeutic approaches. Complement Ther Med. 2010;18:21–7. doi: 10.1016/j.ctim.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun H, Li TJ, Sun LN, Qiu Y, Huang BB, Yi B, et al. Inhibitory effect of traditional Chinese medicine Zi-Shen Pill on benign prostatic hyperplasia in rats. J Ethnopharmacol. 2008;115:203–8. doi: 10.1016/j.jep.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 52.de Souza PA, Palumbo A, Jr, Alves LM, de Souza VP, Cabral LM, Fernandes PD, et al. Effects of a nanocomposite containing Orbignya speciosa lipophilic extract on Benign Prostatic Hyperplasia. J Ethnopharmacol. 2011;135:135–46. doi: 10.1016/j.jep.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Ventura S. Extracts of bark from the traditional Chinese herb Phellodendron amurense inhibit contractility of the isolated rat prostate gland. J Ethnopharmacol. 2010;127:196–9. doi: 10.1016/j.jep.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 54.Fujita R, Liu J, Shimizu K, Konishi F, Noda K, Kumamoto S, et al. Anti-androgenic activities of Ganoderma lucidum. J Ethnopharmacol. 2005;102:107–12. doi: 10.1016/j.jep.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 55.Zlotta AR, Teillac P, Raynaud JP, Schulman CC. Evaluation of male sexual function in patients with lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) treated with a phytotherapeutic agent (Permixon), tamsulosin or finasteride. Eur Urol. 2005;48:269–76. doi: 10.1016/j.eururo.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 56.Franklin C. The role of serenoa repens in the clinical management of lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2009;8:894–7. [Google Scholar]
- 57.Bach D. Und der interdisziplinä rer Arbeitskreis Prostata. Placebokontrollierte Langzeittherapiestudie mit Kü rbissamenextrakt bei BPH-bedingten Miktionsbeschwerden. J Clin Endocrinol Metab. 2000;56:139–46. [Google Scholar]
- 58.Gasco M, Villegas L, Yucra S, Rubio J, Gonzales GF. Dose-response effect of Red Maca (Lepidium meyenii) on benign prostatic hyperplasia induced by testosterone enanthate. Phytomedicine. 2007;14:460–4. doi: 10.1016/j.phymed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Chirumamilla MM. Prostate gland enlargement and ayurvedic treatment. [Last cited on 2012 May 15]. Available from: http://www.muralimanohar.com/Articles,%20English/Diseases%20and%20Conditions/Prostate%20Gland%20Enlargement.htm .
- 60.Ayurvedic treatment for enlarged prostate. [Last cited on 2012 May 15]. Available from: http://ayurveda-foryou.com/treat/prostate1.html .
- 61.Ayurvedic medicines Tablets 1. [Last cited on 2012 May 15]. Available from: http://ayurveda-foryou.com/paypalstore/tablet1.html#chandraprabha .
- 62.Planet Ayurveda. Effective prostate herbal remedies. [Last cited on 2012 May 15]. Available from: http://www.planetayurveda.com/prostateherbal-remedies.htm .
- 63.Express pharma. [Last updated in 2008 Jan 1-15, Last cited on 2012 May 15]. Available from: http://www.expresspharmaonline.com/20080115/management05.shtml .
- 64.Himalaya pharmaceuticals. Himplasia. [Last cited on 2012 May 15]. Available from: http://www.himalayahealthcare.com/products/himplasia.htm .
- 65.Joseph MK. Bangshil and Fortege in prostatic congestion. Curr Med Pract. 1980;24:311–5. [Google Scholar]
- 66.Tavani A, Longoni E, Bosetti C, Maso LD, Polesel J, Montella M, et al. Intake of selected micronutrients and the risk of surgically treated benign prostatic hyperplasia: A case-control study from Italy. Eur Urol. 2006;50:549–54. doi: 10.1016/j.eururo.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 67.Chen-Yuan K, Ming-Jung W. The synthesis of N-phenoxyethyl-1-substituted-1,2,3,4-tetrahydroisoquinolines and their α1 -adrenoceptor blocking activity. Eur J Med Chem. 2009;44:1271–7. doi: 10.1016/j.ejmech.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Kuo GH, Prouty C, Murray WV, Shah RD. An improved synthesis of enantiomerically pure RWJ 69442, a development candidate for the treatment of benign prostatic hyperplasia. J Heterocycl Chem. 2001;38:1003–6. [Google Scholar]
- 69.Smith CP. Botulinum toxin in the treatment of OAB, BPH, and IC. Toxicon. 2009;54:639–46. doi: 10.1016/j.toxicon.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 70.Kuo HC. Prostatic botulinum A toxin injection - an alternative treatment for benign prostatic obstruction in poor surgical candidates. Urology. 2005;65:670–4. doi: 10.1016/j.urology.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 71.Romeiro LA, da Silva Ferreira M, da Silva LL, Castro HC, Miranda AL, Silva CL, et al. Discovery of LASSBio-772, a 1,3-benzodioxole N-phenylpiperazine derivative with potent alpha 1A/D-Adrenergic receptor blocking properties. Eur J Med Chem. 2011;46:3000–12. doi: 10.1016/j.ejmech.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 72.Rohrer CK, Page RL, 2nd, Shakar SF, Lindenfeld J. Carvedilol for the treatment of benign prostatic hypertrophy in patients with heart failure? J Card Fail. 2011;17:875–7. doi: 10.1016/j.cardfail.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 73.McLaren ID, Jerde TJ, Bushman W. Role of interleukins, IGF and stem cells in BPH. Differentiation. 2011;82:237–43. doi: 10.1016/j.diff.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tubaro A, De Nunzio C, Mariani S, Trucchi A, Miano R, Vicentini C, et al. Prostatic diseases and male voiding dysfunction: Reduction of prostate-specific antigen after tamsulosin treatment in patients with elevated prostate-specific antigen and lower urinary tract symptoms associated with low incidence of prostate cancer at biopsy. Urology. 2010;76:436–41. doi: 10.1016/j.urology.2009.12.083. [DOI] [PubMed] [Google Scholar]


