Abstract
Polysaccharides have been finding, in the last decades, very interesting and useful applications in the biomedical and, specifically, in the biopharmaceutical field. Locust bean gum is a polysaccharide belonging to the group of galactomannans, being extracted from the seeds of the carob tree (Ceratonia siliqua). This polymer displays a number of appealing characteristics for biopharmaceutical applications, among which its high gelling capacity should be highlighted. In this review, we describe critical aspects of locust bean gum, contributing for its role in biopharmaceutical applications. Physicochemical properties, as well as strong and effective synergies with other biomaterials are described. The potential for in vivo biodegradation is explored and the specific biopharmaceutical applications are discussed.
KEY WORDS: Controlled release, gelling capacity, locust bean gum, polysaccharides, synergy
Nature provides an impressive array of polymeric materials that have been finding very interesting applications in the biomedical field, mainly because they are known to perform a diverse set of functions in their native environment. Polysaccharides, for instance, play well-known functions in membranes and intracellular communication, while proteins function as structural materials and catalysts.[1] In addition, the current trend is to mimic nature and there are no better candidates to such a task than the proper materials from nature. Natural biopolymers illustrate, as an impressive example, how all the properties displayed by biological materials and systems are exclusively determined by the physicochemical properties of the monomers and their sequence.[2] These natural materials have some remarkable merits over synthetic ones, namely improved capacity for cell adhesion and mechanical properties similar to natural tissues.[3] Moreover, they are economical, readily available, non-toxic, usually biodegradable and, with few exceptions, also biocompatible.[4,5] On the other hand, some intrinsic limitations are also to take into account, such as the highest possibility of immunogenicity and polymer variability related to both origin and supplier.[3] Another relevant aspect contributing for the increasing interest in polysaccharides relies on the discovery of new synthetic routes for their chemical modification, which permit the promotion of new biological activities and the modification of their properties for specific purposes.[6,7] Additionally, the biological activity of polysaccharides is being increasingly recognized for human applications.[8] Polysaccharides have been marking a strong position in the biomedical field, as their different chemical structures and physical properties comprise a large source of materials that can be used in different applications, varying from tissue engineering and preparation of drug vehicles for controlled release, to imaging techniques and associated diagnosis. In a general manner, polysaccharides play leading roles as thickening, gelling, emulsifying, hydrating, and suspending agents, finding diverse applications in the above-mentioned areas.[8]
The most common basic unit of polysaccharides is the monosaccharide D-glucose although D-fructose, D-galactose, L-galactose, D-mannose, L-arabinose, and D-xylose are also frequently present. Some polysaccharides comprise monosaccharide derivatives in their structure, like the amino sugars D-glucosamine and D-galactosamine, as well as their derivatives N-acetylneuraminic acid and N-acetylmuramic acid, and simple sugar acids (glucuronic and iduronic acids). In some cases, polysaccharides are collectively named for the sugar unit they contain, so glucose-based polysaccharides are called glucans, while mannose-based polysaccharides are mannans.[6]
Locust bean gum (LBG) is a neutral polysaccharide composed of mannose and galactose units and, therefore, belongs to the category of galactomannans. This natural polymer has been registering increased interest in the biopharmaceutical field, particularly in oral drug delivery. In this context, it has been showing its application in the design of drug delivery systems, providing the delivery of a defined dose, at a chosen rate, to a targeted biological site. In this review, critical aspects of LBG are exposed, with particular emphasis on the properties that closely affect its biopharmaceutical application, such as its chemical structure, solubility and molecular weight. The most effective synergies with other polysaccharides are described and the reported biopharmaceutical applications are explored and discussed.
Locust Bean Gum Origin and Processing
LBG is extracted from the seeds of the carob tree (Ceratonia siliqua), which is very abundant in the Mediterranean region although its localization also extends to different regions of North Africa, South America, and Asia. The polysaccharide is also referred in the literature by several other synonyms, such as carob bean gum, carob seed gum, carob flour, or even ceratonia.[9]
Carob seeds, which represent approximately 10% of the weight of the fruit, are industrially processed by hull cracking, sifting, and milling operations to isolate and grind the endosperms, which are then sold as crude flour.[10,11] The seeds are mainly composed of galactomannan, which comprises approximately 80%, the rest corresponding to proteins and impurities.[10,12] The protein content of LBG was reported to include approximately 32% albumin and globulin, while the remaining 68% correspond to glutelin.[13] Impurities mainly refer to ash and acid-insoluble matter.[10] After seed processing, crude galactomannan can be further submitted to several processes to eliminate both the protein content and impurities. These procedures include enzymatic or alkaline hydrolysis, precipitation with ethanol or isopropanol, and purification by methanol, or by copper or barium complexes.[10,12,14] Impurities usually remain insoluble even when heating at temperatures up to 70°C.[15] Precipitation with isopropanol revealed to be quite efficient in the elimination of proteins. In a general manner, purification steps have demonstrated to result in higher mannose/galactose (M/G) ratios and in a decrease of protein and impurities.[10]
As shown in Figure 1, a recent growing interest for LBG has been observed, with an increasing number of papers reporting its use in various fields. The strongest application of LBG concerns its use as a thickening and stabilizing agent in both food and cosmetic industries[16,17] and first references to the study of its properties date to more than 50 years ago.[18,19] In food industry it is a food additive, coded as E-410 in the European Union.[20] However, recently it has been pointed as a very useful excipient for pharmaceutical applications, as detailed in Section 6 of this review. The observed increase of interest is mainly due to its ability as controlled release excipient in tablets. However, reports of biodegradability, low toxicity, and availability at low cost[16,17,21] also contribute for its increasing use.
Figure 1.
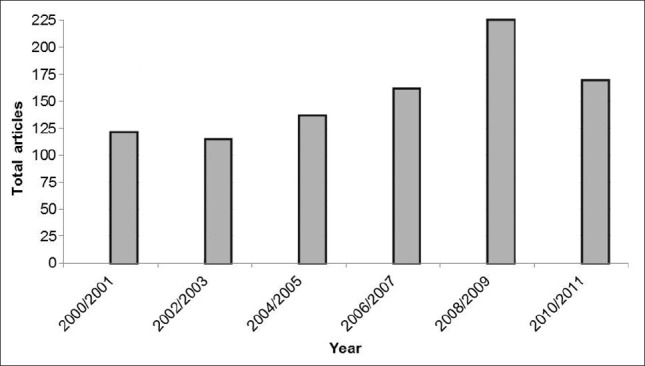
Number of scientific publications published on the topic of “Locust bean gum” as a function of publication years. Taken from ISI Web of Knowledge
Chemical Structure and Physicochemical Properties
Galactomannans are plant reserve carbohydrates present in large quantities in the endosperm of the seeds of many leguminosae such as Ceratonia siliqua (locust bean gum), Cyamopsis tetragonoloba (guar gum), and Caesalpinia spinosa (tara gum).[22,23] Chemically, they consist of a (1-4)-linked β-D-mannose backbone with (1-6)-linked side chains of α- D-galactose,[8,9] being thus neutral polymers.[4] The various galactomannans can be differentiated by the displayed M/G ratio, the substitution pattern of side-chain units and their molecular weight, the latter being influenced by harvesting and manufacturing practices, among other factors.[24] The M/G ratio varies, therefore, depending on the distribution of the galactose units over the mannose backbone, being approximately 4:1 for LBG [Figure 2],[25] 3:1 for tara gum and 2:1 for guar gum.[12] These ratios are always referred as approximate, due to their dependence upon the varying origins of gum materials and plant growth conditions during production.[4] It is important to mention that it is generally recognized that galactose grafts to the mannose chain are not spaced regularly, but instead placed randomly on the linear backbone.[23] This ratio is the main characteristic affecting galactomannans solubility, as higher water solubility is afforded by higher galactose content,[8] an effect that has been justified by the introduction of an entropic, and perhaps steric, barrier to the ordered mannose chain.[24] This observation makes guar gum the most soluble and also the most widely used of the galactomannans, as mentioned in a recently published broad review on applications of guar gum.[26]
Figure 2.
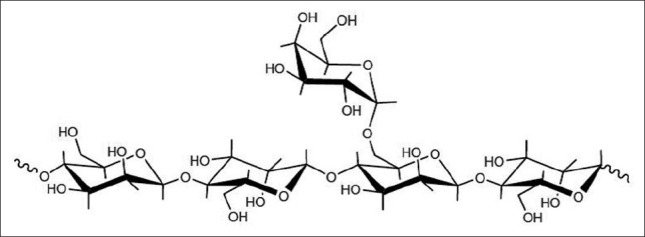
Structure of locust bean gum showing a linear polysaccharide (1-4)-βb-linked backbone of mannose units with single (1-6)-α-d-galactose units attached. Adapted with permission from Coviello et al.
One of the most important pros of plant resources is the fact that they are renewable and, if cultivated or harvested in a sustainable manner, it is possible to obtain a constant supply of raw material. However, plant-based materials also pose potential challenges that include the production of small quantities, usually structurally complex, which may differ according to the location of the plants. Other variables, such as the collecting season, might affect material properties. This may result in a slow and expensive isolation and purification process.[4] In this context, several studies have evidenced that the chemical structure and molecular weight of LBG vary systematically with the type of cultivar, growth condition,s or other unknown biological factors.[3,4] This probably justifies, at least in part, why LBG is considered polydisperse from a chemical point of view. Polydispersion is, in this case, a direct result of three types of structural variation: degree of galactose substitution, patterning of galactose side groups, and chain length or degree of polymerization, all directly related with biosynthesis mechanisms.[3] Importantly, different degrees of substitution of the mannose chain will affect the polymer solubility, for instance, among different suppliers.[20,22] This is possibly one of the major drawbacks hindering a more frequent application of this polysaccharide in the biopharmaceutical field, as solubility is one of the major properties to be controlled and which should be necessarily stable under defined conditions.
Solubility and viscosity
Although galactomannans are hydrophilic molecules, their solubility is in many cases reduced. When in solution, these polysaccharides have an extended rod-like conformation and occupy a large volume of gyration. These gyrating molecules collide with each other and with clusters of solvent molecules to produce solutions of high viscosity, a process that is reported to be dependent on the polymer molecular weight.[23] LBG has the capacity to form very viscous solutions at relatively low concentrations, which are almost unaffected by pH, salts, or temperature.[27] In fact, being a neutral polymer, its viscosity and solubility are little affected by pH changes within the range of 3–11.[4] As said before, the M/G ratio is the main property affecting the solubility of galactomannan polysaccharides, which increases with higher galactose substitution. This effect is attributed to the fact that mannose chains are relatively hydrophobic and galactose units more hydrophilic. In this manner, displaying an M/G ratio of approximately 4:1, LBG presents limited solubility, having propensity to form aggregates in cold water, as the long segments of unsubstituted mannose are prone to undergo aggregation.[3,24] These unsubstituted blocks of the backbone can be as large as 50 mannose units and the mentioned relative hydrophobicity, and consequent low solubility, derives from the proximity between these smooth regions of the mannose backbone. These zones permit, consequently, the formation of strong intramolecular hydrogen bonds that reduce the hydration of the gum.[23] In this context, a gum with higher percentage of galactose has good cold water dispersibility and higher viscosity, but poor gelling properties.
Several studies report the solubilization pattern of LBG. It is generally considered that the polysaccharide is only partially soluble at room temperature, achieving 50% solubilization after 1 h stirring (initial dispersion of 0.1% w/w), and a maximum of 70–85% solubilization can be obtained upon 30 min stirring at 80°C.[11,28] This difference of stabilization as a function of temperature has been attributed to the fact that, at high temperature, some molecules, such as high-molecular-weight components and galactomannan with lower galactose substitution, are dissolved, which does not occur at low temperature, reinforcing the idea of locust bean gum polydispersity.[11,29]
In order to overcome the solubility limitations exhibited by galactomannans, carboxyl, hydroxyl, and phosphate derivatives of these polymers have been proposed.[29] In our group, we have also synthesized several LBG derivatives (sulfate, carboxylate, and aminate). However, although in some cases a strong improvement of solubility was observed (for instance, for sulfated LBG), our main goal was to produce charged derivatives for application in the production of drug delivery systems by polyelectrolyte complexation with other polymers.[30]
Molecular weight and industrial depolymerization
In a general manner, the reported molecular weight of LBG situates between 50 and 1000 kDa.[11] Over recent years there has been an increasing interest in LBG and the need to explore specific industrial applications has demanded the development of strategies that provide the hydrolysis of high-molecular-weight molecules. Acid hydrolysis[31,32] and enzymatic hydrolysis have been described, but owing to the fact that LBG, and in a general manner galactomannans, display β-(1,4)-linked mannose residues, the enzyme β-mannanase has been widely explored to provide this effect.[33] The referred enzyme is mainly extracted and purified from bacteria and fungi, which have demonstrated to be excellent producers of extracellular β-mannanase.[33–36] Importantly, the fungus Aspergillus niger is reported as GRAS organism and, thus, the products of this strain are authorized for food applications,[33] converting it in the main source of the enzyme.
Nevertheless, the conversion of any polysaccharide into its basic oligosaccharides involves the breaking of all its units and, in the case of LBG, both the mannose and the galactose units need to be decomposed. Figure 3 displays a schematic representation of the pathways of LBG enzymatic degradation. β-mannanase catalyzes the random cleavage of β-(1,4)-d-mannopyranosyl linkages within the main chain of galactomannans,[37,38] thus providing only a partial depolymerization of LBG.[23] When acting on this polysaccharide, β-mannanase originates mannobiose, mannotriose, and galactomannobiose.[39,40] The affinity of β-mannanase by LBG decreases with the increasing substitution of the mannose chain[37,40] because galactose units cause steric hindrance to the enzyme and, therefore, it only attacks galactose-unsubstituted mannose blocks.[41]
Figure 3.
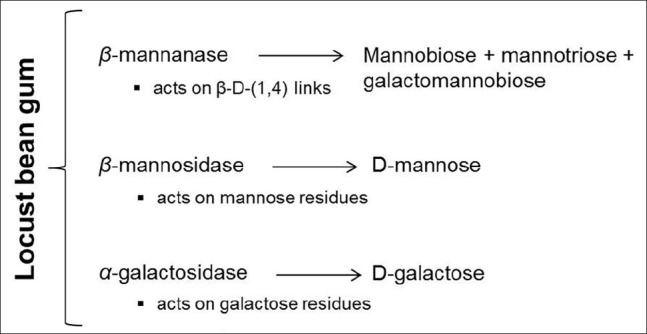
Schematic representation of locust bean gum enzymatic degradation
The complete conversion of galactomannan into the oligosaccharides D-mannose and D-galactose requires the action of two other enzymes, β-mannosidase and α-galactosidase, which can be also obtained from fungi, such as Aspergillus niger[40,42] or bacteria.[34] These enzymes catalyze the cleavage of β-(1,4)-linked d-mannopyranosyl and terminal α-(1,6)-linked D-galactosyl residues, respectively.[42]
Considering the difficulty in depolymerizing LBG, due to the steric effect of galactose residues over β-mannanase, it is usual to start the depolymerization reaction with α-galactosidase, removing some galactose grafts and exposing the mannose chain to the attack of β-mannanase.[23] Industrial depolymerization is mainly performed with α-galactosidase of plant, bacterial, and fungal origin[43] although that of fungal origin is reported as more suitable for technological applications, mainly due to its acidic optimal pH and broad stability profile.[44]
Synergistic Interaction of Locust Bean Gum with Other Polysaccharides
The study of synergistic effects between polysaccharides has a potential industrial interest, as many products involve the formulation of mixed polysaccharide systems. In addition, synergies provide the potential to modulate the rheology of products, which is very important in pharmaceutical formulation.[45] These systems provide a wide variety of structural conditions, which result inevitably in the absence of linearity in their macroscopic properties.
When two macromolecules (with gelling capacity or not) are mixed, it is possible that a synergic interaction takes place, so that it results in the formation of a gel. This gelation process occurs when the polymeric chains of both substances establish a more specific interaction, leading to an increased capacity for water absorption, compared with the sum of the absorption of each substance separately. It can also happen that adding a small amount of a non-gelling polymer to a gelling one induces a strengthening of the resulting gel or, even, some polymers that are individually non-gelling can yield gels upon mixing. This non-additive behavior is termed synergism. Several mechanisms might assist synergistic gel formation. A chemical gel network is formed when covalent bonds are established between the polymer chains. On the other side, physical gels are mediated by the formation of mixed junction zones between segments of the different polymers or by electrostatic interactions occurring between different polymeric chains.[5]
LBG exhibits a significant capacity to form synergistic interactions with other polysaccharides, mainly because of the numerous OH groups present in the molecule structure.[46] This synergy increases the flexibility of the polymer and, in many cases, permits the production of gelling structures with important biopharmaceutical applications. The most important synergies of LBG are observed in contact with xanthan gum and carrageenan.[8,47–49] These interactions will be described in detail below. Interactions between LBG and guar gum have also been reported although they only result in increased viscosity of the solution and not in the formation of a true gel.[50]
Synergy with xanthan gum
Xanthan gum produces high viscosity solutions at low concentration, but it does not naturally gel at any concentration, being insensitive to a broad range of pH, temperature, and electrolyte concentration.[51] These weak gel properties are known to be enhanced by the presence of certain β-(1,4) linked polysaccharides, namely those which normally exist in water solution as random coils and in the condensed phase as stiff, extended ribbons, like the galactomannans. The synergy between LBG and xanthan gum is the most effective and results in a firm, thermoreversible gel.[5] A synergic behavior was observed even in dilute gum solution.[52]
Rocks was the first to report the synergistic interaction between the two polysaccharides, observing the formation of a thermally reversible gel.[53] Subsequent studies indicated that the interaction occurs between the side chains of xanthan and the backbone of LBG as in a lock-and-key model, in which one xanthan chain could associate with one, two, or more locust bean gum molecules.[14,54,55] A study using X-ray diffraction suggested the need to denature xanthan at temperatures exceeding the helix-coil transition temperature for binding between both polymers to occur, leading to strong elastic gels.[56] Furthermore, it was reported that stronger gels, in terms of hardness and elastic modulus, were obtained when the two polymers were mixed in the same weight ratio. The same research group also suggested that the association between xanthan and LBG occurred because of disordered xanthan chains.[57] In contrast, a work with calorimetry and rheological methods revealed that the association between the polysaccharides was triggered by xanthan conformational changes.[58] The interaction between the polymers was later reported to be mediated by two distinct mechanisms. One takes place at room temperature, results in weak gels, and presents little dependence upon the galactose content. In turn, the second mechanism requires significant heating of the polymeric mixture and results in stronger gels, which formation is highly dependent upon the specific galactomannan composition.[59] There are reports on the dependence of gelation upon the temperature of reaction and the specific M/G ratio of galactomannan. For low galactose contents, such as that of LBG, interactions have been described at temperatures usually higher than 45°C. In addition, these interactions do not depend on the ionic concentration of the solutions and are suggested to involve xanthan chains in their ordered as well as disordered conformation. As the number of disordered xanthan chains depends on the total ionic concentration, the occurrence of interactions is strongly affected by the external salt concentration and also by ionic impurities present in the solution or in galactomannan samples, such as proteins.[14]
Another study demonstrated that the stability of xanthan helical structure or xanthan chain flexibility played a critical role in the interaction with LBG. It was shown that the destabilization of xanthan helical structure by deacetylation and heating facilitated the intermolecular binding between xanthan and LBG.[60] However, a study in dilute solution conditions suggested that the synergy is a result of a conformational change of the complex xanthan-LBG, in which LBG should play a significant role.[52]
A more recent work studied the possibility of modulating the gel mechanical properties varying the polymeric ratios and the temperature of reaction, the latter being known to affect xanthan chain conformation. It was observed that a LBG/xanthan ratio of 1:1 always produces a gel, while a ratio of 1:3 results in a weak gel at 75°C and a ratio of 1:9 never results in the formation of a real gel.[61] These results indicated that the properties of the complex polysaccharide gel might be tuned by varying the preparation temperature and/or the weight ratio between the two polymers.
As can be seen, information on LBG/xanthan gum synergy and gelling mechanism is varied and somewhat contradictory. In fact, although many efforts continue to elucidate the interaction, with some recent works providing new evidences, a wide debate is still open in the subject.
The synergy between both polymers is so effective that gels have been proposed in pharmaceutical applications for slow release purposes and tablet formulations already exist comprising of these polysaccharides.[62,63] The specific approaches will be detailed in the section addressing biopharmaceutical applications of LBG.
Synergy with k-carrageenan
The gelation of k-carrageenan is preceded by a coil–helix transition followed by aggregation and network formation. The coil–helix transition is known to be influenced by the presence of electrolytes, salt concentration, temperature, and polymer length.[54,64,65] In addition, it has been reported that k-carrageenan gels are prone to syneresis.[66]
The first studies on the polymeric interaction between LBG and k-carrageenan suggested that k-carrageenan helix interacted with unsubstituted regions of LBG,[67] but several contradictory studies were published after that. Nevertheless, it seems now to be accepted that the enhanced gel properties observed when the two polysaccharides are mixed, are a consequence of particular interactions occurring between the macromolecules. Specific junction zones between the chains of both polymers were reported to be established, resulting in increased gel strength and elasticity and reduced tendency to syneresis.[65,68,69] However, it was suggested that above a total polysaccharide concentration of 8–10 g/L, self-association of LBG chains could also take place.[68]
By comparison with gels formed only with k-carrageenan, it was suggested that LBG interferes with the gel structure by the formation of a secondary network.[70] The synergic effect has been shown to be affected by the polymers molecular weight, as well as by LBG degree of substitution, where the lowest degree of galactose substitution is reported to be more effective in gel formation.[71]
A study based on dynamic viscoelastic measurements and compression tests suggests that LBG adheres non-specifically to k-carrageenan network.[66]
Although less applied than the previous mixture of xanthan gum and LBG, this synergy of carrageenan and LBG served as the basis for the formulation of gel beads for encapsulation and stabilization of lactic acid bacteria[72] and microparticles for sustained release of gentamicin.[73]
Potential for Locust Bean Gum in vivo Biodegradation
When the main goal is to develop polymeric-based materials for biopharmaceutical applications, the in vivo biodegradability of the used polymers is of utmost importance, since their elimination by the organism upon administration is required without the need for additional interventions. Biodegradation of natural polymers is known to be carried out by the action of enzymes, microorganisms and pH action, entailing complex biological, physical, and chemical processes. These processes result in the breakdown of polymer chains, leading to the modification of properties such as molecular weight and solubility.
In which concerns LBG, its biodegradation is expected to be mainly driven by enzymatic activity, as several enzymes exist in the organism that might cleave LBG macromolecule. Oral administration is perhaps the route that most easily ensures an effective degradation of the polysaccharide. This is due to the presence of β-mannanase in the human colonic region[38,74] thus serving as the basis for the development of several strategies of colonic drug delivery that include LBG, as will be detailed in the section of LBG application in buccal drug delivery. As explained before in the section of Molecular Weight and Industrial Depolymerization, this enzyme acts on β-(1,4)-D links of mannose chains, converting these units into mannobiose, mannotriose, and galactomannobiose.[39,40] As observed in Figure 3, the complete degradation of LBG involves two other enzymes, β-mannosidase and α-galactosidase, which act, respectively, on mannose and galactose residues, producing d-mannose and D-galactose. These enzymes were also detected in human fecal contents,[75] reinforcing the potential of this polysaccharide in colonic delivery applications, but also ensuring its degradation upon any modality of oral administration. Actually, there are reports on the in vivo degradation of LBG mediated by colonic bacteria,[76] an effect that has been specifically attributed to bacteroides and ruminococci.[75]
Biopharmaceutical Applications of Locust Bean Gum
Many natural polymers have already demonstrated effectiveness in food, cosmetic, and pharmaceutical applications. The natural origin, as well as some specific individual characteristics, is an asset to make products more appealing to consumers. In the field of drug delivery many efforts have been devoted, in the last decades, to the development of appropriate delivery systems that avoid or minimize side effects, while improving the therapeutic efficacy. The application of natural polymers in pharmaceutical formulations is extremely varied, comprising the production of solid monolithic matrix systems, implants, films, beads, microparticles, nanoparticles, inhalable, and injectable systems, as well as viscous liquid and gel formulations. Within these dosage forms, polymeric materials have different functions such as binders, matrix formers, drug release modifiers, coatings, thickeners, or viscosity enhancers, stabilizers, disintegrators, solubilizers, emulsifiers, suspending agents, gelling agents, and bioadhesives.[4]
Owing to particular features of LBG specifically related with its gelling capacity and synergies with other polysaccharides, a growing interest is being observed regarding its biopharmaceutical use. Our group tested the effect of LBG on Caco-2 cell viability by the thiazolyl blue tetrazolium bromide (MTT) assay, finding very acceptable levels of cell viability between 73% and 81% for LBG concentrations varying between 0.1 and 1 mg/mL (data not shown), which are considered quite realistic for drug delivery applications. These observations were rather expected, as no detrimental effects are known to be obtained from compounds that are composed of basic sugar units.
The most usual biopharmaceutical application of LBG is in the formulation of oral delivery systems, although some works report its use in topical, ocular, buccal and colonic delivery, as will be described in the following sections. In this manner, the almost total of formulations is based on tablets although in a few cases hydrogels and multiparticulate systems are described.
LBG bioactivity
Several works describe the potential of LBG as a bioactive material. In 1983, LBG was referred for the first time as having a hypolipidemic effect, decreasing low density lipoprotein (LDL) cholesterol[77] as a consequence of the high content of insoluble fiber. Several subsequent studies confirmed that ability,[78–80] demonstrating the beneficial effects of LBG in the control of hypercholesterolemia. This potential benefit has inclusive led some authors to propose a daily consumption of food products enriched with the fiber.[80] In addition, it was also demonstrated that the polymer could reduce the rate of hepatic synthesis of cholesterol, although other galactomannans were more efficient in that task.[78] LBG has also been proposed for the treatment of diabetes.[81,82]
Furthermore, due to the high gelling ability and the fact that the formed gel is not assimilated by the gastrointestinal tract, ingestion causes a sensation of satiety resulting in decreased absorption of nutrients, thus rendering LBG adequate for inclusion in dietary products.[20,49] Indeed, a formulation of LBG-based capsules is available in the market for appetite suppression (Carob gum - Arkopharma Arkocápsulasâ).
LBG application in oral drug delivery
Among the various routes of administration, the oral has been the most convenient and commonly used in drug delivery. The application of LBG in oral delivery systems is mainly focused on its use as matrix forming material in tablets, benefiting from the fact that polysaccharides are generally considered to play an important role in drug release mechanisms from matrixes.[83] In these systems, usually intended to provide systemic drug absorption, LBG contributes with its swelling ability to afford a controlled release of the drug. Moreover, in most cases it is observed that the association of LBG with a second polymer affords an improved effect, benefiting from specific interactions occurring between the polymers.
To our knowledge, the first work reporting LBG application in tablet formulation as single polysaccharide excipient dates to 1998, when Sujja-areevath et al. reported the production of sodium diclofenac mini-matrixes containing 49.5% LBG. Optimized tablets had a drug/polymer ratio of 1/1, as higher ratios led to loss of matrix integrity. The formulation containing LBG evidenced lower swelling (50% in 6 h) than those containing xanthan (250% in 6 h) or karaya gum (150% in 6 h) and the swelling rate was observed to approximately follow Fick's diffusion law. However, drug release kinetics and polymer erosion were non-Fickian and, as compared with the other gums, tablets based on LBG displayed the fastest erosion in a phosphate buffer pH 7.0 (65% versus 45% and 25% for xanthan and karaya gum, respectively).[16] Another work consisted in the design of an LBG matrix tablet for the incorporation and release of theophylline and myoglobin. The tablet was further cross-linked with glutaraldehyde in an attempt to provide the network with the potential for an effective controlled release. However, that effect was not observed as release rate is similar in the presence and absence of the cross-linker (80% in 8 h). This observation is justified by the fact that LBG has only a few side chains and only a reduced number of cross-linkages take place within the polymer network which is not enough to affect the drug release mechanism. In contrast, tablets composed of guar gum, which has a higher number of side chains and thus allows stronger cross-linking, evidence a strong difference in drug release profile between cross-linked and non-cross-linked matrixes.[25] In a different approach, but still in the ambit of using LBG as single polysaccharide in the formulations, two very recent works revealed the ability of LBG to act as superdisintegrant in orodispersible tablets. One formulation consisted in nimesulide tablets, in which the incorporation of 10% LBG resulted in a disintegration time of 13 seconds. This time doubled if a standard superdisintegrant, cross-carmellose sodium, was used instead of LBG.[21] The other formulation comprised ofloxacin-loaded Eudragit® microspheres which were later used to prepare orodispersible tablets using LBG. The polysaccharide was used in a concentration varying between 2.5 and 10% and the observed disintegration time varied between 12 and 20 seconds, decreasing with the increase of LBG concentration. Ofloxacin was first encapsulated in microspheres in order to mask its bitter taste.[84]
Even before being used as single polysaccharide excipient, a tablet formulation called TIMERx® was designed using a combination of LBG and xanthan gum, being presented as a novel polysaccharide-based controlled release matrix technology, although this first work was just a preliminary exploration, providing the system characterization but without associating a drug to the system.[85] Later on, TIMERxâ was demonstrated to have controlled release potential both in vitro and in vivo, which was attributed to the high synergy between the polymers. At a LBG/xanthan gum ratio of 1/1 and at 50% polymer concentration, with cumulative presence of 50% glucose, a strong gel is obtained in contact with water.[86] The technology is nowadays commercially available, as a product from Penwest Pharmaceuticals, and was first developed for twice-a-day dosing of oxymorphone in patients with moderate to severe pain. A very complete review on TIMERx technology and its applications is available on Staniforth and Baichwal[86] and a very recent general review on chronotherapeutics is offered by Sunil et al.[87]
The combination of LBG with other polysaccharides is a frequent approach in the design of systems, in many cases benefiting from material synergies, as in the previous example. In this context, LBG/xanthan gum hydrogels were loaded with myoglobin, being then freeze-dried and compressed to produce tablets. Release behavior was mainly governed by LBG, which inhibits drug diffusion from the matrix. Even when a LBG/xanthan gum ratio of 1/9 was used, only 44% of the drug was released in water in 24 h.[63] In another study, LBG by itself was reported to not control the release of diltiazem hydrochloride from tablets, but the controlled release effect was observed upon the addition of karaya gum to the matrix (LBG/karaya gum ratio of 1/1). However, this effect demanded the presence of the polymeric mixture in a concentration which doubles that of the drug (drug/polymer ratio = 1/2).[88]
A different approach consists in the production of multi-layered matrix tablets, which also provide modified release behavior. These dosage forms are drug delivery devices comprising of a matrix core containing the active solute and one or more modulating layers, which are incorporated during tableting process. The modulating layers enable controlling the rate of hydration of the matrix core, thereby restricting the surface area available for diffusion of the drug and at the same time controlling solvent penetration rate.[89,90] In this context, sodium diclofenac tablets with matrix based on LBG, xanthan gum, or a 1:1 mixture of both polymers were produced. These matrix tablets released more than 90% of the drug in 12 h, the system containing LBG evidencing the fastest release. The addition of a triple external layer of carboxymethyl cellulose, which acted as release retardant for the hydrophilic matrix core, controlled diclofenac release, which was of approximately 70% for LBG core tablets in the same period of time.[91]
Considering the set of works available in the ambit of LBG tablet formulation for oral drug delivery, some contradictory results are found, mainly related to the capacity of LBG to provide a controlled release effect. This could be either related with differences in the used LBG molecules, considering different providers, or with the fact that secondary and tertiary components are also included in some formulations, affecting their behavior and making direct comparisons a difficult task.
Notwithstanding the prevalence of tablet formulations, other different approaches have been described. Solid dispersions of LBG and lovastatin were formulated to increase drug solubility. LBG was previously submitted to a thermal treatment to decrease its viscosity, which was reported to not affect its swelling capacity. This treatment had, by itself, a clear effect on lovastatin solubility, which was tested for 2 h in pH 7. Lovastatin released approximately 53% from native LBG solid dispersion and 65% from solid dispersions formulated with treated LBG. The solubility improvement is remarkable, taking into account that unformulated lovastatin only released 35% in the same period. Different methods of solid dispersion preparation were also tested, and the method of modified solvent evaporation demonstrated the better results, followed by spray-drying. Solid dispersions tested in vivo also demonstrated better therapeutic results as compared to unformulated lovastatin.[92] In another study, hydrogels prepared with LBG and xanthan gum were formulated to control the release of prednisolone. An increase in gum concentration resulted in decreased drug release rate from the hydrogels, suggesting that the drug diffusion was mainly controlled by the density of the three-dimensional network structure of the matrix. Glycerin and sucrose were tested as hydrogel additives, demonstrating to provide a significant decrease in drug release rate.[93]
A rather different approach concerns the use of multiparticulate systems, namely microspheres. LBG/alginate[94] and LBG-xanthan gum/alginate[95] microspheres loaded with sodium diclofenac were prepared by ionic cross-linking mediated by calcium. Drug association efficiency was above 90% and demonstrated to increase with increasing amounts of LBG or the mixture of gums. In both cases microspheres displayed a controlled release profile for 12 h in simulated gastric (pH 1.2; 2 h) and intestinal (pH 7.2; 10 h) fluids. Unfortunately, both articles are very scarce in details and discussion, and it was not possible to conclude on the more adequate formulation for diclofenac delivery and release.
LBG application in buccal drug delivery
The administration of drugs through the buccal mucosa offers two major advantages, which include avoiding pre-systemic elimination within the gastrointestinal tract and first-pass hepatic effect.[96,97] Therefore, buccal drug delivery mainly envisages improving the bioavailability of poorly absorbable drugs in the intestinal area.[98] One of the most important features to be exhibited by buccal delivery systems is a strong mucoadhesiveness, which is usually obtained using mucoadhesive polymers.[97,99] LBG has been reported to have mucoadhesive profile[97] although not as strong as other polysaccharides like chitosan.
Only two works deal with the application of LBG in the design of buccal delivery systems, in both cases involving a combination with a second polysaccharide. Tablets containing LBG or a mixture of LBG and xanthan gum as matrix materials were produced in order to improve the bioavailability of metoprolol, by avoiding an extensive first-pass effect of the drug. Formulations containing only LBG resulted in progressive release of the drug, with 7.5% of polymer leading to 98% release in 45 min. An increase to 15% LBG resulted in decreased release rate, registering approximately 45% in the same period. Combinations of xanthan gum and locust bean gum revealed more effective for tablet formulation, considering physical integrity, hardness and mucoadhesion strength. Tablets with LBG/xanthan gum ratio of 2:1 exhibited complete drug release in 45 min, as desired, but also poor drug permeation. To overcome this limitation, 1% sodium lauryl sulfate was incorporated in the formulation, resulting in improved drug permeation across porcine buccal mucosa.[100]
The second study also uses LBG in association with another polymer, comparing the bioavailability of propranolol hydrochloride formulated in LBG/chitosan tablets of different ratios (2/3, 3/2 and 4/1) administered to human volunteers. All buccal formulations improved drug bioavailability (1.3, 2.1 and 2.3 fold, respectively) as compared to the oral administration of a similar formulation. Naturally, tablets of ratio 2/3 were those exhibiting higher mucoadhesion, because of the highest chitosan content, and were also those evidencing the more complete release (98% in 10 h, as compared to 92% and 90% of formulations 3/2 and 4/1, respectively) although differences are not very relevant.[101]
LBG application in colonic drug delivery
The rationale for the use of polysaccharides in the production of delivery systems aimed at colonic delivery of drugs mainly relies on the presence of large amounts of polysaccharidases in the human colon. This is a consequence of the fact that this region is particularly colonized by a great number of bacteria, which produce many enzymes.[75,102]
Apart from the obvious application in providing a local therapeutic effect, for instance in inflammatory colonic diseases, systemic colonic delivery of drugs is also an option, especially for those drugs observing difficult absorption from the upper gastrointestinal tract. This possibility derives from the fact that the colon lacks various digestive enzymes present in the upper regions, mainly proteinases, thus possessing a less hostile environment in comparison with the stomach and small intestine.[103,104] As mentioned in Section 5, it is known that β-mannanase and other relevant enzymes are present in the human colon, ensuring the in vivo degradation of LBG.[38,74,75] In this context, bacterial species reported to be involved in LBG degradation are bacteroides and ruminococci.[75,105]
A first study on LBG application in colonic drug delivery systems consisted in the production of butanediol diglycidylether cross-linked LBG films, used as coating in theophylline tablets. The films evidenced very high swelling ability (300–500%) and were shown to undergo degradation by colonic microflora, potentiating an application in colonic delivery. However, mechanical instability of the films was observed, especially at higher coating quantities, thereby suggesting their non-suitability for application in colonic carrier production.[106]
Another study investigated the potential of LBG/chitosan mixtures to be used as coating materials, in order to provide protection from the physiological environment of stomach and small intestine, while permitting degradation by colonic bacterial enzymes, enabling drug release. Different LBG/chitosan ratios were studied and applied as coating over mesalazine core tablets. LBG capacity to hydrate and form a viscous gel layer was intended to provide a slower dissolution towards the core tablet. The ratio 4/1 demonstrated the most adequate behavior, showing cumulative release of 98% after 26 h incubation, which corresponded to 2 h HCl, 3 h in pH 7.4 buffer and 21 h in pH 6.8 PBS containing 4% (w/v) rat caecal contents. In vivo studies conducted in humans showed that drug release only initiated after 5 h, which corresponds to the transit time of the small intestine.[102]
LBG application in ocular drug delivery
A unique work reports the use of LBG in the formulation of a drug delivery system to the eye. LBG/i-carrageenan microparticles encapsulating gentamicin were prepared by emulsification, to be further incorporated in a polyvinyl alcohol gel that is applied on the ocular surface. Formulations without LBG showed an initial burst release within the first 6 h, which decreased by more than 50% by the addition of 10% LBG.[73] Unfortunately, no further studies were reported on this system to allow a larger vision on its potential.
LBG application in topical drug delivery
The use of LBG was also described in a formulation for topical application. The authors prepared a hydrogel with a LBG/xanthan gum ratio of 1/1, which was used to incorporate niosomes.[107] These are non-ionic surfactant vesicles, which offer several advantages over conventional liposomes, including higher chemical stability, lower costs, and greater availability of materials.[108,109] Niosomes were loaded with several distinct drugs, such as calcein, ibuprofen, and caffeine. The subsequent incorporation of niosomes on the hydrogel provided a protective effect on vesicle integrity and a slow release of the drugs from the polysaccharide system up to 50 h.[107]
Conclusions
Excipients have traditionally been included in drug formulations as inert substances whose role mainly relies on aiding the manufacturing process. Nevertheless, in the last decades they have been increasingly included in dosage forms to fulfill specialized functions aimed at improving drug delivery. LBG is being used in biopharmaceutical applications with several distinct functions, varying from controlled release excipient to tablet disintegrant. In most cases, the polysaccharide is associated with a second material, benefiting from strong synergies, and fields of application are varied, comprising oral, buccal, and colonic delivery, but also ocular and topical applications have been described. A substantial amount of research remains to be conducted to unveil the real potential the polysaccharide might possess. Considering the available works, it seems to be particularly interesting in the case of synergies established with other polysaccharides, namely xanthan gum and carrageenan.
Acknowledgments
This work was supported by National Portuguese funding through FCT - Foundation for Science and Technology, project PEst-OE/EQB/LA0023/2011 and PTDC/SAU-FCF/100291/2008.
Footnotes
Source of Support: This work was supported by National Portuguese funding through FCT – Foundation for Science and Technology, project PEst-OE/EQB/LA0023/2011 and PTDC/SAU-FCF/100291/2008.
Conflict of Interest: None declared.
References
- 1.Yu L, Dean K, Li L. Polymer blends and composites from renewable resources. Prog Polym Sci. 2006;31:576–602. [Google Scholar]
- 2.Malafaya P, Silva G, Reis R. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering application. Adv Drug Deliv Rev. 2007;59:207–33. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Pollard M, Kelly R, Fischer P, Windhab E, Eder B, Amadò R. Investigation of molecular weight distribution of LBG galactomannan for flours prepared from individual seeds, mixtures, and commercial samples. Food Hyd. 2008;22:1596–606. [Google Scholar]
- 4.Beneke C, Viljoen A, Hamman J. Polymeric plant-derived excipients in drug delivery. Molecules. 2009;14:2602–20. doi: 10.3390/molecules14072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copetti G, Grassi M, Lapasin R, Pricl S. Synergistic gelation of xanthan gum with locust bean gum: A rheological investigation. Glycoconjugate J. 1997;14:951–61. doi: 10.1023/a:1018523029030. [DOI] [PubMed] [Google Scholar]
- 6.d’Ayala GG, Malinconico M, Laurienzo P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules. 2008;13:2069–106. doi: 10.3390/molecules13092069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurienzo P. Marine polysaccharides in pharmaceutical applications: An overview. Mar Drugs. 2010;8:2435–65. doi: 10.3390/md8092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int. 2008;57:397–430. [Google Scholar]
- 9.Rowe R, Sheskey P, Owen S. Handbook of Pharmaceutical Excipients. 5th ed. London: Pharmaceutical Press; 2006. [Google Scholar]
- 10.Bouzouita N, Khaldi A, Zgoulli S, Chebil L, Chekki R, Chaabouni M, et al. The analysis of crude and purified locust bean gum: A comparison of samples from different carob tree populations in Tunisia. Food Chem. 2007;101:1508–15. [Google Scholar]
- 11.Dakia P, Blecker C, Robert C, Whatelet B, Paquot M. Composition and physicochemical properties of locust bean gum extracted from whole seeds by acid or water dehulling pre-treatment. Food Hyd. 2008;22:807–18. [Google Scholar]
- 12.Andrade C, Azero E, Luciano L, Gonçalves M. Solution properties of the galactomannans extracted from the seeds of Caesalpinia pulcherrima and Cassia javanica: Comparison with locust bean gum. Int J Biol Macromol. 1999;26:181–5. doi: 10.1016/s0141-8130(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 13.Smith B, Bean S, Schober T, Tilley M, Herald T, Aramouni F. Composition and molecular weight distribution of carob germ protein fractions. J Agric Food Chem. 2010;58:7794–800. doi: 10.1021/jf101523p. [DOI] [PubMed] [Google Scholar]
- 14.Bresolin TM, Milas M, Rinaudo M, Reicher F, Ganter JL. Role of galactomannan composition on the binary gel formation with xanthan. Int J Biol Macromol. 1999;26:225–31. doi: 10.1016/s0141-8130(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 15.Kök M, Hill S, Mitchell J. A comparison of the rheological behaviour of crude and refined locust bean gum preparations during thermal processing. Carbohyd Polym. 1999;38:261–5. [Google Scholar]
- 16.Sujja-areevath J, Munday D, Cox P, Khan K. Relationship between swelling, erosion and drug release in hydrophilic natural gum mini-matrix formulations. Eur J Pharm Sci. 1998;6:207–17. doi: 10.1016/s0928-0987(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 17.Pollard M, Kelly R, Wahl C, Fischer KP, Windhab E, Eder B, et al. Investigation of equilibrium solubility of a carob galactomannan. Food Hyd. 2007;21:683–92. [Google Scholar]
- 18.Hart R. Carob-seed gum: Its use for the detection and estimation of boric acid and borates. Ind Eng Chem. 1930;2:329–31. [Google Scholar]
- 19.Barry J, Halsey G. Dilute solution properties of a neutral polysaccharide. J Phys Chem. 1963;67:2821–6. [Google Scholar]
- 20.Urdiain M, Doménech-Sánchez A, Albertí S, Benedí V, Rosselló J. Identification of two additives, locust bean gum (E-410) and guar gum (E- 412), in food products by DNA-based methods. Food Addit Contamin. 2004;21:619–25. doi: 10.1080/02652030410001713889. [DOI] [PubMed] [Google Scholar]
- 21.Malik K, Arora G, Singh I. Locust bean gum as superdisintegrant - Formulation and evaluation of nimesulide orodispersible tablets. Polym Med. 2011;41:17–28. [PubMed] [Google Scholar]
- 22.Daas P, Grolle K, van Vliet T, Schols H, de Jongh H. Toward the recognition of structure-function relationships in galactomannans. J Agric Food Chem. 2002;50:4282–9. doi: 10.1021/jf011399t. [DOI] [PubMed] [Google Scholar]
- 23.Mathur V, Mathur N. Fenugreek and other less known legume galactomannan-polysaccharides: Scope for developments. J Sci Ind Res. 2005;64:475–81. [Google Scholar]
- 24.Picout D, Ross-Murphy S, Jumel K, Harding S. Pressure cell assisted solution characterization of polysaccharides. 2. Locust bean gum and tara gum. Biomacromol. 2002;3:761–7. doi: 10.1021/bm025517c. [DOI] [PubMed] [Google Scholar]
- 25.Coviello T, Alhaique F, Dorigo A, Matricardi P, Grassi M. Two galactomannans and scleroglucan as matrices for drug delivery: Preparation and release studies. Eur J Pharm Biopharm. 2007;66:200–9. doi: 10.1016/j.ejpb.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Mudgil D, Barak S, Khatkar B. Guar gum: Processing, properties and food applications - A review 2011; doi: 10. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0522-x. doi: 10.1007/s13197-011-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alves MM, Antonov YA, Gonçalves MP. The effect of structural features of gelatin on its thermodynamic compatibility with locust bean gum in aqueous media. Food Hyd. 1999;13:157–66. [Google Scholar]
- 28.Richarsdon P, Willmer J, Foster T. Dilute properties of guar and locust bean gum in sucrose solutions. Food Hyd. 1998;12:339–48. [Google Scholar]
- 29.Garcia-Ochoa F, Casas J. Viscosity of locust bean (Ceratonia siliqua) gum solutions. J Sci Food Agric. 1992;59:97–100. [Google Scholar]
- 30.Braz L, Grenha A, Ferreira D, Rosa da Costa A, Sarmento B. Locust bean gum derivatives for nanometric drug delivery. Rev Port Farm. 2011;52(6 Suppl):127–8. [Google Scholar]
- 31.Vaheed H, Shojaosadati S, Galip H. Evaluation and optimization of ethanol production from carob pod extract by Zymomonas mobilis using response surface methodology. J Ind Microbiol Biotechnol. 2011;38:101–11. doi: 10.1007/s10295-010-0835-1. [DOI] [PubMed] [Google Scholar]
- 32.Grössl M, Harrison S, Kaml I, Kenndler E. Characterisation of natural polysaccharides (plant gums) used as binding media for artistic and historic works by capillary zone electrophoresis. J Chromatogr A. 2005;1077:80–9. doi: 10.1016/j.chroma.2005.04.075. [DOI] [PubMed] [Google Scholar]
- 33.Naganagouda K, Salimath P, Mulimani V. Purification and characterization of endo-β-1,4 mannanase from Aspergillus niger gr for application in food processing industry. J Microbiol Biotechnol. 2009;19:1184–90. [PubMed] [Google Scholar]
- 34.Talbot G, Sygusch J. Purification and characterization of thermostable beta-mannanase and alpha-galactosidase from Bacillus stearothermophilus. Appl Environ Microbiol. 1990;56:3505–10. doi: 10.1128/aem.56.11.3505-3510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blibech M, Ghorbel R, Fakhfakh I, Ntarima P, Piens K, Bacha A, et al. Purification and characterization of a low molecular weight of β-mannanase from Penicillium occitanis Pol6. Appl Biochem Biotechnol. 2010;160:1227–40. doi: 10.1007/s12010-009-8630-z. [DOI] [PubMed] [Google Scholar]
- 36.Wu M, Tang C, Li J, Zhang H, Guo J. Bimutation breeding of Aspergillus niger strain for enhancing β-mannanase production by solid-state fermentation. Carbohyd Re s. 2011;346:2149–55. doi: 10.1016/j.carres.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 37.McCleary B, Matheson N. Action patterns and substrate-binding requirements of β-D-mannanase with mannosaccharides and mannan-type polysaccharides. Carbohyd Res. 1983;119:191–219. [Google Scholar]
- 38.Alonso-Sande M, Teijeiro-Osorio D, Remuñán-López C, Alonso MJ. Glucomannan, a promising polysaccharide for biopharmaceutical purposes. Eur J Pharm Biopharm. 2009;72:453–62. doi: 10.1016/j.ejpb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Kurakake M, Komaki T. Production of β-mannanase and β-mannosidase from Aspergillus awamori K4 and their properties. Curr Microb. 2001;42:377–80. doi: 10.1007/s002840010233. [DOI] [PubMed] [Google Scholar]
- 40.Civas A, Eberhard R, Le Dizet P, Petek F. Glycosidases induced in Aspergillus tamarii: Secreted α-D-galactosidase and β-D-mannanase. Biochem J. 1984;219:857–63. doi: 10.1042/bj2190857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurakake M, Sumida T, Masuda D, Oonishi S, Komaki T. Production of galacto-manno-oligosaccharides from guar gum by β-Mannanase from Penicillium oxalicum SO. J Agric Food Chem. 2006;54:7885–89. doi: 10.1021/jf061502k. [DOI] [PubMed] [Google Scholar]
- 42.Ademark P, Varga A, Medve J, Harjunpää V, Drakenberg T, Tjerneld F, et al. Softwood hemicellulose-degrading enzymes from Aspergillus niger: Purification and properties of a β-mannanase. J Biotechnol. 1998;63:199–210. doi: 10.1016/s0168-1656(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 43.Kotiguda G, Kapnoor S, Kulkarni D, Mulimani V. Degradation of raffinose oligosaccharides in soymilk by immobilized alpha-galactosidase of Aspergillus oryzae. J Microbiol Biotechnol. 2007;17:1430–6. [PubMed] [Google Scholar]
- 44.Ferreira J, Reis A, Guimarães V, Falkoski D, Fialho L, de Rezende S. Purification and characterization of Aspergillus terreus α-galactosidases and their use for hydrolysis of soymilk oligosaccharides. Appl Biochem Biotechnol. 2011;164:1111–25. doi: 10.1007/s12010-011-9198-y. [DOI] [PubMed] [Google Scholar]
- 45.Pinheiro AC, Bourbon AI, Rocha C, Ribeiro C, Maia JM, Gonçalves MP, et al. Rheological characterization of κ-carrageenan/galactomannan and xanthan/galactomannan gels: Comparison of galactomannans from non-traditional sources with conventional galactomannans. Carbohyd Polym. 2011;83:392–9. [Google Scholar]
- 46.Srivastava M, Kapoor V. Seed galactomannans: An overview. Chem Biodiv. 2005;2:295–317. doi: 10.1002/cbdv.200590013. [DOI] [PubMed] [Google Scholar]
- 47.Sewall C. Gelling interactions of phycocolloids extracted from red algae with a galactomannan from locust bean and a glucomannan from konjac tuber. J Appl Phycol. 1992;4:347–51. [Google Scholar]
- 48.Wang J, Somasundaran P. Study of galactomannose interaction with solids using AFM, IR and allied techniques. J Colloid Interf Sci. 2007;309:373–83. doi: 10.1016/j.jcis.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 49.Dakia PA, Wathelet B, Paquot M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chem. 2007;102:1368–74. [Google Scholar]
- 50.Goycoolea F, Morris E, Gidley M. Viscosity of galactomannans at alkaline and neutral pH: Evidence of “hyperetanglement” in solution. Carbohyd Polym. 1995;27:69–71. [Google Scholar]
- 51.Becker A, Katzen F, Pühler A, Ielpi L. Xanthan gum biosynthesis and application: A biochemical/genetic perspective. Appl Microbiol Biotechnol. 1998;50:145–52. doi: 10.1007/s002530051269. [DOI] [PubMed] [Google Scholar]
- 52.Higiro J, Herald TJ, Alavi S. Rheological study of xanthan and locust bean gum interaction in dilute solution. Food Res Int. 2006;39:165–75. [Google Scholar]
- 53.Rocks J. Xanthan gum. Food Technol. 1971;25:476–83. [Google Scholar]
- 54.Morris E, Rees D, Robinson G, Young G. Competitive inhibition of interchain interactions in polysaccharide systems. J Mol Biol. 1980;138:363–74. doi: 10.1016/0022-2836(80)90292-2. [DOI] [PubMed] [Google Scholar]
- 55.Tako M, Asato A, Nakamura S. Rheological aspects of the intermolecular interaction between xanthan and locust bean gum in aqueous media. Eur Polym J. 1984;48:2995–3000. [Google Scholar]
- 56.Cairns P, Miles MJ, Morris VJ, Brownsey GJ. X-Ray fibre-diffraction studies of synergistic, binary polysaccharide gels. Carbohyd Res. 1987;160:411–23. [Google Scholar]
- 57.Cairns P, Miles M, Morris V. Intermolecular bonding of xanthan gum and carob gum. Nature. 1986;322:89–90. [Google Scholar]
- 58.Williams P, Clegg S, Day D, Phillips G, Nishinari K. Mixed gels formed with konjac mannan and xanthan gum. In: Dickinson E, editor. Food polymers, gels and colloids. Cambridge: Royal Society of Chemistry; 1991. pp. 339–48. [Google Scholar]
- 59.Mannion R, Melia C, Launay B, Cuvelier G, Hill S, Harding S, et al. Xanthan/locust bean gum interactions at room temperature. Carbohyd Polym. 1992;19:91–7. [Google Scholar]
- 60.Wang F, Wang YJ, Sun Z. Conformational role of xanthan in its interaction with locust bean gum. J Food Sci. 2002;67:2609–14. [Google Scholar]
- 61.Sandolo C, Bulone D, Mangione MR, Margheritelli S, Di Meo C, Alhaique F, et al. Synergistic interaction of locust bean gum and xanthan investigated by rheology and light scattering. Carb Polym. 2010;82:733–41. [Google Scholar]
- 62.Vendruscolo CW, Andreazza IF, Ganter JL, Ferrero C, Bresolin TM. Xanthan and galactomannan (from M.scabrella) matrix tablets for oral controlled delivery of theophylline. Int J Pharm. 2005;296:1–11. doi: 10.1016/j.ijpharm.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Sandolo C, Coviello T, Matricardi P, Alhaique F. Characterization of polysaccharide hydrogels for modified drug delivery. Eur Biophys J. 2007;36:693–700. doi: 10.1007/s00249-007-0158-y. [DOI] [PubMed] [Google Scholar]
- 64.Lundin L, Hermansson AM. Influence of locust bean gum on the rheological behaviour and microstructure of K-k-carrageenan. Carbohyd Polym. 1995;28:91–9. [Google Scholar]
- 65.Chronakis IS, Borgström J, Piculell L. Conformation and association of κ-carrageenan in the presence of locust bean gum in mixed NaI/CsI solutions from rheology and cryo-TEM. Int J Biol Macromol. 1999;25:317–28. doi: 10.1016/s0141-8130(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 66.Andrade CT, Azero EG, Luciano L, Gonçalves MP. Rheological properties of mixtures of κ-carrageenan from Hypnea musciformis and galactomannan from Cassia javanica. Int J Biol Macromol. 2000;27:349–53. doi: 10.1016/s0141-8130(00)00139-2. [DOI] [PubMed] [Google Scholar]
- 67.Dea IC, McKinnon AA, Rees DA. Tertiary and quaternary structure in aqueous polysaccharide systems which model cell wall cohesion: Reversible changes in conformation and association of agarose, carrageenan and galactomannans. J Mol Biol. 1972;68:153–72. doi: 10.1016/0022-2836(72)90270-7. [DOI] [PubMed] [Google Scholar]
- 68.Turquois T, Doublier J, Taravel F, Rochas C. Synergy of the kappa-carrageenan-carob galactomannan blend inferred from rheological studies. Int J Biol Macromol. 1994;16:105–7. doi: 10.1016/0141-8130(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 69.Lundin L, Hermansson AM. Multivariate analysis of the influences of locust bean gum, αs-casein, κ-casein on viscoelastic properties of Na-κ-carrageenan gels. Food Hyd. 1998;12:175–87. [Google Scholar]
- 70.Fernandes PB, Gonçalves MP, Doublier JL. Effect of galactomannan addition on the thermal behaviour of κ-carrageenan gels. Carbohyd Polym. 1992;19:261–9. [Google Scholar]
- 71.Fernandes PB, Gonçalves MP, Doublier JL. A rheological characterization of kappa-carrageenan/galactomannan mixed gels: A comparison of locust bean gum samples. Carbohyd Polym. 1991;16:253–74. [Google Scholar]
- 72.Arnaud J, Lacroix C, Castaigne F. Counter-diffusion of lactose and lactic acid in kappa-carrageenan/locust bean gum gel beads with or without entrapped lactic acid bacteria. Enzyme Microb Technol. 1992;14:715–24. doi: 10.1016/0141-0229(92)90111-z. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki S, Lim JK. Microencapsulation with carrageenan-locust bean gum mixture in a multiphase emulsification technique for sustained drug release. J Microencapsulation. 1994;11:197–203. doi: 10.3109/02652049409040451. [DOI] [PubMed] [Google Scholar]
- 74.Nakajima N, Matsuura Y. Purification and characterization of konjac glucomannan degrading enzyme from anaerobic human intestinal bacterium, Clostridium butyricum-Clostridium beijerinckii group. Biosci Biotechnol Biochem. 1997;61:1739–42. doi: 10.1271/bbb.61.1739. [DOI] [PubMed] [Google Scholar]
- 75.Jain A, Gupta Y, Jain S. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J Pharm Pharm Sci. 2007;10:86–128. [PubMed] [Google Scholar]
- 76.Bauer A, Kesselhut A. Novel pharmaceutical excipients for colon targeting. STP Pharm Sci. 1995;5:54–9. [Google Scholar]
- 77.Zavoral J, Hannan P, Fields D, Hanson M, Frantz I, Kuba K, et al. The hypolipidemic effect of locust bean gum food products in familial hypercholesterolemic adults and children. Am J Clin Nut. 1983;38:285–94. doi: 10.1093/ajcn/38.2.285. [DOI] [PubMed] [Google Scholar]
- 78.Evans AJ, Hood RL, Oakenfull DG, Sidhu GS. Relationship between structure and function of dietary fibre: A comparative study of the effects of three galactomannans on cholesterol metabolism in the rat. Br J Nutr. 1992;68:217–29. doi: 10.1079/bjn19920079. [DOI] [PubMed] [Google Scholar]
- 79.Ruiz-Roso B, Quintela J, de la Fuente E, Haya J, Pérez-Olleros L. Insoluble carob fiber rich in polyphenols lowers total and LDL cholesterol in hypercholesterolemic sujects. Plant Foods Human Nut. 2010;65:50–6. doi: 10.1007/s11130-009-0153-9. [DOI] [PubMed] [Google Scholar]
- 80.Zunft HJ, Lüder W, Harde A, Haber B, Graubaum HJ, Koebnick C, et al. Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur J Nut. 2003;42:235–42. doi: 10.1007/s00394-003-0438-y. [DOI] [PubMed] [Google Scholar]
- 81.Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nut Food Res. 2005;49:560–70. doi: 10.1002/mnfr.200500025. [DOI] [PubMed] [Google Scholar]
- 82.Tsai A, Peng B. Effects of locust bean gum on glucose tolerance, sugar digestion, and gastric motility in rats. J Nut. 1981;111:2152–6. doi: 10.1093/jn/111.12.2152. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman A. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 84.Malik K, Arora G, Singh I. Taste masked microspheres of ofloxacin: Formulation and evaluation of orodispersible tablets. Sci Pharm. 2011;79:653–72. doi: 10.3797/scipharm.1104-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tobyn MJ, Staniforth JN, Baichwal AR, McCall TW. Prediction of physical properties of a novel polysaccharide controlled release system. I. Int J Pharm. 1996;128:113–22. [Google Scholar]
- 86.Staniforth JN, Baichwal AR. TIMERx®: Novel polysaccharide composites for controlled/programmed release of drugs in the gastrointestinal tract. Expert Opin Drug Deliv. 2005;2:587–95. doi: 10.1517/17425247.2.3.587. [DOI] [PubMed] [Google Scholar]
- 87.Sunil SA, Srikanth MV, Rao NS, Uhumwangho MU, Latha K, Murthy KV. Chronotherapeutic drug delivery systems - An approach to circardian rhythms diseases. Curr Drug Deliv. 2011;8:622–33. doi: 10.2174/156720111797635559. [DOI] [PubMed] [Google Scholar]
- 88.Moin A, Shivakumar H. Formulation and in vitro evaluation of sustained-release tablet of diltiazem: Influence of hydrophilic gums blends. J Pharm Res. 2010;3:600–4. [Google Scholar]
- 89.Colombo P, Conte U, Gazzaniga A, Maggi L, Sangalli ME, Peppas NA, et al. Drug release modulation by physical restrictions of matrix swelling. Int J Pharm. 1990;63:43–8. [Google Scholar]
- 90.Conte U, Maggi L. Modulation of the dissolution profiles from Geomatrix® multi-layer matrix tablets containing drugs of different solubility. Biomaterials. 1996;17:889–96. doi: 10.1016/0142-9612(96)83284-4. [DOI] [PubMed] [Google Scholar]
- 91.Ahmed S, Mangamoori L, Rao Y. Formulation and characterization of matrix and triple-layer matrix tablets for oral controlled drug delivery. Int J Pharm Pharm Sci. 2010;2:137–43. [Google Scholar]
- 92.Patel M, Tekade A, Gattani S, Surana S. Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. AAPS PharmSciTech. 2008;9:1262–9. doi: 10.1208/s12249-008-9171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe K, Yakou S, Takayama K, Machida Y, Nagai T. Factors affecting prednisolone release from hydrogels prepared with water-soluble dietary fibers, xanthan and locust bean gums. Chem Pharm Bull. 1992;40:459–62. doi: 10.1248/cpb.40.459. [DOI] [PubMed] [Google Scholar]
- 94.Deshmukh V, Sakarkar D, Wakade R. Formulation and evaluation of controlled release alginate microspheres using locust bean gum. J Pharm Res. 2009;2:458–61. [Google Scholar]
- 95.Deshmukh V, Jadhav J, Masirkar V, Sakarkar D. Formulation, optimization and evaluation of controlled release alginate microspheres using synergy gum blends. Res J Pharm Technol. 2009;2:324–7. [Google Scholar]
- 96.Mujoriya R, Dhamande K, Wankhede U, Angure S. A review on study of buccal drug delivery system. Inn Syst Design Eng. 2011;2:1–13. [Google Scholar]
- 97.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery — A promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 98.Şenel S. Potential applications of chitosan in oral mucosal delivery. J Drug Deliv Sci Technol. 2010;20:23–32. [Google Scholar]
- 99.Remuñán-López C, Portero A, Vila-Jato JL, Alonso MJ. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J Control Release. 1998;55:143–52. doi: 10.1016/s0168-3659(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 100.Yamagar M, Kadam V, Hirlekar R. Design and evaluation of buccoadhesive drug delivery system of metoprolol tartrate. Int J PharmTech Res. 2010;2:453–62. [Google Scholar]
- 101.Vijayaraghavan C, Vasanthakumar S, Ramakrishnan A. In vitro and in vivo evaluation of locust bean gum and chitosan combination as a carrier for buccal drug delivery. Pharmazie. 2008;63:342–7. [PubMed] [Google Scholar]
- 102.Raghavan CV, Muthulingam C, Leno Jenita JA, Ravi TK. An in vitro and in vivo investigation into the suitability of bacterially triggered delivery system for colon targeting. Chem Pharm Bull. 2002;50:892–5. doi: 10.1248/cpb.50.892. [DOI] [PubMed] [Google Scholar]
- 103.Sinha V, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224:19–38. doi: 10.1016/s0378-5173(01)00720-7. [DOI] [PubMed] [Google Scholar]
- 104.Chourasia M, Jain S. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004;11:129–48. doi: 10.1080/10717540490280778. [DOI] [PubMed] [Google Scholar]
- 105.Kumar R, Patil M, Patil S, Paschapur M. Polysaccharides based colon specific drug delivery: A review. Int J PharmTech Res. 2009;1:334–46. [Google Scholar]
- 106.Hirsch S, Binder V, Schehlmann V, Kolter K, Bauer KH. Lauroyldextran and crosslinked galactomannan as coating materials for site-specific drug delivery to the colon. Eur J Pharm Biopharm. 1999;47:61–71. doi: 10.1016/s0939-6411(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 107.Marianecci C, Carafa M, Di Marzio L, Rinaldi F, Di Meo C, Alhaique F, et al. New vesicle-loaded hydrogel system suitable for topical applications: Preparation and characterization. J Pharm Pharm Sci. 2011;14:336–46. doi: 10.18433/j3160b. [DOI] [PubMed] [Google Scholar]
- 108.Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6:813–25. doi: 10.1517/17425240903071029. [DOI] [PubMed] [Google Scholar]
- 109.Lilia Romero E, Morilla MJ. Topical and mucosal liposomes for vaccine delivery. Wiley Interdisc Rev Nanomed Nanobiotechnol. 2011;3:356–75. doi: 10.1002/wnan.131. [DOI] [PubMed] [Google Scholar]


