Abstract
Nanotechnology is the science of manipulating atoms and molecules in the nanoscale - 80,000 times smaller than the width of a human hair. The world market for products that contain nanomaterials is expected to reach $2.6 trillion by 2015. The use of nanotechnology has stretched across various streams of science, from electronics to medicine and has now found applications in the field of cosmetics by taking the name of nanocosmetics. This widespread influence of nanotechnology in the cosmetic industries is due to the enhanced properties attained by the particles at the nano level including color, transparency, solubility etc. The different types of nanomaterials employed in cosmetics include nanosomes, liposomes, fullerenes, solid lipid nanoparticles etc. Recently, concerns over the safety of such nanocosmetics are raised and have forced the cosmetic industries to limit the use of nanotechnology in cosmetics and for enforcing laws to undergo a full-fledged safety assessment before they enter into the market. In this review, emphasis is made on the types of nanomaterials used in cosmetics by the various cosmetic brands, the potential risks caused by them both to human life and also to the environment and what all regulations have been undertaken or can be taken to overcome them.
KEY WORDS: Dendrimers, health risks, hydrogel, nanocosmeceuticals, safety controls
Nanotechnology is an innovative science that includes the design, characterization, production and application of structures, devices and systems by controlling shape and size at the nanometer scale, which covers the size range from 1 nanometer to 100 nanometer (nm), where 1 nanometer is 1 billionth of a meter. It is not news to cosmetic companies that nanotechnology is the way of the future and is considered as the hottest and emerging technology available. Cosmetic manufacturers use nanoscale versions of ingredients to provide better UV protection, deeper skin penetration, long-lasting effects, increased color and finish quality etc. The global market for cosmetics using nanotechnology is projected to reach an estimated $155.8 million in 2012.[1] This widespread use of nanoscale materials in cosmetics is due to the fact that these nanoparticles obtain newer properties which differ from the large-scale particles. These altered properties include color, transparency, solubility and chemical reactivity, making the nanomaterials attractive to the cosmetics and personal care industries.[2]
Front-running brands of nanocosmetics
It has been found out from different surveys that almost all the major cosmetic manufacturers use nanotechnology in their various products. Cosmetics giant Estee Lauder entered the NanoMarket in 2006 with a range of products containing “NanoParticles”. L’Oreal, the world's largest cosmetics company, is devoting about $600 million dollars, of its $17 billion dollar revenues, to Nano patents, and has patented the use of dozens of “nanosome particles”. It ranks number 6 in nanotech patent holders in the U.S.[3] Other examples include Freeze 24/7, DDF (Doctor's Dermatologic Formula), and Colorescience.[4] An estimation of how the top 10 cosmetic companies of the world rank in terms of nano-related patents, based on Espacenet database, is depicted in Graph 1.[5]
Graph 1.
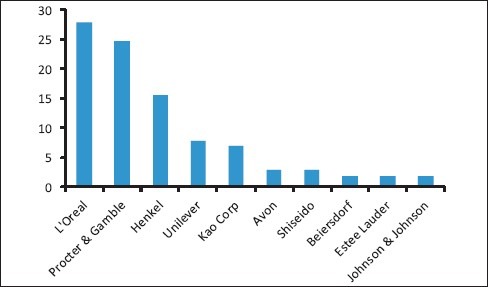
Ranking of top 10 beauty companies in terms of number of nano-related patents
Nano-variegation in cosmetics
Mineral-based cosmetic ingredients with nano-sized dimensions
Some cosmetic products, such as sunscreens, use mineral-based materials and their performance depends on their particle size. In sunscreen products, titanium dioxide and zinc oxide, in the size range of 20 nm, are used as efficient UV filters. Their main advantage is that they provide broad UV-protection and do not cause cutaneous adverse health effects.[6,7]
Other nano-sized materials employed in cosmetics
Many of the leading cosmetic companies claim their products to contain various types of nano-sized materials like fullerenes, nanotubes, liposomes, quantum dots etc.[6,7]
Types of nanomaterials used in cosmetics are the following
Liposomes
Liposomes are concentric bilayered vesicles in which the aqueous volume is entirely enclosed by a lipid bilayer composed of natural or synthetic phospholipids which are GRAS (generally regarded as safe) products. The lipid bilayer of liposomes can fuse with other bilayers such as the cell membrane, which promotes release of its contents, making them useful for cosmetic delivery applications. Their ease of preparation, enhanced absorption of active ingredients by skin and continuous supply of agents into the cells over a sustained period of time make them suitable for cosmetic applications.[8] Vesicles, other than liposomes are being used these days that claim to further enhance the penetration of substances across the skin, such as transferosomes,[9,10] niosomes[11,12] and ethosomes.[13]
Nanoemulsions
They are dispersions of nanoscale droplets of one liquid within another.[14] They are metastable systems whose structure can be manipulated based on the method of preparation. The components used for their preparation are GRAS products and are safe to use. Their smaller particle size provide higher stability and better suitability to carry active ingredients; they also increase the shelf life of the product.[15,16]
Nanocapsules
Nanocapsules are submicroscopic particles that are made of a polymeric capsule surrounding an aqueous or oily core. It has been found that the use of nanocapsules decreases the penetration of UV filter octyl methoxycinnamate in pig skin when compared with conventional emulsions.[17]
Solid lipid nanoparticles
They are oily droplets of lipids which are solid at body temperature and stabilized by surfactants. They can protect the encapsulated ingredients from degradation, used for the controlled delivery of cosmetic agents over a prolonged period of time and have been found to improve the penetration of active compounds into the stratum corneum.[18] In vivo studies have shown that an SLN-containing formulation is more efficient in skin hydration than a placebo.[19] They have also been found to show UV-resistant properties, which were enhanced when a molecular sunscreen was incorporated and tested. Enhanced UV blocking by 3,4,5-trimethoxybenzoylchitin (a good UV absorber) was seen when incorporated into SLNs.[20]
Nanocrystals
They are aggregates comprising several hundred to tens of thousands of atoms that combine into a “cluster”. Typical sizes of these aggregates are between 10 and 400 nm and they exhibit physical and chemical properties somewhere between that of bulk solids and molecules. They allow safe and effective passage through skin.[21]
Nanosilver and Nanogold
Cosmetic manufacturers are harnessing the enhanced antibacterial properties of nanosilver in a range of applications. Some manufacturers are already producing underarm deodorants with claims that the silver in the product will provide up to 24-hour antibacterial protection. Nano-sized gold, like nanosilver, is claimed to be highly effective in disinfecting the bacteria in the mouth and has also been added to toothpaste.[22]
Dendrimers
Dendrimers are unimolecular, monodisperse, micellar nanostructures, around 20 nm in size, with a well-defined, regularly branched symmetrical structure and a high density of functional end groups at their periphery. They contain large number of external groups suitable for multifunctionalization.[23,24]
Cubosomes
Cubosomes are discrete, sub-micron, nanostructured particles of bi-continuous cubic liquid crystalline phase.[25] It is formed by the self assembly of liquid crystalline particles of certain surfactants when mixed with water and a microstructure at a certain ratio. Cubosomes offer a large surface area, low viscosity and can exist at almost any dilution level. They have high heat stability and are capable of carrying hydrophilic and hydrophobic molecules.[26] Combined with the low cost of the raw materials and the potential for controlled release through functionalization, they are an attractive choice for cosmetic applications as well as for drug delivery.
Hydrogels
They are 3D hydrophilic polymer networks that swell in water or biological fluids without dissolving as a result of chemical or physical cross-links. They can predict future changes and change their property accordingly to prevent the damage.[27]
Buckyballs
Buckminster fullerene, C60, is perhaps the most iconic nanomaterial and is approximately 1 nm in diameter. It has found its way into some very expensive face creams. The motivation is to capitalize on its capacity to behave as a potent scavenger of free radicals.[28]
Manufacturers employing nanotechnology in their marketed products as per a study conducted by the ′Friends of the Earth’ are shown in Table 1.[2]
Table 1.
Manufacturers employing nanotechnology in their marketed products[2]

The given Graph 2 shows the principle nanomaterials used in various cosmetics.[29]
Graph 2.
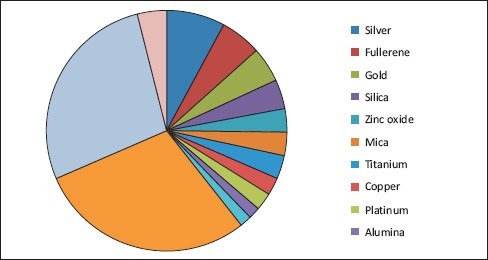
Principle nanomaterials used in cosmetics
Black-box warnings for nanocosmetics – how and why?
Nanoparticles have been found to cause a large number of risks both to humans as well as to the environment. The toxicity of nanomaterials is affected by their properties, which are attributable to their smaller size, chemical composition, surface structure, solubility, shape and aggregation. The various reasons for this nanotoxicity are summarized below:-
Smaller size of nanoparticles
The main characteristic of nanoparticles is their small size. This can alter their physicochemical properties when compared with their larger counterparts and can create the opportunity for increased uptake and interaction with the biological tissues. Toxicity is mainly concerned with the production of reactive oxygen species, including free radicals which will result in oxidative stress, inflammation, and consequent damage to proteins, membranes and DNA. Because of their size, these nanoparticles can easily gain access to the blood stream via skin or inhalation and from there they will be transported to the various organs. The high dose and long residence time of the nanoparticles in the vital organs can lead to their dysfunction.[30,31] Carbon nanotubes have been shown to cause the death of kidney cells and to inhibit further cell growth.[32] Whereas 500 nm titanium dioxide particles have only a small ability to cause DNA strand breakage, 20 nm particles of titanium dioxide are capable of causing complete destruction of super-coiled DNA, even at low doses and in the absence of exposure to UV.[33] In another study, it was found that mice which were subacutely exposed to 2–5 nm TiO2 nanoparticles showed a significant but moderate inflammatory response.[34]
Shape of nanoparticles
Nanoparticles are produced in a variety of shapes like spheres, tubes, sheets etc. and this may be a major cause for the health risks caused by them. A study has shown that exposing the abdominal cavity of mice to long carbon nanotubes are linked with inflammation of the abdominal wall.[35]
Surface area of nanoparticles
As the size of the particle decreases, their surface area increases leading to an increase in their reactivity. Nanomaterials are also highly reactive due to their high surface area-to-mass ratio, providing more area by weight for chemical reactions to occur. Studies have revealed that because of this increased reactivity, some nanoscale particles may be potentially explosive and/ or photoactive. For example, some nanomaterials—such as nanoscale titanium dioxide and silicon dioxide—may explode if finely dispersed in the air and they come into contact with a sufficiently strong ignition source.[36]
Penetration of nanoparticles via skin
Scientific studies have shown that nanoparticles can penetrate skin, especially if skin is flexed.[37] Broken skin is a direct route for the penetration of particles even up to a size of 7000 nm. The presence of acne, eczema and wounds may enhance the absorption of nanoparticles into the blood stream and may lead to further complications. A preliminary study found that nanoparticle penetration was deeper in skin affected by psoriasis than in unaffected skin.[38] Recently, the base carriers are being modified in order to enhance the skin penetration by incorporating certain penetration enhancers, both physical and chemical, and also by preparing newer vesicular systems with increased skin penetrability like ethosomes and transferosomes. Even flexing and massage can increase the skin penetration of nanoparticles. One study found that even particles up to 1000 nm in size can be taken up through intact skin to reach living cells, when skin is flexed.[39]
Cellular toxicity of zinc oxide and titanium dioxide nanoparticles
In a study published by Minghong Wu and co-workers at Shanghai University, they have discovered that zinc oxide (ZnO) nanoparticles used in sunscreens can damage or kill the stem cells in the brains of mice.[40] To investigate the potential neurotoxicity of ZnO nanoparticles, Wu et al. prepared cultures of mouse neural stem cells (NSCs), and treated them with zinc oxide nanoparticles ranging from 10 to 200 nanometers in size. After 24 hours, the cell viability assay indicated that ZnO nanoparticles manifested dose-dependent, but not size-dependent toxic effects on NSCs. Through analysis using confocal microscopy, transmission electron microscopy examination, and flow cytometry, many of the NSCs showed clear signs of apoptosis. This zinc oxide nanoparticle toxicity was found to be the effect of the dissolved zinc ions in the culture medium or inside cells.[41] In another work by Arnaud Magrez at the NN Research Group, it was found that titanium dioxide based nanofilaments were found to be cytotoxic, which was affected by their geometry and also enhanced by the presence of defects on the nanofilament surface, resulting from chemical treatment. Nanofilament internalization and alterations in cell morphology were observed.[32]
Occupational risks of nanoparticles
Workers may be accidentally exposed to nanomaterials during the production of nanomaterials or products containing them, as well as during use, disposal or recycling of these products. Exposure may also occur in cleaning and maintaining research, production and handling facilities.[42] A higher potency of nanomaterials compared to microsized particles was detected by Kaewamatawong et al.[43] At present, there is insufficient information on the number of workers exposed to nanomaterials in the work place or the effects on human health of such exposure, according to the European Agency for Safety and Health at Work. In addition, because nanomaterials have applications in many consumer products and the use of such materials in products is increasing, consumers have an increasing chance of exposure to these materials.
Route and extent of exposure[44–46]
health risks that nanoparticles pose to the humans also depend on the route and extent of exposure to such materials. Nanomaterials enter the body mainly through 3 routes.
Inhalation
It is the most common route of exposure of airborne nanoparticles according to the National Institute of Occupational Health and Safety. For example, workers may inhale nanomaterials while producing them if the appropriate safety devices are not used, while consumers may inhale nanomaterials when using products containing nanomaterials, such as spray versions of sunscreens containing nanoscale titanium dioxide. According to officials at the National Institutes of Health, although the vast majority of inhaled particles enter the pulmonary tract, evidence from studies on laboratory animals suggest that some inhaled nanomaterials may travel via the nasal nerves to the brain and gain access to the blood, nervous system, and other organs, according to studies we reviewed.
Ingestion
Ingestion of nanomaterials may occur from unintentional hand-to-mouth transfer of nanomaterials or from the intentional ingestion of nanomaterials. A large fraction of nanoparticles, after ingestion, rapidly pass out of the body; however, according to some of the studies we reviewed, a small amount may be taken up by the body and then migrate into organs.
Through skin
Studies have shown that certain nanomaterials have penetrated layers of pig skin within 24 hours of exposure.[37] According to some of the studies reviewed by the US Government Accountability Office (GAO), concerns have been raised that nanomaterials in sunscreens could penetrate damaged skin.
Environmental risks of nanoparticles
The environment is also at risk due to the exposure of nanomaterials through release into the water, air, and soil, during the manufacture, use, or disposal of these materials. These nanomaterials, if antibacterial in nature and if released in sufficient amounts, could potentially interfere with beneficial bacteria in sewage and waste water treatment plants and could also contaminate water intended for reuse, according to some of the studies reviewed by US GAO. For example, studies have revealed the toxicity of TiO2 nanoparticles to the main body systems of rainbow trout.[47] In a study conducted by the University of Toledo, the researchers discovered that nano-titanium dioxide used in personal care products reduced biological roles of bacteria after less than an hour of exposure. These findings suggest that these particles, which end up at municipal sewage treatment plants could eliminate microbes that play vital roles in ecosystems and help treat wastewater.[48] In one of the studies done on carbon fullerenes, it has been found out that they can cause brain damage in largemouth bass,[49] a species accepted by regulatory agencies as a model for defining ecotoxicological effects. Fullerenes have also been found to kill water fleas and have bactericidal properties.[50] Rice University's Centre for Biological and Environmental Nanotechnology has pointed out the tendency for nanoparticles to bind to contaminating substances already pervasive in the environment like cadmium and petrochemicals. This tendency would make nanoparticles a potential mechanism for long range and widespread transport of pollutants in groundwater.[51,52] Certain studies have even suggested that nanoparticles have the potential for biomagnification.[53] An interdisciplinary team of researchers at the UC (University of California) Santa Barbara produced a groundbreaking observation on how nanoparticles are able to biomagnify in a simple microbial food chain.[54]
Toxicity produced by carbon fullerenes (buckyballs)
Various studies have shown that carbon fullerenes, which are currently being used in moisturizers and some face creams, have the potential to cause brain damage in fishes[55,56] kill water fleas and have bactericidal properties. In a work done by Dhawan et al., he proved that stable aqueous suspensions of colloidal C60 fullerenes have demonstrated genotoxicity with a strong correlation between fullerene concentration and genotoxic response.[57] Fullerenes have even been found to be toxic to the vascular endothelial cells.[58]
Characterization methods for safety assessment of nanoparticles in cosmetics
The opinions of the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) deals with the risk assessment methodologies available for evaluating the possible adverse health and environmental effects of nanotechnology products[59] and also on the investigation of nanomaterials.[60] The specific characteristic of nanomaterials will require new test strategies to determine the mechanisms of potential injury that they may cause. The main parameters that are evaluated for the safety of nanomaterials are the following:
Physical-chemical properties
Physical properties like size, shape, specific surface area, agglomeration state, size distribution, surface morphology, structure, solubility and chemical properties like structural formula/molecular structure, composition of nanomaterial, phase identity, surface chemistry, hydrophilicity/lipophilicity have to be analyzed.[59]
Mathematical modeling
These predictive models range from simple, empirical algorithms to complex mathematical equations which sometimes require knowledge and estimation of experimentally inaccessible parameters. But, since, in none of these models, data relating to macromolecular compounds or particle structures have been included, they cannot be used with any confidence to predict what might happen when such entities contact the skin.[61]
Microscopic techniques
More useful information from the in vitro studies can be obtained by microscopic examination of the skin posttreatment. While absolute quantification may not be possible, visualization of the tissue to which an active has been applied can provide valuable insight.[61,62]
The methods used for microscopic evaluation are shown in Table 2.[61]
Table 2.
Methods employed for microscopic techniques[61]
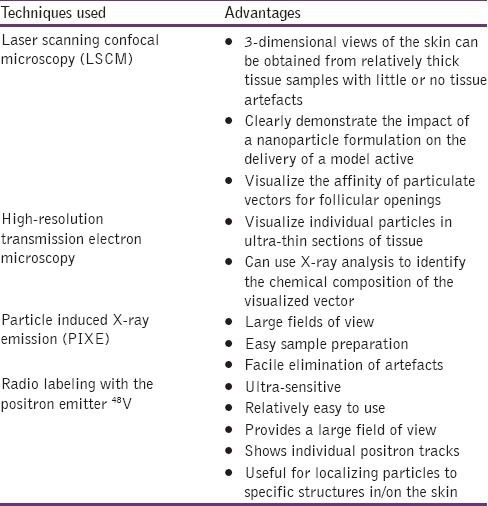
In vitro methods
Though there are a number of alternative methods and technologies for studying the molecular mechanisms involved in the biological activity of compounds, only validated methods are permitted for cosmetic products. These validated methods must be used when testing is required, for the safety assessment of cosmetic ingredients.[61,62]
The different validated in vitro tests employed are depicted in Figure 1.[61]
Figure 1.
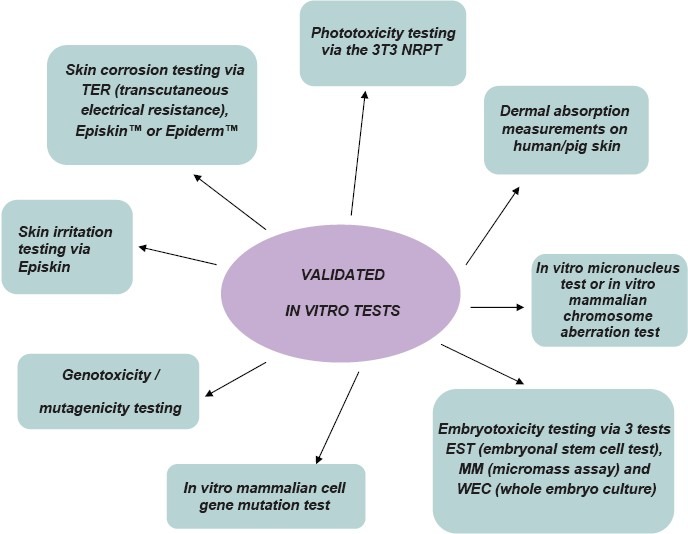
Validated in vitro Methods employed[61]
The relevant toxicological end points considered important for nanomaterials are given in Figure 2.[61]
Figure 2.
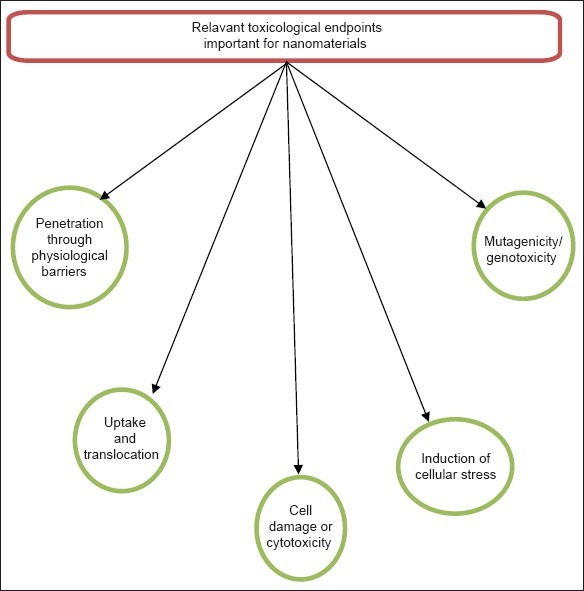
Relevant toxicological endpoints important for nanomaterials
Safety requisites for a blooming beauty
Cosmetic manufacturers using nanotechnology confront an uncertain future from both consumer response and a regulatory standpoint. Eminent scientific bodies like the Royal Society, Britain's most prestigious scientific body, and the US Food and Drug Administration warn that the health risks of nanocosmetics require a thorough investigation before product commercialization.[63] One of the major problems is that there is no much evidence about how much or what type of safety assessments are done by the various cosmetic manufacturers on their products.
Though there are increasing number of cosmetics and personal care products containing nanomaterials in the market, there are no specific regulations regarding their safety assessment. In Australia, the National Industry Chemicals Notification and Assessment Scheme (NICNAS) regulates the safety of ingredients in cosmetics and personal care products and the Therapeutic Goods Administration (TGA) regulates sunscreens. However these regulators fail to distinguish between nanoparticles and larger sized particles. The EU's Scientific Committee on Consumer Products (SCCP) looked at the safety evaluation of nanomaterials for use in cosmetic products and considered the implications on animal testing and whether the previous opinions on nanomaterials currently used in sunscreen products would need to be revised.[64]
The European Parliament approved the amended recast of the EU Cosmetics Directive, introducing the mention of ‘nanomaterials’ into an EU legislation. As requested by the European Parliament, the new regulation introduces a safety assessment procedure for all products containing nanomaterials, which could lead to a ban on a substance if there is a risk to human health.[65] The major excerpts from the act include the following[66,67]:-
The ruling defines nanomaterial as “an insoluble or bio-persistent and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100 nm”.
The responsible person shall ensure compliance with safety, GMP, safety assessment, product information file, sampling and analysis, notification, restrictions for substances listed in Annexes, CMR, nanomaterial traces, animal testing and labeling, claims, information to the public, communication of SUE, information on substances.
-
Prior to placing the cosmetic product on the market, the responsible person should submit the following information to the Commission:
- The presence of substances in the form of nanomaterials
- Their identification including the chemical name (IUPAC) and other descriptors
- The reasonably foreseeable exposure conditions
In case the Commission has concerns regarding the safety of the nanomaterial, the Commission shall, without delay, request the SCCS to give its opinion on the safety of these nanomaterials for the relevant categories of cosmetic products and the reasonably foreseeable exposure conditions.
All ingredients present in the form of nanomaterials shall be clearly indicated in the list of ingredients. The names of such ingredients shall be followed by the word “nano” in brackets.
-
Particular consideration shall be given to any possible impacts on the toxicological profile due to
- Particle sizes, including nanomaterials;
- Impurities of the substances and raw material used; and
- Interaction of substances
In a nutshell
The use of engineered nanomaterials has hiked in today's world. It has also captured the hearts of the cosmetic industries with its enhanced properties and they are shifting their focus from cosmeceuticals to nanocosmeceuticals by incorporating nanotechnology in most of their manufacturing processes. But all these nanocosmetics have raised a great concern regarding their safety for humans and environment. In order to ensure the safety and efficacy of such products, the European Union has incorporated a new amendment in its Cosmetics Directive which will become active from 2012 onwards. This new regulation will allow only the safer nanocosmetic products to enter into the market, safeguarding the beauty and health of the consumers.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Law 360. Nano-cosmetics: Beyond skin deep. 2011. [Last accessed on 2011]. Available from: http://www.shb.com/newsevents/2011/NanoCosmeticsBeyondSkinDeep.pdf .
- 2.Friends of the Earth Report – Nanomaterials, Sunscreens and Cosmetics: Small Ingredients Big Risks. [Last accessed on 2006]. Available from: http://www.nano.foe.org.au. http://www.foe.org .
- 3.Nano Science Institute. Scientific Committee Rules on the Safety of Nanocosmetics. [Last accessed on 2008]. Available from: http://www.nanoscienceinstitute.com/NanoCosmetics.htm .
- 4.Schueller R, Romanowski P. Emerging Technologies and the Future of Cosmetic Science, Cosmetics and Toiletries. [Last accessed on 2006]; Available from: http://www.specialchem4cosmetics.com/services/articles.aspx?id=957 . [Google Scholar]
- 5.Nano and the top 10 big cosmetic companies: L’Oreal, Procter and Gamble and Henkel on the podium for patents. [Last cited on 2009]. Available from: http://www.nanocolors.wordpress.com/2009/10/29/nano-the-top-10-big-cosmetics-companies-L’Oreal-Procter-Gamble-and-Henkel .
- 6.Pierfrancesco M. Use and potential of nanotechnology in cosmetic dermatology. Clin Cosmet Investig Dermatol. 2010;3:5–13. doi: 10.2147/ccid.s4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SCCP (Safety Committee on Consumer Products), Opinion on Safety of Nanomaterials in Cosmetic Products. [Last accessed on 2007]. Available from: http://www.ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_123.pdf .
- 8.Jain S, Sapee R, Jain NK. Proultraflexible lipid vesicles for effective transdermal delivery of norgesterol. USA: Proceedings of 25th conference of C.R.S; 1998. pp. 32–5. [Google Scholar]
- 9.Cevc G. Transfersomes, liposomes and other lipid suspensions on the skin: Permeation enhancement, vesicle penetration, and transdermal drug delivery. Crit Rev Ther Drug Carrier Syst. 1996;13:257–388. doi: 10.1615/critrevtherdrugcarriersyst.v13.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 10.Thong HY, Zhai H, Maibach HI. Percutaneous penetration enhancers: An overview. Skin Pharmacol Physiol. 2007;20:272–82. doi: 10.1159/000107575. [DOI] [PubMed] [Google Scholar]
- 11.Uchegbua IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. [Google Scholar]
- 12.Balakrishnan P, Shanmugam S, Lee WS, Lee WM. Formulation and in vitro assessment of minoxidil niosomes for enhanced skin delivery. Int J Pharm. 2009;377:1–8. doi: 10.1016/j.ijpharm.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes-novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J Control Release. 2000;65:403–18. doi: 10.1016/s0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 14.Nanoemulsion based on phosphoric acid fatty acid esters and its uses in the cosmetics, dermatological, pharmaceutical, and/or ophthalmological fields. L’Oréal, US Patent 6274150 [Google Scholar]
- 15.Fred Z, Esther B, Daniel S, Christina L, Franz S. Preparation and Properties of Coenzyme Q10 Nanoemulsions, Cosmetic Science Technology. [Last accessed on 2006]. Available from: http://www.mibellebiochemistry.com/pdfs/Preparation_and_Properties_of_Coenzyme_Q10_Nanemulsions_Cosm_Sci_Technol_2006.pdf .
- 16.Sonneville-Aubrun O, Simonnet JT, L’Alloret F. Nanoemulsions: A new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108-109:145–9. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SL, Kim JC. In vivo hair growth promotion effects of cosmetic preparations containing hinokitiol-loaded poly(epsilon-caprolacton) nanocapsules. J Microencapsul. 2008;25:351–6. doi: 10.1080/02652040802000557. [DOI] [PubMed] [Google Scholar]
- 18.Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–55. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 19.Wissing SA, Müller RH. Cosmetic applications for solid lipid nanoparticles (SLN) Int J Pharm. 2003;254:65–8. doi: 10.1016/s0378-5173(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 20.Song C, Liu S. A new healthy sunscreen system for human: Solid lipid nanoparticles as carrier for 3,4,5- trimethoxybenzoylchitin and the improvement by adding vitamin E. Int J Biol Macromol. 2005;36:116–9. doi: 10.1016/j.ijbiomac.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Petersen R. Nanocrystals for use in topical cosmetic formulations and method of production thereof. Abbott GmbH and Co., US Patent 60/866233. 2008 [Google Scholar]
- 22.Nanotechnology in cosmetics analysed. [Last accessed on 2010]. Available from: http://www.personalcaremagazine.com/Story.aspx?Story=7419 .
- 23.Cosmetic or dermatological topical compositions comprising dendritic polyesters. L’Oréal, US Patent 6287552. 2001 [Google Scholar]
- 24.Michael F. Cosmetic Compositions For Hair Treatment Containing Dendrimers Or Dendrimer Conjugates - Patent 6068835. 2010 [Google Scholar]
- 25.Spicer PT, Lynch ML, Visscher M, Hoath S. Bicontinuous Cubic Liquid Crystalline Phase and Cubosome Personal Care Delivery Systems. In: Rosen M, editor. Personal Care Delivery Systems and Formulations. Berkshire, UK: Noyes Publishing; 2003. [Google Scholar]
- 26.Kesselman E, Efrat R, Garti N, Danino D. Formation of cubosomes as vehicles of biologically active substances. [Last accessed on 2007]. Available from: http://www.materials.technion.ac.il/ism/Docs/2007/Life-Abstracts/Poster/E_Kesselman.pdf .
- 27.Morales ME, Gallardo V, Clarés B, García MB, Ruiz MA. Study and description of hydrogels and organogels as vehicles for cosmetic active ingredients. J Cosmet Sci. 2009;60:627–36. [PubMed] [Google Scholar]
- 28.Rania B, Rainer MV, Muhammad N, Matthias R, Zoltan S, Christian WH, et al. Medicinal applications of fullerenes. Int J Nanomedicine. 2007;2:639–49. [PMC free article] [PubMed] [Google Scholar]
- 29.PEN Consumer Products Inventory. [Last accessed on 2008]. Available from: http://www.nanotechprojects.org/consumer .
- 30.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royal Society. “Nanoscience and nanotechnologies: Opportunities and uncertainties”, Royal Society London. [Last accessed on 2004]. Available from: http://www.nanotec.org.uk/finalReport.htm .
- 32.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–5. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 33.Donalson K, Beswick P, Gilmour P. Free radical activity associated with the surface of particles: A unifying factor in determining biological activity? Toxicol Lett. 1996;88:293–8. doi: 10.1016/0378-4274(96)03752-6. [DOI] [PubMed] [Google Scholar]
- 34.Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation Exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poland CA, Duffin R, Kinloch I. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 36.Pritchard DK. Health and safety laboratory, literature review – explosion hazards associated with nanopowders. [Last accessed on 2004]. Available from: http://www.hse.gov.uk/research/hsl_pdf/2004/hsl04-12.pdf .
- 37.Ryman-Rasmussen J, Riviere J, Monteiro-Riviere N. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci. 2006;9:159–65. doi: 10.1093/toxsci/kfj122. [DOI] [PubMed] [Google Scholar]
- 38.Tarl WP, Jeffrey EG, Lynlee LL, Rokhaya F, Margaret B. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63:470–91. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Rouse J, Yang J, Ryman-Rasmussen J, Barron A, Monteiro-Riviere N. Effects of mechanical flexion on the penetration of fullerene amino acid derivatized peptide nanoparticles through skin. Nano Lett. 2007;7:155–60. doi: 10.1021/nl062464m. [DOI] [PubMed] [Google Scholar]
- 40.Minghong W. Toxicity of nanoparticles: Zinc oxide on the brain, NPG Asia Materials. [Last accessed on 2009]. Available from: http://www.natureasia.com/asia-materials/highlight.php?id=438 .
- 41.Wu W, Samet JM, Peden DB, Bromberg PA. Phosphorylation of p65 Is required for zinc oxide nanoparticle–induced interleukin 8 expression in human bronchial epithelial cells. Environ Health Perspect. 2010;118:982–7. doi: 10.1289/ehp.0901635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran C, Donaldson K, Stones V, Fernandez T, Ford A, Christofi N, et al. A scoping study to identify hazard data needs for addressing the risks presented by nanoparticles and nanotubes. Research Report. Institute of Occupational Medicine. 2005 [Google Scholar]
- 43.Kaewamatawong T, Kawamura N, Okajima M, Sawada M, Morita T, Shimada A. Acute pulmonary toxicity caused by exposure to colloidal silica: Particle size dependent pathological changes in mice. Toxicol Pathol. 2005;33:743–9. doi: 10.1080/01926230500416302. [DOI] [PubMed] [Google Scholar]
- 44.Schulte P, Geraci C, Zumwalde R, Hoover M, Kuempel E. Occupational risk management of engineered nanoparticles. J Occup Environ Hyg. 2008;5:239–49. doi: 10.1080/15459620801907840. [DOI] [PubMed] [Google Scholar]
- 45.Institute of Occupational Medicine for the Health and Safety. [Last accessed on 2004]. Available from: http://www.hse.gov.uk .
- 46.Claude O, Brigitte R. Engineered nanoparticles current knowledge about OHS risks and prevention measures, chemical substances and biological agents, Studies and Research Projects, REPORT R-656. [Last accessed on 2010]. Available from: http://www.irsst.qc.ca/media/documents/PubIRSST/R-656.pdf .
- 47.Chen J, Dong X, Zhao J, Tang G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J Appl Toxicol. 2009;29:330–7. doi: 10.1002/jat.1414. [DOI] [PubMed] [Google Scholar]
- 48.Matthew C. Environmental health news, Nanoparticles from sunscreens damage microbes, scientific american. [Last accessed on 2009]. Available from: http://www.scientificamerican.com/article.cfm?id=nanoparticles-insunscreen&page=2 .
- 49.Ernie H. Fullerenes and fish brains: Nanomaterials cause oxidative Stress. Environ Health Perspect. 2004;112:A568–9. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.L’enabrunet, Delinay L, Ernestm H, Pedroj JA, Markr W. Comparative photoactivity and antibacterial properties of C60 fullerenes and titanium dioxide nanoparticles. Environ Sci Technol. 2009;43:4355–60. doi: 10.1021/es803093t. [DOI] [PubMed] [Google Scholar]
- 51.Center for Biological and Environmental Nanotechnology (CBEN), Rice University, ‘Nanorust’ cleans arsenic from drinking water. [Last accessed on 2006]. Available from: http://www.rice.edu/media/nanorust_arsenic.html .
- 52.Michael SW, Pedro JA, Yu-lun F, Nurg A, Michael ON, Jeffrey TM, et al. Cleaner water using bimetallic nanoparticle catalysts. J Chem Technol Biotechnol. [Last accessed on 2008]. Available from: http://www.pure-t.net/tce_paper.pdf. www.interscience.com.
- 53.Jonathan DJ, Jason M, Jason M.U, Paul MB. Evidence for biomagnification of gold nanoparticles within a terrestrial food chain. Environ Sci Technol. 2011;45:776–81. doi: 10.1021/es103031a. [DOI] [PubMed] [Google Scholar]
- 54.UCSB, UCSB scientists demonstrate biomagnifications of nanomaterials in simple food chain, nanotechnology today. [Last accessed on 2011]. Available from: http://www.nanotechnologytoday.blogspot.com/2011/01/ucsbscientists-demonstrate.html .
- 55.Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–8. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberdorster E. 227th American Chemical Society National Meeting, Anaheim. CA. Washington DC: American Chemical Society IandEC 21; [Last accessed on 2004]. “Toxicity of nC60 fullerenes to two aquatic species: Daphnia and largemouth bass” [Abstract] Available from: http://www.oasys2.confex.com/acs/227nm/techprogram/P719002.HTM . [Google Scholar]
- 57.Dhawan A, Taurozzi JS, Pandey AK, Shan W, Miller SM, Hashsham SA, et al. Stable colloidal dispersions of C60 fulleres in water: Evidence for genotoxicity. Environ Sci Technol. 2006;40:7394–401. doi: 10.1021/es0609708. [DOI] [PubMed] [Google Scholar]
- 58.Hideyuki Y, Naoharu I. Cytotoxicity of water-soluble fullerene in vascular endothelial cells. Am J Physiol Cell Physiol. 2006;290:C1495–502. doi: 10.1152/ajpcell.00481.2005. [DOI] [PubMed] [Google Scholar]
- 59.SCENIHR, Risk Assessment of Products of Nanotechnologies. [Last accessed on 2009]. Available from: http://www.ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf .
- 60.SCENIHR, Opinion on The Appropriateness of The Risk Assessment Methodology in Accordance with The Technical Guidance Documents for New and Existing Substances for Assessing The Risks Of Nanomaterials. [Last accessed on 2007]. Available from: http://www.ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_004c.pdf .
- 61.SCCP (Scientific Committee on Consumer Products), Preliminary Opinion On Safety of nanomaterials in cosmetic products. [Last accessed on 2007]. Available from: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_099.pdf .
- 62.Report of the ICCR Joint Ad Hoc Working Group on Nanotechnology in Cosmetic Products: Criteria and Methods of Detection, ICCR-4. [Last accessed on 2010]. Available from: http://www.fda.gov/downloads/InternationalPrograms/HarmonizationInitiatives/UCM235485.pdf .
- 63.The Royal Society, Industry should disclose nano safety testing methods. [Last accessed on 2006]. Available from: http://www.royalsociety.org/News.aspx?id=1369 .
- 64.SCCP, The SCCP's Notes of Guidance for The Testing of Cosmetic Ingredients and Their Safety Evaluation, 6th Revision. [Last accessed on 2006]. Available from: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_03j.pdf .
- 65.European Parliament Press Release - MEPs approve new rules on safer cosmetics. [Last accessed on 2009]. Available from: http://www.europarl.europa.eu/news/expert/infopress_page/066-52333-082-03-13-911-20090323IPR52331-23-03-2009-2009-true/default_en.htm .
- 66.NIA (Nanotechnology Industries Association), European Parliament approves labelling and notification of nanomaterials in cosmetics. [Last accessed on 2009]. Available from: http://www.nanotechia.org/news/global/europeanparliament-approves-labelling-and-notific .
- 67.Anna G, Lefteris C. Nanotechnology in the EU Cosmetics Regulation, Nanotechnology, Household and Personal Care today. [Last accessed on 2009]. Available from: http://www.steptoe.com/assets/attachments/3915.pdf .


