Abstract
Context:
Evidence suggests that diets with high contents of cholesterol will increase serum lipoproteins and apolipoproteins, thereby increase risk of atherosclerosis. According to literature, some plants show hypolipidemic, hypocholestrolemic, and antiatherosclerotic activities.
Aims:
In this study, antiatherosclerotic effect of Hypericum perforatum hydroalcoholic extract on hypercholesterolemic rabbits was compared with that of lovastatin.
Materials and Methods:
Twenty five mature male New Zealand rabbits were randomly divided into five groups of five and were fed for 60 days as follows: Standard diet (GroupI), standard diet and hydroalcoholic extract of Hypericum perforatum (150 mg/kg daily)(GroupII), standard diet, hydroalcoholic extract of Hypericum perforatum (150 mg/ kg daily) and cholesterol (1% of food content) (Group III), standard diet and cholesterol (1% of food content)(GroupIV), and finally standard diet, lovastatin (10 mg/kg), and cholesterol (1% of foodcontent) (GroupV).
Results:
Hypericum perforatum extract significantly decreased the levels of apolipoprotein B(apoB), apolipoprotein B/apolipoprotein A (apoB/apoA), triglyceride, cholesterol, low density lipoprotein cholesterol, oxidized LDL, malondialdehyde, and C-reactive protein (CRP) as well as atherosclerosis index, and increased high density lipoprotein and apoA in rabbits of Group III compared to the rabbits of Group IV. The effect of Hypericum perforatum extract in decreasing the level of some biochemical factors like apoB, apoB/apoA, and CRP was meaningfully more than that of lovastatin. Histopathological findings confirmed that hydroalcoholic extract of Hypericum perforatum restricted the atherosclerotic lesions.
Conclusions:
This study indicates that hydroalcoholic extract of Hypericum perforatum possesses hypolipidemic and anti-atherosclerotic effects and could be beneficial in the management of hyperlipidemia and atherosclerosis.
KEY WORDS: Apolipoprotein, atherosclerosis, Hypericum perforatum, lovastatin
Hypericum is a genus of about 400 species of flowering plants in the family Clusiaceae, Different species of Hypericum exist worldwide with a native geographical distribution including temperate and subtropical regions of North America, Europe, Asia, Russia, India, and China. The major active constituents are considered to be hyperforin, hypericin, pseudohypericin, flavonoids, and tannins.[1] Hypericum perforatum is best known for its effect on mild-to-moderate depression. Investigations proved several pharmacological activities for Hypericum perforatum including antipyretic, analgesic, antispasmodic, antiviral, and antimicrobial activities as well as its role in treatment of psychiatric and neurologic disorders such as depression and migraine.[1,2] There have long been efforts in pharmaceutical industry to find a solution to prevent or treat atherosclerosis by managing elevated lipid level which plays an important role in atheroma plaque formation. Unfortunately, none of these efforts were completely satisfactory, and the doubts about the safety of synthetic drugs have produced an increasing demand by patients to use natural products with cholesterol-lowering activity. Therefore, the scientists are looking for cholesterol-lowering and antiatherogenic activities of different plant products and their active ingredients to prevent or control atherosclerosis.[3–5] Atherosclerosis is a complex inflammatory disease involving the endothelium, lipid engorged macrophages and smooth muscle cells.[6] Low density lipoprotein cholesterol (LDL-C)is a major risk factor for atherosclerosis.[7] LDL-C oxidation is a progressive process leads to the formation of oxidized LDL and plays an important role to initiate atheroma plaque formation.[8,9]
Although hyperlipidemia is a distinct factor in cardiovascular diseases, in nearly half the patients no change happens in their lipid profiles, so it is suggested that apolipoprotein B (apoB), apolipoprotein B/apolipoprotein A (apoB/apoA), and apolipoprotein A(apoA) should also be regarded as highly predictive in evaluation of cardiac risk.[10]
Many now believe that there is a link between the inflammatory process and coronary atherosclerosis. One of the indications of inflammation is the presence of C-reactive protein (CRP) in the blood. Levels of this marker are elevated when ischemic heart disease is present, because the plaque in diseased arteries typically contains inflammatory cells.[11] In a recent study, the effect of ethanol extract of Hypericum lysimachioides to decrease the atherosclerotic lesions in hypercholesterolemia rabbits has been demonstrated;[12] hence, in this study, the effect of another species of Hypericum (Hypericum perforatum) extract on traditional and specially novel serum risk factors (apoB, apoB/apoA) of cardiovascular diseases as well as fatty streak formation has been evaluated. Also, in order to compare its effect with current medications, we have used lovastatin as a chemical drug used in prevention and treatment of atherosclerosis.[13]
Materials and Methods
Plant material
Hypericum perforatum was provided from Isfahan Natural Resource Institute and authenticated by Dr. Lili Ghaemmaghami at the Biology Department, School of Sciences Isfahan University. The voucher specimen was deposited in Isfahan University Herbarium under the number 13648.
Preparation of hydro alcoholic extract
Above-ground parts of the plant were dried for 10 days at room temperature. The dried plants were ground by an electric blender. One hundred gram of Hypericum perforatum plant's powder was soaked in 96% ethanol for 72 h and then filtered and concentrated by a distiller in a vacuum. The concentrated solution was decanted in three consecutive steps (once with 100 ml and twice with 50 ml of chloroform). The resulting solution was vaporized and desiccated in 50°C under sterile conditions.[14] The dried powder obtained from the last step kept in dark glass bottle at 4°C until use. The flavonoid content was measured by spectrophotometery in 424 nm wavelength,[15] and anthocyanines were measured by the same methods in 535 nm wave length.[16]
Grouping and feeding of the rabbits
Twenty-five mature male New Zealand rabbits with average body weight of 2000 g were used. In order to adapt with their environment, animals were housed for two weeks in an air-conditioned animal room at 23±2°C, relative humidity 40–70%, with 12 h/12 h light/dark photoperiod, and provided with Super Fosskorn Standard Rabbit Chow, purchased from Pasteur Institute of Iran.
Group I: Fed with 100 g standard laboratory diet daily.
Group II: Standard diet 100 g + Hypericum perforatum hydroalcoholic extract (150 mg/kg daily).
Group III: Standard diet 100 g + Hypericum perforatum hydroalcoholic extract (150 mg/kg daily) + cholesterol suspended in olive oil and added to the diet (1% of food content daily).
Group IV: Standard diet + cholesterol added to the diet (1% of food content daily).
Group V: Standard diet + lovastatin (10 mg/kg daily) + cholesterol added to the diet (1% of food content daily) The Hypericum perforatum hydroalcoholic extract, cholesterol and lovastatin were given by feeding tube for 60 days. The animals were provided from Razi Institute, Karaj, Iran.
Isfahan Cardiovascular Research Center Ethics Committee which is a member of office for human research protections, US department of health and human services, approved the present study, and the animals were handled according to guidelines of Isfahan University of Medical Sciences for Laboratory Animal Sciences for the care and use of laboratory animals.
Measuring the biochemical factors
Prior to the beginning of diets, on the 30th and 60th day, the rabbits were put on fasting conditions for 12 h and taken blood samples. We took blood samples from group I (control group) only at the beginning and the end of study. Blood samples were taken from the marginal auricular vein. The plasma was obtained by centrifuging the blood samples at 2000 rpm for 15 min. Total cholesterol triglyceride (TG), LDL-C, and high density lipoprotein (HDL-C) were measured using special kits (DiaSys, Germany) which utilized the colorimetric method, in an auto analyzer (Hitachi auto analyzer, Hitachi Co., Tokyo). Concentrations of apoA and apoB were also measured using special kits (DiaSys, Germany) in an auto analyzer (Hitachi auto analyzer, Hitachi Co., Tokyo) according to the turbid metric method; CRP was also measured by rabbit CRP ELISA (Rapidbio, USA). We measured oxidized LDL (Ox-LDL) by rabbit Ox-LDL ELISA (Rapidbio, USA). malondialdehyde (MDA) was estimated by the double heating method of Draper and Hadley.[17] The principle of the method is the spectrophotometric measurement of the color generated by the reaction of thiobarbituric acid (TBA) with MDA. All these factors were measured at the beginning, 30th and 60th day except for Ox-LDL which was checked only at 60th day. Atherosclerosis index (AI) was calculated according to the following formula: AI=LDL-C/HDL-C.[18]
Histopathological analysis of aorta
On the 60th day, all the groups of the animals were sacrificed by rapid intracardiac pentobarbital (5%) injection for histopathological analysis. After incising the chest wall, the aorta was cut and removed, rinsed with normal saline solution and put in 10% formalin to be preserved for the next step. Cuts of aortic tissue were stained by Hematoxyline-Eosin method. Chekanov scale was used for grading of atherosclerotic plaques and the results were determined on a scale of 1–4 in relation to the thickness of media layer as follows:
Grade 1. Plaque less than half as thick as the media with some form of endothelial dysfunction.
Grade 2: Plaque at least half as thick as media with accumulation of intracellular lipid, macrophages, and smooth muscle cells.
Grade 3: Plaque as thick as the media with an abundance of macrophages, smooth muscle cells, and connective tissue.
Grade 4: Plaque thicker than the media with a large intracellular intimal lipid core and inflammatory cell infiltration.[19]
All histopathological evaluations were done by a pathologist blinded to the experimental design.
Statistical analysis
Differences between groups were statistically analyzed by one-way ANOVA, and the differences between the means of groups were separated by least significant difference (LSD) test. All data were presented as mean±SD. values of P<0.05 were considered as significant. A computer program (SPSS 13.0, SPSS Inc. Chicago, IL, USA) was used for statistical analysis.
Results
The results showed that each 100 g powder of Hypericum perforatum plant results in 8.33 ± 0.033 g hydroalcoholic extract powder of Hypericum perforatum. It was also demonstrated that the amount of flavonoids and anthocyanins in 100 g of Hypericum perforatum extract is 0.435 ± 0.0031 mg and 2.299 ± 0.99 mg, respectively At the onset, no statistically significance was found between the mean values among the study groups [Table 1]. In the group fed with high cholesterol diet (Group IV), on the 30th day, a meaningful increase in concentrations of total cholesterol, TG, LDL-C, HDL–C, apoB, AI, CRP in comparison with Group II [Table 2] and the beginning of the study [Table 1] were observed. Keeping these rabbits on a high cholesterol diet until 60th day shows the same results as compared to Group I and II [Table 3] and the start day of experiment [Table 1].
Table 1.
Selective biochemical parameters in all five groups on the start day
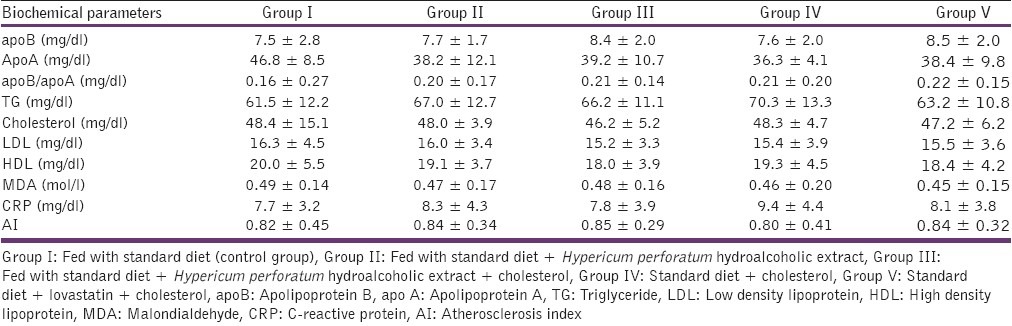
Table 2.
Effects of hydroalcoholic extract of Hypericum perforatum on selective cardiovascular risk factors in rabbits fed with high cholesterol diets on 30th day
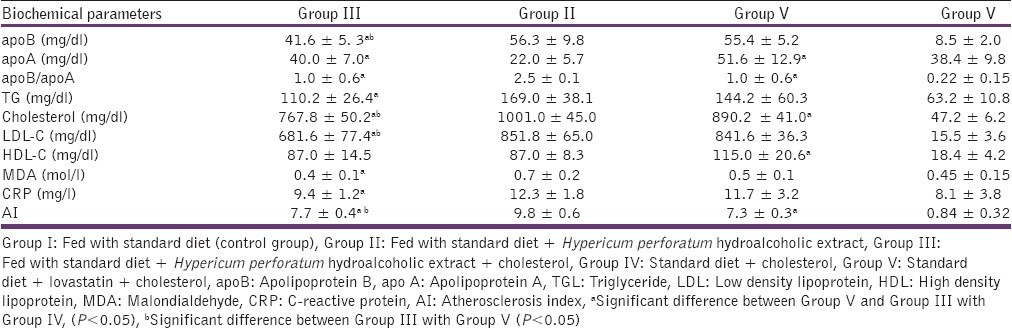
Table 3.
Effects of hydroalcoholic extract of Hypericum perforatum on selective cardiovascular risk factors in rabbits fed with high cholesterol diets on 60th day

Tables 2 and 3 summarize the results of comparing the biochemical factors in Group III (received Hypericum perforatum + high cholesterol diet), Group IV (received high cholesterol diet) and Group V (received lovastatin + high cholesterol diet) on the 30th and 60th day respectively. As shown in Table 2, on the 30th day, Group III (received Hypericum perforatum + high cholesterol diet) had meaningfully lower levels of cholesterol, apoB, apoB/apoA, LDL-C AI, TG, MDA and CRP, and higher levels of apoA as compared to Group IV (received high cholesterol diet). Also, the rabbits fed with high cholesterol diet plus Hypericum perforatum extract showed to have significantly lower levels of cholesterol, LDL-C, AI, apoB, and TG in comparison with the rabbits of Group V (received lovastatin + high cholesterol diet). On 60th day, in the group fed with Hypericum perforatum in addition to high cholesterol diet (Group III) the levels of apoB, apoB/apoA, TG, cholesterol, LDL-C, Ox-LDL, MDA, CRP, and AI were found to be decreased compared to the rabbits fed with high cholesterol diet (Group IV). On the other hand, the levels of apoA, HDL-C increased in Group III. The differences were statically significant. There was also a meaningful decrease in CRP, apoB/apoA, and apoB in Group III as compared to Group V as shown in Table 3.
At the end of present study there was no significant difference in biochemical factors and fatty streak formation between Group I and II, except a meaningful increase in HDL-C in later group. (30.75 ± 7.4 vs. 18.0 ± 3.9) (Supplementary Table 3) On the basis of histology reports, the aorta was completely normal in Group I (control) and Group II (received only hypericum perforatum hydroalcoholic extracts), and no pathologic lesion was seen in intima and media layers.
Group IV (fed with high cholesterol diet) showed atheromatous plaques, in which macrophages were full of fat and created foam cells, also smooth muscle cell proliferation was observed [Figure 1].
Figure 1.
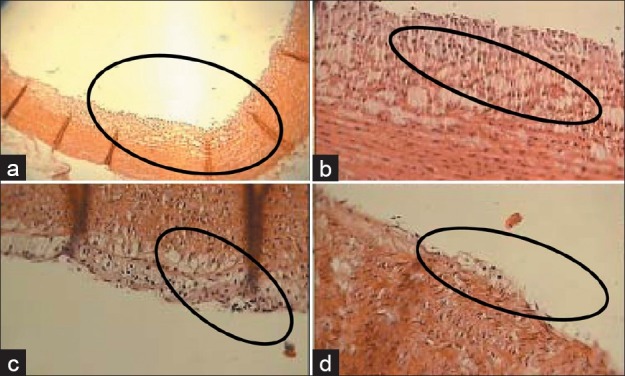
Cross sections of the aorta: (a) Group fed with high cholesterol diet, magnification ×10, (b) group fed with high cholesterol diet, magnification ×40, (c) group fed with high cholesterol diet+ Hypericum perforatum extract, magnification ×40; (d) Group fed with high cholesterol diet and lovastatin, magnification ×40
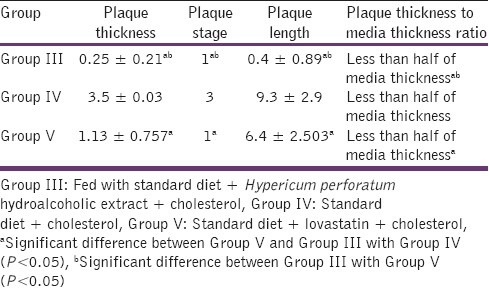
The comparison between Groups III and V (Hypericum perforatum and lovastatin) showed that Hypericum perforatum extract was more efficacious in preventing atheromatic plaque formation [Table 4].
Table 4.
Comparison of atheroma plaque in aortic cuts of three groups of rabbits fed with high cholesterol diets
Discussion
Evidence from epidemiologic studies and clinical trials has conclusively demonstrated that patients with high levels of total cholesterol and LDL-C and low levels of HDL-C are at increased risk for coronary heart disease.[20]
It is also clear that the traditional risk factors, such as hypertension and dyslipidemia, are important in formation and progression of atherosclerosis. But it appears that identification and understanding of so-called novel risk factors may further improve our ability to predict future risks when these are included along with the traditional risk factors in evaluating the global risk profile.[21] Regardless of lots of efforts in pharmaceutical industry to find drug therapy for atherosclerosis, a definite treatment strategy has still to be established. Moreover, as people are learning more about the nutritional and medicinal value of plants, herbal and natural medicines are becoming increasingly more popular by the day.[22]
It is now widely accepted ox-LDL rather than LDL-C is mainly responsible for initiation of atherosclerosis.[8,9,23] Several lines of evidence suggest that antioxidant compounds of some plants including Hypericum perforatum are able to scavenge free radicals and thereby prevent lipid peroxidation and reduce the risk of ischemic heart disease.[24–27]
Also, hypocholesterolemic activity of different plants and their active compounds have been shown in some studies, and it was suggested that this effect might be associated with the plants’ antioxidant properties.[3–5] Effects of several species of Hypericum in addition to their anti-oxidant properties have been examined and reported previously.[1,2]
While some studies have investigated Hypericum perforatum and Hypericum lysimachioides for their hypocholesterolemic effects,[4,12] and some looked for interactions between Hypericum perforatum and statins,[28,29] none examined effects of Hypericum perforatum on novel cardiovascular risk factors such as apoB, apoB/apoA, and CRP. In this study, effects of hydroalcoholic extract of Hypericum perforatum on selected traditional and novel cardiovascular risk factors as well as formation and progression of atheromatous plaque were investigated. Five groups of rabbits were examined, and after 60 days, treatments with Hypericum perforatum and lovastatin were compared.
The present study showed that in the group fed with Hypericum perforatum hydroalcoholic extract in addition to the high cholesterol diet group, this extract effectively decreased apoB, cholesterol and LDL-C and increased apoA and HDL-C as compared to the high cholesterol diet group, furthermore it significantly decreased the level of apoB even in comparison to the group fed with high cholesterol diet plus lovastatin. The decrease in cholesterol level after administration of Hypericum perforatum extract is probably caused by inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) or increased fecal excretion of biliary acids and cholesterol.[4]
Although high LDL-C and low HDL-C are known risk factors of atherosclerosis, studies shows that apoB, apoB/apoA, and apoA should also be considered as highly predictive in evaluation of cardiac risk.[10]
Over 90% of LDL-C particle is composed of apoB. It serves the role of solubalizing cholesterol within the LDL-C complex; hence, it can easily transport and deposit on the arterial wall.[30]
ApoA is primarily found in HDL particle. It prevents the accumulation of cholesterol loaded macrophages which deposit on the arterial wall.[31] It has also been reported that level of serum TG has a direct association with the risk of cardiovascular diseases.[32] Using Hypericum perforatum extract markedly decreases the level of TG in our study, which may be due to decreased absorption and increased excretion of TG via feces.[4] Hypercholesterolaemia results in excessive production of oxygen-free radicals which lead to produce endothelial cell injury by lipid peroxidatioin.[33] It is widely accepted that LDL-C oxidation is considered to be the hallmark of early atherosclerosis.[34] MDA is a highly reactive three carbon dialdehyde formed during oxidative degeneration as a product of free oxygen radicals,[35] which is accepted as an indicator of lipid peroxidation.[36]
In this study, plasma MDA and Ox-LDL both decreased in the group received high cholesterol diet plus Hypericum perforatum compared to the group received high cholesterol diet.
Hypericum perforatum possess some active constituents such as flavonoids, hypericin and pseudohypericin, which are structurally phenolic compounds.[1] It is assumed that as hypericin can bind to LDL-C. It can act as an antioxidant for LDL-C oxidation.[26] According to Zou et al., the antioxidant mechanism of flavonoid rich extract of Hypericum perforatum might be attributed to its free radical scavenging activity, metal-chelation activity, and reactive oxygen quenching activity.[37] Dietary antioxidants are thought to be beneficial in reducing the incidence of coronary heart disease.[38]
Some investigations showed there is a positive correlation between the antioxidation and cardioprotective activities of phenolic compounds.[39,40] Hypericum perforatum founds to have anthocyanins which are water-soluble flavonoid pigments that appear red to blue, according to pH.[41] Antocyanins might trap reactive oxygen in plasma and interstitial fluid of the arterial wall, hence preventing oxidation of LDL-C and acting as an antiatherogenic particle in the plants.[42]
In addition to antioxidant effects, the extract has anti-inflammatory effect, probably exerted by the flavonoid quercetin. The anti-inflammatory effect of Hypericum perforatum may be related to its inhibitory effect on primary proinflammatory transcriptional factors.[43,44] It has been shown that quercetin, apigenin and several other flavonoids prevent expression of intercellular adhesion molecule-1 (ICAM), vascular cell adhesion molecule-1 (VCAM) and E-selectin on endothelial cells and hence inhibition of lymphocytes adhesion to endothelial cells.[45] One of the major indicators of inflammation in the body is CRP. On the other hand, many now believe that there is a link between the inflammatory process and atherosclerosis.[6] After 2 months of administration of Hypericum perforatum extract in the present study, the rabbits fed with high cholesterol diet plus Hypericum perforatum extract showed significantly lower levels of CRP in comparison with the rabbits fed with high cholesterol diet, also when compared to the rabbits fed with high cholesterol diet plus lovastatin Histological study demonstrated that the length and thickness of atherosclerotic lesions in the group fed with high cholesterol diet plus Hypericum perforatum extract (Group III) decreased compared to high cholesterol diet group (Group IV) and group fed with high cholesterol diet plus lovastatin (V). Hypericum perforatum extract, like lovastatin, remarkably decreases the atherosclerotic lesions in aorta, compared to the high cholesterol group. In our study, the group fed with Hypericum perforatum extract had meaningfully less severe lesions than the control group or the group received high cholesterol diet plus lovastatin Our study showed that, hydroalcoholic extract of Hypericum perforatum in hypercholesterolemic rabbits might prevent even decrease atherosclerotic deposition despite continuation of high cholesterol diet, and this might be due to its ability to lower the levels of cholesterol, LDL-C, apoB, and TG as well as to slow lipid peroxidation process (lower Ox-LDL and MDA), and finally to enhance the inflammatory response of the endothelial cells. Further studies are needed to identify the exact components of Hypericum perforatum which are responsible for its hypolipidemic, anti oxidant and anti inflammatory actions.
As simvastatin is extensively metabolized by CYP3A4 and this plant is an inducer of this enzyme therefore Hypericum perforatum decreases plasma concentrations of simvastatin but not pravastatin.[46]
Further studies are needed to detect the active ingredients and their structure, to study the pharmacodymacis and pharmacokinetics of the extract and to approve the effect by arranging suitable clinical trials.
Acknowledgment
This work was supported by a grant (Grant No. 84143) from Isfahan Cardiovascular Research Center (ICRC) affiliated to Isfahan University of Medical Sciences and performed at ICRC after approval of Animal Ethics Committee.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Barnes J, Anderson LA, Phillipson JD. St John's wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53:583–600. doi: 10.1211/0022357011775910. [DOI] [PubMed] [Google Scholar]
- 2.Gerard J. The herbal or general history of plants: The complete 1633 edition as revised and enlarged by Thomas Johnson. First edition. New York: Dover Publications; 1975. 1595. [Google Scholar]
- 3.Ling WH, Wang LL, Ma J. Supplementation of the black rice outer layer fraction to rabbits decreases atherosclerotic plaque formation and increases antioxidant status. J Nutr. 2002;132:20–6. doi: 10.1093/jn/132.1.20. [DOI] [PubMed] [Google Scholar]
- 4.Zou Y, Lu Y, Wei D. Hypocholesterolemic effects of a flavonoid-rich extract of Hypericum perforatum L. in rats fed a cholesterol-rich diet. J Agric Food Chem. 2005;53:2462–6. doi: 10.1021/jf048469r. [DOI] [PubMed] [Google Scholar]
- 5.Shukla R, Gupta S, Gambhir JK, Prabhu KM, Murthy PS. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis in hypercholesterolaemic rabbits. J Ethnopharmacol. 2004;92:47–51. doi: 10.1016/j.jep.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Canto JG, Iskandrian AE. Major risk factors for cardiovascular disease: Debunking the “only 50%” myth. JAMA. 2003;290:947–9. doi: 10.1001/jama.290.7.947. [DOI] [PubMed] [Google Scholar]
- 8.Stulnig TM, Jurgens G, Chen Q, Moll D, Schonitzer D, Jarosch E, et al. Properties of low density lipoproteins relevant to oxidative modifications change paradoxically during aging. Atherosclerosis. 1996;126:85–94. doi: 10.1016/0021-9150(96)05896-0. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura YK, Read MH, Elias JW, Omaye ST. Oxidation of serum low-density lipoprotein (LDL) and antioxidant status in young and elderly humans. Arch Gerontol Geriatr. 2006;42:265–76. doi: 10.1016/j.archger.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet. 2001;358:2026–33. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 11.Elias-Scale SE, Kardys I, Oudkerk M, Hofman A, Witteman JC. C-reactive protein is related to extent and progression of coronary and extra-coronary results from the Rotterdam study. Atherosclerosis. 2007;195:e195–202. doi: 10.1016/j.atherosclerosis.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Hakimoğlu F, Kizil G, Kanay Z, Kizil M, Isi H. The effect of ethanol extract of hypericum lysimachioides on lipid profile in hypercholesterolemic rabbits and its in vitro antioxidant activity. Atherosclerosis. 2007;192:113–22. doi: 10.1016/j.atherosclerosis.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Blake GJ, Ridker PM. Are statins anti-inflammatory? Curr Control Trials Cardiovasc Med. 2000;1:161–5. doi: 10.1186/cvm-1-3-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eseyin O, Ebong P, Ekpo A, Igboasoiyi A, Oforah E. Hypoglycemic effect of the seed extract of Telfairia occidentalis in Rat. Pak J Biol Sci. 2007;10:498–501. doi: 10.3923/pjbs.2007.498.501. [DOI] [PubMed] [Google Scholar]
- 15.Petry RD, Ortega GG, Silva WB. Flavonoid content assay: Influence of the reagent concentration and reaction time on the spectrophotometric behavior of the aluminium chloride-flavonoid complex. Pharmazie. 2001;56:465–70. [PubMed] [Google Scholar]
- 16.Schutz K, Persike M, Carle R, Scriber A. Characterization and quantification of anthocyanins in selected artichoke (Cynara scolymus L.) cultivars by HPLC-DAD-ESI-MSn. Anal Bioanal Chem. 2006;384:1511–7. doi: 10.1007/s00216-006-0316-6. [DOI] [PubMed] [Google Scholar]
- 17.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 18.Mertz DP. “Atherosclerosis-index” (LDL/HDL): Risk indicator in lipid metabolism disorders. Med Klin. 1980;75:159–61. [PubMed] [Google Scholar]
- 19.Chekanov VS. Low frequency electrical impulses reduce atherosclerosis in cholesterol fed rabbits. Med Sci Monit. 2003;9:BR302–9. [PubMed] [Google Scholar]
- 20.Sacks FM. Why cholesterol as a central theme in coronary artery disease? Am J Cardiol. 1998;82:14T–17T. doi: 10.1016/s0002-9149(98)00717-6. [DOI] [PubMed] [Google Scholar]
- 21.Frohlich J, Lear SA. Old and new risk factors for atherosclerosis and development of treatment recommendations. Clin Exp Pharmacol Physiol. 2002;29:838–42. doi: 10.1046/j.1440-1681.2002.03733.x. [DOI] [PubMed] [Google Scholar]
- 22.Guenther E, Mendoza J, Crouch BI, Moyer-Mileur LJ, Junkins EP., Jr Differences in herbal and dietary supplement use in the hispanic and non-hispanic pediatric populations. Pediatr Emerg Care. 2005;21:507–14. doi: 10.1097/01.pec.0000173343.22777.a5. [DOI] [PubMed] [Google Scholar]
- 23.Gaut JP, Heinecke JW. Mechanisms for oxidizing low-density lipoprotein.Insights from patterns of oxidation products in the artery wall and from mouse models of atherosclerosis. Trends Cardiovasc Med. 2001;11:103–12. doi: 10.1016/s1050-1738(01)00101-3. [DOI] [PubMed] [Google Scholar]
- 24.Gugliucci A, Menini T. Three different pathways for human LDL oxidation are inhibited in vitro by water extracts of the medicinal herb Achyrocline satureoides. Life Sci. 2002;71:693–705. doi: 10.1016/s0024-3205(02)01734-4. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn K, McClain C, Carrier DJ, Wallace S, King J, Nagarajan S, et al. Effect of Albizia julibrissin water extracts on low-density lipoprotein oxidization. J Agric Food Chem. 2007;55:4704–9. doi: 10.1021/jf063458e. [DOI] [PubMed] [Google Scholar]
- 26.Laggner H, Schreier S, Hermann M, Exner M, Muhl A, Gmeiner BM, et al. The main components of St John's Wort inhibit low-density lipoprotein atherogenic modification: A beneficial “side effect” of an OTC antidepressant drug? Free Radic Res. 2007;41:234–41. doi: 10.1080/10715760600978831. [DOI] [PubMed] [Google Scholar]
- 27.Lapointe A, Couillard C, Lemieux S. Effects of dietary factors on oxidation of low – density lipoprotein particles. J Nutr Biochem. 2006;17:645–58. doi: 10.1016/j.jnutbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Andren L, Andreasson A, Eggertsen R. Interaction between a commercially available St.John's wort product (Movina) and atorvastatin in patients with hypercholesterolemia. Eur J Clin Pharmacol. 2007;63:913–6. doi: 10.1007/s00228-007-0345-x. [DOI] [PubMed] [Google Scholar]
- 29.Eggertsen R, Andreasson A, Andren L. Effects of treatment with a commercially available St John's Wort product (Movina(R)) on cholesterol levels in patients with hypercholesterolemia treated with simvastatin. Scand J Prim Health Care. 2007;25:1549. doi: 10.1080/02813430701442768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young SG. Recent progress in understanding apolipoprotein B. Circulation. 1990;82:1574–94. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]
- 31.Colvin PL, Parks JS. Metabolism of high density lipoprotein subfractions. Curr Opin Lipidol. 1999;10:309–14. doi: 10.1097/00041433-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Davignon J, Cohn JS. Triglycerides: A risk factor for coronary heart disease. Atherosclerosis. 1996;124(Suppl):S57–64. doi: 10.1016/0021-9150(96)05858-3. [DOI] [PubMed] [Google Scholar]
- 33.Prasad K, Kalra J. Experimental atherosclerosis and oxygen free radicals. Angiology. 1989;40:835–43. doi: 10.1177/000331978904000911. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan M, Aviram M. Oxidized low density lipoprotein: Atherogenic and proinflammatory characteristics during macrophage foam cell formation. An inhibitory role for nutritional antioxidants and serum paraoxonase. Clin Chem Lab Med. 1999;37:777–87. doi: 10.1515/CCLM.1999.118. [DOI] [PubMed] [Google Scholar]
- 35.Valenzuela A. The biological significance of malondialdehyde determination in the assessment of tissue oxidative stress. Life Sci. 1991;48:301–9. doi: 10.1016/0024-3205(91)90550-u. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 37.Zou Y, Lu Y, Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52:5032–9. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]
- 38.Tribble DL. AHA Science Advisory. Antioxidant consumption and risk of coronary heart disease: Emphasison vitamin C, vitamin E, and beta-carotene: A statement for healthcare professionals from the American Heart Association. Circulation. 1999;99:591–5. doi: 10.1161/01.cir.99.4.591. [DOI] [PubMed] [Google Scholar]
- 39.Kalt W, Forney CF, Martin A, Prior R. Antioxidant capacity, vitamin C, phenolics and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999;47:4638–44. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- 40.Taubert D, Berkels R, Klaus W, Roesen R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: Essential structural features. J Cardiovasc Pharmacol. 2002;40:701–13. doi: 10.1097/00005344-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Harborne JB, Williams CA. Anthocyanins and other flavonoids. Nat Prod Rep. 2001;18:310–33. doi: 10.1039/b006257j. [DOI] [PubMed] [Google Scholar]
- 42.Yamakoshi J, Kawaka S, Koga T, Arial T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1999;142:139–49. doi: 10.1016/s0021-9150(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 43.Tedeschi E, Menegazzi M, Margotto D, Suzuki H, Fortran U, Kleinert H. Anti-inflammatory actions of St. John's wort: Inhibition of human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha (STAT-1alpha) activation. J Pharmacol Exp Ther. 2003;307:254–61. doi: 10.1124/jpet.103.054460. [DOI] [PubMed] [Google Scholar]
- 44.Winkler C, Wirleitner B, Schroecksnadel K, Schennach H, Fuchs D. St.John's wort (Hypericum perforatum) counteracts cytokine-induced tryptophan catabolism in vitro. Biol Chem. 2004;385:1197–202. doi: 10.1515/BC.2004.155. [DOI] [PubMed] [Google Scholar]
- 45.Gerritsen ME, Carley WW, Ranges GE, Shen CP, Phan SA, Ligon GF, et al. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995;147:278–92. [PMC free article] [PubMed] [Google Scholar]
- 46.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, Kawaguchi A, Harada K, et al. Different effects of St john's wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther. 2001;70:518–24. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]


