Abstract
Background:
Neuroimmune factors have been proposed as contributors to the pathogenesis of depression. Beside other therapeutic effects including neuroprotective, antioxidant, anticonvulsant and analgesic effects, Nigella sativa and its main ingredient, thymoquinone (TQ), have been shown to have anti-inflammatory effects. In the present study, the effects of Nigella sativa hydro-alcoholic extract and thymoquinone was investigated on lipopolysaccharide- induced depression like behavior in rats.
Materials and Methods:
50 male Wistar rats were divided into 5 groups: Group 1 (control group) received saline instead of NS extract, thymoquinone or lipopolysaccharide. The animals in group 2 (lipopolysaccharide (LPS)) were treated by saline instead of NS extract and were injected LPS (100μg/kg, ip) 2 hours before conducting each forced swimming test. Groups 3 (LPS + NS 200) and 4 (LPS + NS 400) were treated by 200 and 400 mg/kg of NS (ip), respectively, from the day before starting the experiments and before each forced swimming test. These animals were also injected LPS 2hours before conducting each swimming test. The animals in group 5 received TQ instead of NS extract. Forced swimming test was performed 3 times for all groups (in alternative days), and immobility time was recorded. Finally, the animals were placed in an open- field apparatus, and the crossing number on peripheral and central areas was observed.
Results:
The immobility time in the LPS group was higher than that in the control group in all 3 times (P<0.001). The animals in LPS + NS 200, LPS + NS 400 and LPS + TQ had lower immobility times in comparison with LPS groups (P<0.01, and P<0.01). In the open- field test, the crossing number of peripheral in the LPS group was higher than that of the control one (P<0.01) while the animals of LPS + NS 200, LPS + NS 400 and LPS + TQ groups had lower crossing number of peripheral compared with the LPS group (P <0.05, and P<0.001). Furthermore, in the LPS group, the central crossing number was lower than that of the control group (P<0.01). In the animals treated by NS or TQ, the central crossing number was higher than that of the LPS group (P<0.05, and P<0.001).
Conclusions:
The results of the present study showed that hydro-alcoholic extract of Nigella sativa can prevent LPS-induced depression like behavior in rats. These results support the traditional belief on the beneficial effects of Nigella sativa in the nervous system. Moreover, further investigations are required in order to better understand this protective effect.
KEY WORDS: Depression, forced swimming test, lipopolysaccharide, Nigella sativa, open-field, thymoquinone
Depression, the second most common chronic disease, is expanding in the world while about half of the patients with depression are unaware of their disease, or their disease are diagnosed else.[1,2] Depression occurs in children, adolescents, adults and in elderly as a result of the combination of states of sadness, loneliness, irritability, absurdity, despair, confusion and shame and reveals some physical symptoms.[1] Fortunately, proper diagnosis and treatment can significantly reduce the symptoms of depression.[3] From among the depressive patients who have been treated by drugs or psychotherapeutic methods, about 5% and 15% had complete and partial recovery and between 20% and 35% did not respond at all.[3,4]
Neuroimmune factors have been proposed as contributors to the pathogenesis of depression.[5,6] It has been shown that increased levels of the inflammatory markers such as C-reactive (CRP), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-a) are associated with depression.[7,8] It has also been shown that antidepressant medications affect cytokine levels, and that this mechanism appears to influence the treatment outcome in depression.[9,10] Neuroinflammation, the process, which is accompanied by brain immune response and glial cell activation, has been shown to play an important role in depression.[11] Moreover, antidepressant agents have anti-neuroinflammatory properties.[11] In vivo studies using animal models have demonstrated that different types of antidepressants exert on the expression of inflammatory mediator, such as cytokines, microgliosis and astrogliosis in the nervous system. In vitro studies on rodent glial cells have also demonstrated that some types of antidepressants decrease glial generation of inflammatory molecules.[11] Considering these findings, an inhibition for neuroinflammation seems to be a new mechanism of action of antidepressant treatment.
NS L. (NS) is an annual flowering plant, native to different regions of southern Europe and some parts of Asia. The flowers are delicate and are usually colored pale blue and white with small black seeds.[12] NS seeds are the source of active components such as 30-40% fixed oil, 0.5-1.5% essential oil, various sugars and proteins and pharmacologically active components containing thymoquinone (TQ), ditimoquinone (DTQ) and nigellin.[12–17] In traditional medicine, this herb was indentified to have healing power so that it has been used in the middle east and far east for treating diseases such as asthma, headache, dysentery, infections, obesity, back pain, hypertension and gastrointestinal problems. Finally, there is a common Islamic opinion that the NS is useful for all diseases except death.[18] The results of previous experimental studies have supported the pharmacological effects of the seeds of NS or thymoquinone. It has been reported that the extract of NS seeds and thymoquinone inhibit nitric oxide production and inducible nitric oxide synthase expression.[12,19,20] The anti oxidant effects of NS and thymoquinone in CCl4-induced oxidative injury in rat liver,[21] isolated rat hepatocytes,[22] hypercholesterolemic rats[23] and gentamicin[24] and cyclosporine induced kidney injury have been reported as well.[24,25] Hosseinzadeh et al.[26] found that NS oil and thymoquinone have antioxidant effects during cerebral ischemia-reperfusion injury in rat hippocampus.[26] Anticancer activity was reported against malignant cells in mice and humans.[27,28] There is a line of evidence that NS and thymoquinone have anti-inflammatory activity and affect the immune system.[29,30]
Based on [the properties of NS, which has been reported in traditional medicine and in experimental studies, the present study was designed in order to evaluate possible effects of hydro-alcoholic extract of NS and thymoquinone on lipopolysaccharide-induced depression-like behavior in rats.
Materials and Methods
Preparing the plant extract
Powdered seeds (100 g) of NS were extracted in a Soxhlet extractor with ethanol (70%). The resulting extract was concentrated under reduced pressure and kept at -20°C until being used (yielded 32%). Nigella sativa extract was dissolved in saline.[31,32]
Animals and the experimental protocol
50 male Wistar rats (8 weeks old and weighted 230 ± 20 g) were kept at 22 ± 2°C and 12 h light/dark cycle at 7:00 am. They were randomly divided into 5 groups, and treated according to the experimental protocol for 1 week. All measurements were performed between 10 and 14 am.
Rats in group 1 (control group) received saline instead of both NS extract and thymoquinone from the day before starting the experiments. The animals of this group were injected saline instead of lipopolysaccharide as well. Group 2 (lipopolysaccharide; LPS) animals were injected by saline instead of NS extract and thymoquinone from the day before starting the experiments. The animals in this group were injected lipopolysaccharide (Sigma Chemical Co.) 2 hours before conducting the swimming test. Groups 3 (lipopolysaccharide + NS 200; LPS + NS 200) and 4 (lipopolysaccharide + NS 400; LPS + NS 400) animals were treated by 200 and 400 mg/kg of NS extract from the day before conducting the swimming test. The animals of this group were injected LPS (100 μg/kg) 2 hours before doing the swimming test. Group 5 (lipopolysaccharide + thymoquinone; LPS + TQ) animals were injected by thymoquinone (40 mg/kg) instead of NS extract. Forced swimming test (FST) was carried out 3 times for all the groups (in alternative days). Finally, the animals were placed in the open-field and were observed for 5 min, and the peripheral and central crossing number was recorded.
Behavioral procedures
Forced swimming test
All animals were compromised to the test room environment for 1 h before the beginning of the experiment. During the FST, the animals were placed in a glass cylindrical tank with 60 cm height and 38 cm width, which was filled with water (24 ± 1°C) to the depth of 40 cm. The water was changed between each animal. In the first day, the rats were placed inside the water cylinder for 15 min (pre-test), and then the animals were placed individually inside the water cylinder for 5 min for the following 3 alternative days (test days).[33] 2 hours before each test, the rats were injected with either LPS 100 μg/kg (LPS group, LPS + NS 200, LPS + NS 400 and LPS + TQ groups) or saline (control group).[34] The animals of LPS + NS 200, LPS + NS 400 and LPS + TQ groups were treated by 200 mg/kg NS, 400 mg/kg NS and 40 mg/kg thymoquinone from the day before doing the swimming test. The time of floating (immobility) during the FST was recorded for 5 min in each session.[35,36] Rats were considered immobile when they floated in the water; they only performed movements that enabled them to keep their head above the water. After the FST, the rats were dried in a heated cage, and then they were returned to their home cages.[37]
Open-field
Open-field test was carried out for studying the depression-like behaviors of animals after the first FST test.[38] In the present study, the open-field measurement was done by a wooden apparatus with an area of 100 × 100 cm and height of 40 cm. Inside of the apparatus was divided into 16 equal squares using a black line. In addition, within the apparatus was divided into 2 zones called peripheral and central zones.[39–41] All the rats were familiarized with the test environment by being placing in the room for 1-h before beginning the experiment. During the experiment, a low-level light was used to reduce anxiety.[37] Each animal was placed in the central zone, and its movement was recorded by a digital camera for 5 min,[39–42] and the following criteria were calculated: (1) The crossing number in the central zone, (2) the crossing number in the peripheral zone, and (3) rearing counts: The number of vertical movement when the rat stood vertically on its hind paws on the floor and forepaws on the wall.[37] 2 hours before each test, the rats were injected either LPS 100 μg/kg (LPS group, LPS + NS 200, LPS + NS 400 and LPS + TQ groups) or saline (control group).[34] The animals in LPS + NS 200, LPS + NS 400 and LPS + TQ groups were treated by 200 mg/kg NS, 400 mg /kg NS and 40 mg/kg thymoquinone 2 hours before the open-field tests.
Statistical analysis
The data were expressed as mean ± SEM. For the FST, repeated measured ANOVA, and for the data of open-field test, the one-way ANOVA were run, followed by tukey post hoc comparisons test. The criterion for the statistical significance was P < 0.05.
Results
The results of the FST showed that the immobility times in the control group were 13.13 ± 1.92, 10.74 ± 1.3 and 11.58 ± 2.21 sec in the 3 times, respectively. There was no significant difference between these 3 times. The animals in the LPS group had 39.68 ± 7.97, 51.73 ± 6.23 and 56.78 ± 8.11 sec immobility times. As shown in the figure, the immobility times increased in 3 days. The repeated measures ANOVA also showed that immobility times in the LPS group were higher than that of the control group (P < 0.001). In the LPS + NS 200 group, the immobility time was 17.3 ± 3.22, 15.34 ± 4.36 and 18.95 ± 3.66 sec, respectively. There was no significant difference between these 3 times. The comparison using the repeated measure ANOVA showed that the immobility times in the LPS + NS 200 group were lower than those that of the LPS group (P < 0.01); however, there was no significant difference between the LPS + NS 200 and control groups. In the LPS + NS 400 group, the 3 measured immobility times were 16.33 ± 2.50, 19.83 ± 3.56 and 14.64 ± 4.45 sec, respectively, and there was no significant difference between the 3 times. Immobility times were lower than those of the LPS group (P < 0.01) when being compared using the repeated measures ANOVA. In TQ + NS 400 group, the immobility times were 11.81 ± 1.94, 12.93 ± 1.59 and 13.65 ± 2.39 sec, respectively, and they were significantly lower than those of the LPS group (P < 0.001) [Figure 1].
Figure 1.
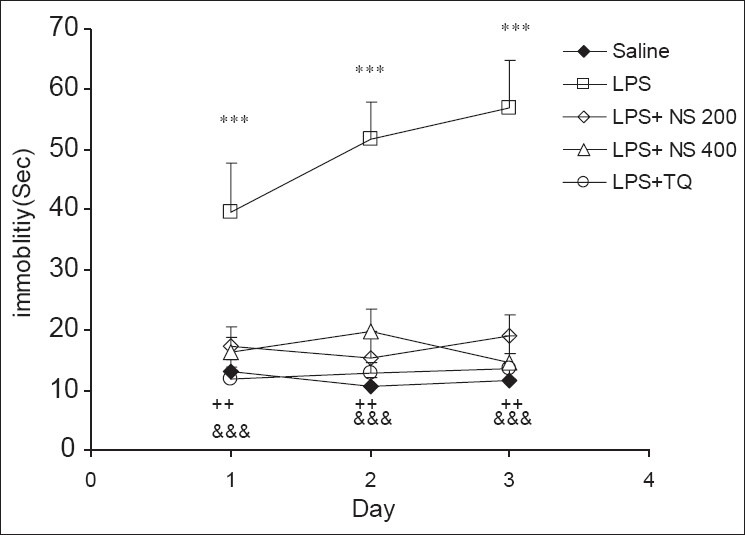
Comparison of immobility times in the forced swimming test between 5 groups. Data are expressed as mean ± SEM (N = 10 in each group). ***P < 0.001 comparison of LPS group with Saline group, ++P < 0.01 comparison of LPS + NS 200 and LPS + NS 400 groups with LPS group, &&&P < 0.001 comparison of LPS + TQ group with LPS group.
The results of the open-field test showed that the number of crossing in the peripheral zone in the LPS group was higher than that of the control group (P < 0.01). As shown in Figure 2, the crossing number of peripheral zone in LPS + NS 200 , LPS + NS 400 and LPS + TQ groups was significantly lower than that in the LPS group (P < 0.001, P < 0.05 and P < 0.001, respectively) [Figure 2].
Figure 2.
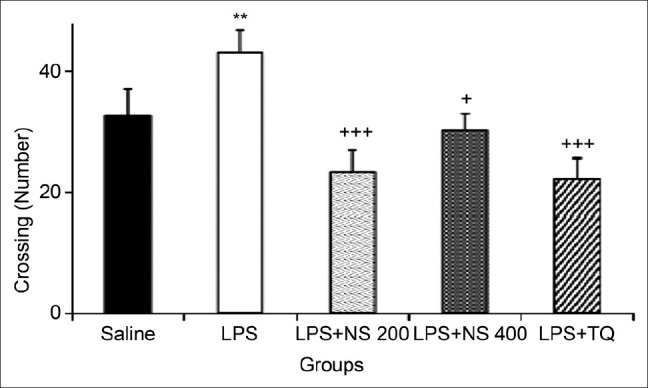
Comparison of the number of crossing in the peripheral zone in the open-field test. Data are expressed as mean ± SEM (N = 10 in each group). **P < 0.01 compared to Saline group, +P < 0.05 and +++P < 0.001 compared to LPS group.
The results also showed that the number of crossing in the central zone in the LPS group was lower than that in the control group (4.38 ± 0.47 vs. 9.5 ± 1.92), (P < 0.01). The crossing number in the central zone by the animals of LPS + NS 200, LPS + NS 400 and LPS + TQ groups was 7.37 ± 0.97, 8.33 ± 0.85 and 11.03 ± 1.31, respectively; they were significantly higher than those of the LPS group (P < 0.05, P < 0.01 and P < 0.001) [Figure 3].
Figure 3.
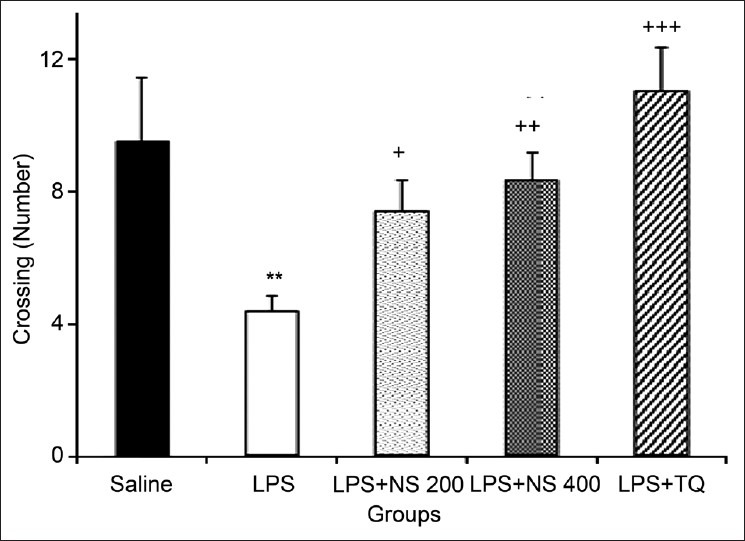
Comparison of the number of crossing in the central zone in the open-field test. Data are expressed as mean ± SEM (N = 10 in each group). **P < 0.01 compared to Saline group, +P < 0.05, ++P < 0.01 and +++P < 0.001 compared to LPS group
As shown in Figure 4, there was no significant difference between the groups in the rearing number.
Figure 4.
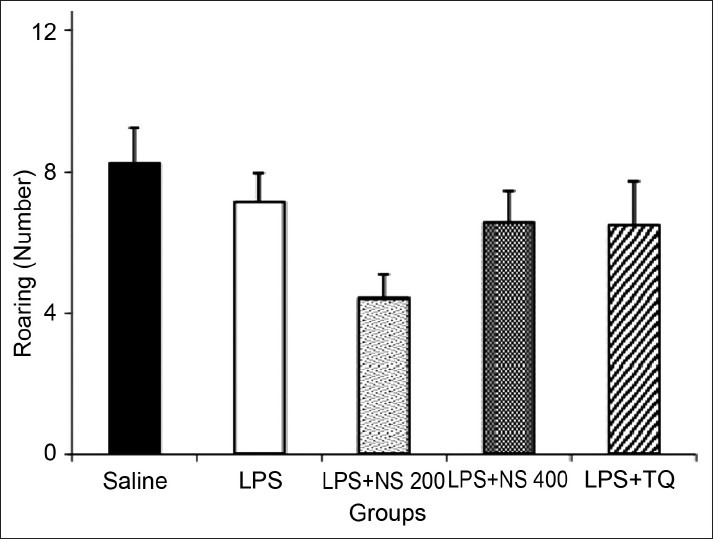
Comparison of the number of rearing in the open-field test. Data are expressed as mean ± SEM (N = 10 in each group)
Discussion
The results of the present study showed that treating of the animals by lipopolysaccharide induces a depression-like behavior in rats. Lipopolysaccharide is a fragment from the cell wall of gram-negative bacteria,[43] which triggers the production of IL-1β, interleukin-6 (IL-6), and TNF-α.[44,45] Both systemic and central administrations of LPS increase the levels of IL-1β and the related pro-inflammatory cytokines in several areas of the brain including the hippocampus, hypothalamus and diencephalic structures.[46,47] The result is a complex of behavioral changes, comparable with the cytokine-induced depression-like behavior in rats.[48] A large number of evidence has confirmed the role of inflammation in depression. Treatment with interleukin (IL)-2 or interferon (IFN)-γ in patients with cancer leads to high depression rates.[49,50] In the present study, the corresponding depressive symptoms were observed in lipopolysaccharide treated rats.[51,52] It has been previously reported that administration of LPS in doses, lower than the doses used in the present study, was resulted in avoidance of the central area of the open-field by arts.[53,54] It has also been shown that peripherally injection of LPS in doses, higher than doses which were used in the present study, induced sickness behavior including reduction in appetite and body weight, suppresses exploratory and social activity, fatigue and malaise, impairment in cognitive abilities, reduced libido and sexual behavior and anhedonia as well as depression like behaviors.[55–58] The results of the present study showed that immobility times in the LPS group were higher than those of the control group in the forced swimming test. It seems that peripherally administration of a medium dose of LPS also induce depressive like behavior. However, the effects of LPS on behavior has been repeatedly evaluated in a period lower than 24 h, it is likely that the injection of LPS in 3 times, which was carried out in the present study, resulted in a cumulative response because it has been previously reported that the effect of LPS may be seen even 28 days after injection.[53–78] The results of present study also showed that the crossing number in the central area of the open-field by the animals of the LPS group was lower than that of the control group, which may be another evidence for depression like behavior due to administration of LPS.
On the other hand, patients with depression were also found to exhibit all the cardinal features of inflammation.[49] Evidence suggests that various anti-inflammatory manipulations have antidepressant effects in experimental animals and humans. For example, genetic knockout of IL-6 in mice reduces depressive-like behavior in the forced swim, tail suspension, learned helplessness, and sucrose preference tests.[59] Knockout of the IL-1 receptor and administration of IL-1 receptor antagonist also blocks stress-induced depressive-like behavior in the sucrose preference, social exploration tests, escape deficits, anhedonia and reduces social behavior.[60–63]
Consistent with the pathophysiology of depression, these cytokine-induced behavioral changes are associated with alterations in the metabolism of serotonin, norepinephrine and dopamine in brain regions, which are essential to the regulation of emotion, including the limbic system (amygdala, hippocampus and nucleus accumbens), as well as the regulation of psychomotor function and reward, including the basal ganglia.[64,65]
The seed extract of NS has been used for many years as a natural remedy for treating of a number of illnesses and conditions due to its pharmacological properties and being an immunostimulant, anti-tumor, anti-inflammatory and respiratory stimulant agent.[18,28,66–68] The volatile oil is extracted from the seeds, and its main active ingredient, thymoquinone has been shown to exert anti-inflammatory effects in a number of diseases including bronchial asthma.[66,69–71] It has been shown that IL-4, IL-5, and IL-13 protein levels in the fluid of bronchial lavages were significantly reduced in allergic mice after TQ administration.[72]
TQ supplementation also could alleviate hepatic toxicity, induced by LPS in the form of normalization of GSH hepatic level and reduction of liver function parameters. Also, LPS-induced hepatic lipid peroxides formation (in the form of MDA) and apoptosis (indicated by hepatic caspase-3 activity) were markedly reduced in rats, which received TQ along with LPS. In addition, serum TNF-α and inflammatory changes in the liver section were markedly reduced in rats, which were co-administered TQ and LPS in comparison with the LPS-treated animals.[73] Tekeoglu et al. (2006) also reported that TQ had anti-inflammatory effects on experimentally-induced arthritis in rats and decreased the levels of TNF-a and IL-1β in circulation.[74] Similarly, Mohamed et al. (2005) showed that treatment of rats with thymoquinone prevented the experimental autoimmune encephalomyelitis,[75] which was probably due to its antioxidant and anti-inflammatory properties.[76] The results of the present study also showed that pretreatment by both NS extract and thymoquinone prevented depression-like behavior induced by LPS in rats. Based on the results of present study, the mechanism(s) for the effect of NS extract or thymoquinone is unknown, but it seems that they can affect depression like behavior induced by LPS with common or different mechanisms.
Besides thymoquinone, the main ingredient, which has an important role in pharmacological effects of NS, other component(s) may also be involved in the results of the present study. More recently, melanin has been shown to occur abundantly in the seed coats of NS.[77] On the other hand, according to a number of botanical sources, melanin has been found to act as an immunologically active modulator of cytokines.[78,79] Therefore, an involvement of this ingredient of NS, which modulates cytokine production,[77] may also be suggested as a possible compound, which has a role in the result of the present study.
Conclusion
The results of the present study showed that the hydro-alcoholic extract of NS prevented lipopolysaccharide-induced depression like behavior in rats. These results support the traditional belief about the beneficial effect of NS on the nervous system. Further studies are required for determining (confirming) the protective effect of NS.
Acknowledgments
Authors would like to thank the Vice President of Research in Mashhad University of Medical Sciences for the financial supports.
Footnotes
Source of Support: Vice President of Research in Mashhad University of Medical Sciences
Conflict of Interest: None declared.
References
- 1.Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001–8. [PubMed] [Google Scholar]
- 2.Spitzer RL, Kroenke K, Linzer M, Hahn SR, Williams JB, deGruy FV, 3rd, et al. Health-related quality of life in primary care patients with mental disorders. Results from the PRIME-MD 1000 Study. JAMA. 1995;274:1511–7. [PubMed] [Google Scholar]
- 3.Coulehan JL, Schulberg HC, Block MR, Madonia MJ, Rodriguez E. Treating depressed primary care patients improves their physical, mental, and social functioning. Arch Intern Med. 1997;157:1113–20. [PubMed] [Google Scholar]
- 4.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: Leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 7.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 9.Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol. 2010;25:201–15. doi: 10.1002/hup.1103. [DOI] [PubMed] [Google Scholar]
- 10.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashioka S. Antidepressants and Neuroinflammation: Can Antidepressants Calm Glial Rage Down? Mini Rev Med Chem. 2011;11:555–64. doi: 10.2174/138955711795906888. [DOI] [PubMed] [Google Scholar]
- 12.Boskabady MH, Shirmohammadi B, Jandaghi P, Kiani S. Possible mechanism(s) for relaxant effect of aqueous and macerated extracts from Nigella sativa on tracheal chains of guinea pig. BMC Pharmacol. 2004;4:3. doi: 10.1186/1471-2210-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boskabady MH, Farhadi J. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: A randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14:1137–44. doi: 10.1089/acm.2008.0049. [DOI] [PubMed] [Google Scholar]
- 14.Boskabady MH, Javan H, Sajady M, Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21:559–66. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 15.Boskabady MH, Keyhanmanesh R, Saadatloo MA. Relaxant effects of different fractions from Nigella sativa L. on guinea pig tracheal chains and its possible mechanism(s) Indian J Exp Biol. 2008;46:805–10. [PubMed] [Google Scholar]
- 16.Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17:707–13. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, Shafei MN, Parsaee H. Effects of aqueous and macerated extracts from Nigella sativa on guinea pig isolated heart activity. Pharmazie. 2005;60:943–8. [PubMed] [Google Scholar]
- 18.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 19.El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N, et al. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2:1603–11. doi: 10.1016/s1567-5769(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 20.Mahmood MS, Gilani AH, Khwaja A, Rashid A, Ashfaq MK. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res. 2003;17:921–4. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- 21.Kanter M, Coskun O, Uysal H. The antioxidative and antihistaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80:217–24. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 22.Mansour MA, Ginawi OT, El-Hadiyah T, El-Khatib AS, Al-Shabanah OA, Al-Sawaf HA. Effects of volatile oil constituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: Evidence for antioxidant effects of thymoquinone. Res Commun Mol Pathol Pharmacol. 2001;110:239–51. [PubMed] [Google Scholar]
- 23.Ismail M, Al-Naqeep G, Chan KW. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010;48:664–72. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Yaman I, Balikci E. Protective effects of nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–90. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Uz E, Bayrak O, Kaya A, Bayrak R, Uz B, Turgut FH, et al. Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: An experimental model. Am J Nephrol. 2008;28:517–22. doi: 10.1159/000114004. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–7. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Salomi NJ, Nair SC, Jayawardhanan KK, Varghese CD, Panikkar KR. Antitumour principles from Nigella sativa seeds. Cancer Lett. 1992;63:41–6. doi: 10.1016/0304-3835(92)90087-c. [DOI] [PubMed] [Google Scholar]
- 28.Worthen DR, Ghosheh OA, Crooks PA. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998;18:1527–32. [PubMed] [Google Scholar]
- 29.I ik H, Cevikbaş A, Gurer US, Kiran B, Uresin Y. Potential adjuvant effects of Nigella sativa seeds to improve specific immunotherapy in allergic rhinitis patients. Med Princ Pract. 2010;19:206–11. doi: 10.1159/000285289. [DOI] [PubMed] [Google Scholar]
- 30.Winkler C, Schroecksnadel K, Ledochowski M, Schennach H, Houcher B, Fuchs D. In vitro Effects of Nigella sativa Seeds Extracts on Stimulated Peripheral Blood Mononuclear Cells. In vitro. 2008;19:101–6. [Google Scholar]
- 31.Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30:591–8. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rakhshandah H, Hosseini M. Potentiation of pentobarbital hypnosis by Rosa damascena in mice. Indian J Exp Biol. 2006;44:910–2. [PubMed] [Google Scholar]
- 33.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 34.Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–34. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 35.Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience. 2004;126:849–57. doi: 10.1016/j.neuroscience.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 36.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–32. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Pitychoutis PM, Nakamura K, Tsonis PA, Papadopoulou-Daifoti Z. Neurochemical and behavioral alterations in an inflammatory model of depression: Sex differences exposed. Neuroscience. 2009;159:1216–32. doi: 10.1016/j.neuroscience.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 38.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: Implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–51. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 39.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–74. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 40.Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol Biochem Behav. 2003;74:433–47. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- 41.Engeland CG, Kavaliers M, Ossenkopp KP. Influence of the estrous cycle on tolerance development to LPS-induced sickness behaviors in rats. Psychoneuroendocrinology. 2006;31:510–25. doi: 10.1016/j.psyneuen.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Boguszewski P, Zagrodzka J. Emotional changes related to age in rats–a behavioral analysis. Behav Brain Res. 2002;133:323–32. doi: 10.1016/s0166-4328(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 43.Kozak W, Conn CA, Kluger MJ. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;266:R125–35. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 45.Kant GJ, Meyerhoff JL, Jarrard LE. Biochemical indices of reactivity and habituation in rats with hippocampal lesions. Pharmacol Biochem Behav. 1984;20:793–7. doi: 10.1016/0091-3057(84)90201-6. [DOI] [PubMed] [Google Scholar]
- 46.Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49:125–34. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 47.Takao T, Culp SG, De Souza EB. Reciprocal modulation of interleukin-1 beta (IL-1 beta) and IL-1 receptors by lipopolysaccharide (endotoxin) treatment in the mouse brain-endocrine-immune axis. Endocrinology. 1993;132:1497–504. doi: 10.1210/endo.132.4.8462448. [DOI] [PubMed] [Google Scholar]
- 48.Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–20. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- 49.Miller AH. Depression and immunity: A role for T cells? Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piser TM. Linking the cytokine and neurocircuitry hypotheses of depression: A translational framework for discovery and development of novel anti-depressants. Brain Behav Immun. 2010;24:515–24. doi: 10.1016/j.bbi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Abdulla D, Renton KW. Beta-adrenergic receptor modulation of the LPS-mediated depression in CYP1A activity in astrocytes. Biochem Pharmacol. 2005;69:741–50. doi: 10.1016/j.bcp.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86:651–9. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Layé S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, et al. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–8. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- 55.Kang A, Hao H, Zheng X, Liang Y, Xie Y, Xie T, et al. Peripheral anti-inflammatory effects explain the ginsenosides paradox between poor brain distribution and anti-depression efficacy. J Neuroinflammation. 2011;8:100. doi: 10.1186/1742-2094-8-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enayati M, Solati J, Hosseini MH, Shahi HR, Saki G, Salari AA. Maternal infection during late pregnancy increases anxiety-and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 2011;10(87(2-3)):295–302. doi: 10.1016/j.brainresbull.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Park SE, Lawson M, Dantzer R, Kelley KW, McCusker RH. Insulin-like growth factor-I peptides act centrally to decrease depression-like behavior of mice treated intraperitoneally with lipopolysaccharide. J Neuroinflammation. 2011;8:179. doi: 10.1186/1742-2094-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sah SP, Tirkey N, Kuhad A, Chopra K. Effect of quercetin on lipopolysaccharide induced-sickness behavior and oxidative stress in rats. Indian J Pharmacol. 2011;43:192–6. doi: 10.4103/0253-7613.77365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–94. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–82. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- 61.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–28. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 62.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arakawa H, Blandino P, Jr, Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiol Behav. 2009;98:139–46. doi: 10.1016/j.physbeh.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson's disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 65.Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–27. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- 66.Houghton PJ, Zarka R, de las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–6. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 67.Swamy SM, Tan BK. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70:1–7. doi: 10.1016/s0378-8741(98)00241-4. [DOI] [PubMed] [Google Scholar]
- 68.el Tahir KE, Ashour MM, al-Harbi MM. The respiratory effects of the volatile oil of the black seed (Nigella sativa) in guinea-pigs: Elucidation of the mechanism(s) of action. Gen Pharmacol. 1993;24:1115–22. doi: 10.1016/0306-3623(93)90358-5. [DOI] [PubMed] [Google Scholar]
- 69.Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17:1209–14. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 70.Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother Res. 2004;18:195–9. doi: 10.1002/ptr.1390. [DOI] [PubMed] [Google Scholar]
- 71.Mahgoub AA. Thymoquinone protects against experimental colitis in rats. Toxicol Lett. 2003;143:133–43. doi: 10.1016/s0378-4274(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 72.El Gazzar M, El Mezayen R, Nicolls MR, Marecki JC, Dreskin SC. Downregulation of leukotriene biosynthesis by thymoquinone attenuates airway inflammation in a mouse model of allergic asthma. Biochim Biophys Acta. 2006;1760:1088–95. doi: 10.1016/j.bbagen.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Helal GK. Thymoquinone supplementation ameliorates acute endotoxemia-induced liver dysfunction in rats. Pak J Pharm Sci. 2010;23:131–7. [PubMed] [Google Scholar]
- 74.Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21:895–7. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- 75.Mohamed A, Afridi DM, Garani O, Tucci M. Thymoquinone inhibits the activation of NF-kappaB in the brain and spinal cord of experimental autoimmune encephalomyelitis. Biomed Sci Instrum. 2005;41:388–93. [PubMed] [Google Scholar]
- 76.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 77.El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A. Herbal melanin modulates tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine. 2006;13:324–33. doi: 10.1016/j.phymed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Avramidis N, Kourounakis A, Hadjipetrou L, Senchuk V. Antiinflammatory and immunomodulating properties of grape melanin: Inhibitory effects on paw edema and adjuvant induced disease. Arzneimittelforschung. 1998;48:764–71. [PubMed] [Google Scholar]
- 79.Mohagheghpour N, Waleh N, Garger SJ, Dousman L, Grill LK, Tusé D. Synthetic Melanin Suppresses Production of Proinflammatory Cytokines. Cell Immunol. 2000;199:25–36. doi: 10.1006/cimm.1999.1599. [DOI] [PubMed] [Google Scholar]


