Abstract
Introduction:
Schiff bases and azetidinones form an important structural class possessing wide spectrum of biological activities that include antibacterial and antifungal activity. A series of Schiff's bases N’-(substituted benzylidene)-2-(naphthalen-1-yloxy) acetohydrazides (3a-f) and azetidinones N-[3-chloro-2-oxo-4-(substituted phenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamides (4a-b) were synthesized and tested for antimicrobial activity.
Materials and Methods:
The chemical structures of synthesized compounds were elucidated on the basis of IR and 1H NMR spectroscopy. The synthesized compounds were screened for antibacterial activity against E. coli (ESS 2231) and B. subtilis (MTCC 441). The compounds were also tested for antifungal activity against A. niger (NCIM 618) and C. albicans (NCIM 3557) by the cup diffusion method.
Results and Discussion:
The in vitro antimicrobial activity results showed that the N-[3-chloro-2-oxo-4-(4-substitutedphenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamides (4a-b) exhibited better antibacterial activity than the synthesized N′-(substituted benzylidene)-2-(naphthalen-1-yloxy)-acetohydrazides (3a-f). Compound (4b) displayed potent antibacterial activity against the B. subtilis and E. coli (MIC values of 16-64 μg/mL). The antifungal activity of the synthesized compounds (3a-f and 4a-b) against the A. niger and C. albicans was relatively weak, most of the compounds showed poor activities (MIC >128μg/mL).
Conclusion:
The antibacterial activity of the synthesized compounds was moderate to low and antifungal activity was relatively weak. Therefore, a further study with this class of compounds is necessary to elucidate the mechanism and structure activity relationship.
KEY WORDS: Antibacterial, antifungal, azetidinone, naphthalene, Schiff's bases
The incidence of fungal and bacterial infections has increased dramatically in recent years.[1] Emerging infectious diseases and the increasing numbers of multidrug resistant microbial pathogens still make the treatment of infectious diseases an important and challenging problem.[2] In order to overcome this rapid development of drug resistance, new agents showed preferably consist of chemical characteristics that clearly differ from those of existing agents. Schiff bases and azetidinones form an important structural class possessing wide spectrum of biological activities that include antibacterial and antifungal activity.[3–6] In the last few years, naphthalene derivatives have been of great interest in synthetic organic chemistry for their role in antimicrobial and antifungal activity.[7–11] In view of these observations, we herein report the synthesis and biological evaluation of some new Schiff bases and azetidinones of 1-naphthol.
Materials and Methods
Melting points of the synthesized compounds were determined by open capillary method and were uncorrected. The IR spectra of synthesized compounds were recorded in potassium bromide discs on Schimadzu FTIR Spectrophotometer 8300. The 1H NMR spectra of the synthesized compounds were recorded in DMSO-d6 using AV-300 BROKER JEOL Spectrophotometer and tetramethylsilane (TMS) as an internal standard. The signals are quoted as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; bs, broad singlet and are expressed in δ ppm. All reagents were of commercial quality and were used without further purification. The reactions progress was monitored by thin-layer chromatography (TLC) using silica gel G and spots were visualized with iodine.
Chemistry
Synthesis of ethyl-2-(naphthalen-1-yloxy) acetate (1)
A mixture of 1-naphthol (14.4 g, 0.10 mol), ethylchloroacetate (10.7 ml, 0.10 mol) and anhydrous K2CO3 (13.8 g, 0.10 mol) in dry acetone was refluxed on a water bath for 36 h. The mixture was then filtered and solvent was removed under reduced pressure. The residue was washed with 5% sodium hydroxide solution for the removal of traces of 1-naphthol. The resulting residue was washed with water, dried and recrystallized from ethanol to afford ethyl-2-(naphthalen-1-yloxy) acetate (1). Yield: 16.2 g (70.43%); mp: 46-48°C; IR (KBr, cm−1 ): 3012 (CH str. aromatic) 2955 (CH str. aliphatic), 1643 (C=O str.), 1043 (C–O–C str.). 1H NMR (DMSO-d6, δ ppm): 7.80 – 6.06 (m, 7H, Ar–H), 4.73 (s, 2H, -OCH2CO), 4.16 (q, 2H, –CH2), 1.35 (t, 3H, CH3).
Synthesis of 2-(naphthalen-1-yloxy) acetohydrazide (2)
A mixture of 1 (2.3 g, 0.01mol) and hydrazine hydrate (0.98 ml, 0.02 mol) in methanol was refluxed on a water bath for 9 h. After cooling, the solid that separated was washed with water, dried to give crude product. Pure and needle shaped crystals of 2-(naphthalen-1-yloxy) acetohydrazide (2) were obtained after recrystallization from ethanol. Yield: 1.81 g (83.7%); mp: 146-148°C; IR (KBr, cm−1): 3209 (NH str.), 3002 (CH str. aromatic), 2951 (CH str. aliphatic), 1623 (C=O str.), 1518 (NH bend.), 1465 (CH bend.), 1254–1183 (C–N str.), 1050 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 8.43 (s, 1H, NH), 7.68–6.16 (m, 7H, Ar–H), 4.69 (s, 2H, NH2), 4.23 (s, 2H, –CH2).
General procedure for the synthesis of various N′(substituted benzylidene)-2-(naphthalen-1-yloxy) acetohydrazides (3a-f)
A mixture of (2) (2.16 g, 0.01 mol), appropriate aromatic aldehydes (0.01mol) and glacial acetic acid (1 ml) in methanol (50 ml), was refluxed on water bath for 6–10 h. After cooling, the solvent was removed under reduced pressure to give crude product. The product was recrystallized from ethanol to afford N’(substituted benzylidene)-2-(naphthalen-1-yloxy) acetohydrazides (3a-f).
N’-Benzylidene-2-(naphthalen-1-yloxy) acetohydrazide (3a)
Yield: 2.39 g (79%); mp: 177–179°C; IR (KBr, cm−1): 3343 and 3191 (NH str.), 3059 (CH str. aromatic), 2856 (CH str. aliphatic), 2256 (C=N str.), 1690 (C=O str.), 1076 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.68 (s, 1H, NH), 8.03–6.8 (m, 12H, Ar–H), 5.35 (s, 1H, =CH), 4.86 (s, 2H, CH2).
N’-(2-Chlorobenzylidene)-2-(naphthalen-1-yloxy) acetohydrazide (3b)
Yield: 2.92 g (86%); mp: 154–156°C; IR (KBr, cm−1): 3362 and 3191(NH str.), 3048 (CH str. aromatic), 2954 (CH str. aliphatic), 2256 (C=N str.), 1653 (C=O str.), 1090 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.88 (s, 1H, NH), 8.04–6.90 (m, 11H, Ar–H), 5.37 (s, 1H, =CH), 4.88 (s, 2H, CH2).
N’-(3-Hydroxy-4-methoxybenzylidene)-2-(naphthalen-1-yloxy) acetohydrazide (3c)
Yield: 3.0 g (85%); mp: 152–154°C; IR (KBr, cm−1): 3396 and 3173 (NH str.), 3070 (CH str. aromatic), 2968 (CH str. aliphatic), 2265 (C=N str.), 1653 (C=O str.), 1036 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.50 (s, 1H, NH), 9.31 (bs, 1H, OH), 8.19–6.86 (m, 10H, Ar–H), 5.31 (s, 1H, =CH), 4.84 (s, 2H, CH2), 3.79 (s, 3H, OCH3).
N’-(4-(Dimethylamino) benzylidene)-2-(naphthalen-1-yloxy) acetohydrazide (3d)
Yield: 2.82 g (81%); mp: 160–162°C; IR (KBr, cm−1): 3453 and 3195 (NH str.), 3051 (CH str. aromatic), 2927 (CH str. aliphatic), 2235 (C=N str.), 1694 (C=O str.), 1070 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.38 (s, 1H, NH), 8.18-6.71 (m, 11H, Ar–H), 5.30 (s, 1H, =CH), 4.81 (s, 2H, CH2), 3.34 (s, 6H, N(CH3)2).
N’-(4-Nitrobenzylidene)-2-(naphthalen-1-yloxy) acetohydrazide (3e)
Yield: 3.2 g (90%); mp: 193–195°C; IR (KBr, cm−1): 3410 and 3200 (NH str.), 3091 (CH str. aromatic), 2969 (CH str. aliphatic), 2237 (C=N str.), 1698 (C=O str.), 1108 (C–O–C str.); 1H NMR (DMSO-d6 , δ ppm): 11.96 (s, 1H, NH), 8.12-6.91 (m, 11H, Ar-H), 5.39 (s, 1H, =CH), 4.91 (s, 2H, CH2).
N’-(3-Hydroxybenzylidene)-2-(naphthalen-1-yloxy) acetohydrazide (3f)
Yield: 2.8 g (87%); mp: 198–200°C; IR (KBr, cm−1): 3410 and 3195 (NH str.), 3001 (CH str. aromatic), 2963 (CH str. aliphatic), 2242 (C=N str.), 1658 (C=O str.), 1020 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.61 (s, 1H, NH), 9.61(s, 1H, OH) 8.24–6.81 (m, 11H, Ar–H), 5.33 (s, 1H, =CH), 4.85 (s, 2H, CH2).
General procedure for the synthesis of N-[3-chloro-2-oxo-4-(substituted phenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamides (4a-b)
N’-(benzylidene)-2-(naphthalen-1-yloxy) acetohydrazide 3a (1.76 g, 5 mmol) were dissolved in 1, 4-dioxane (100 ml) with constant stirring at room temperature. Triethylamine (1.39 ml, 0.01 mol) was added slowly followed by drop wise addition of chloroacetyl chloride (0.4 ml, 5 mmol). Stirring was continued for further 30 min. Thereafter, the reaction mixture was refluxed for 8 h. The mixture was allowed to cool at room temperature and filtered to remove insoluble salt. Excess of the solvent was removed and the obtained residue was washed with water, dried and recrystallized from ethanol to afford N-[3-chloro-2-oxo-4-(substituted phenyl)-azetidin-1-yl]-2-(naphthalene-1-yloxy) acetamides (4a-b).
N-(3-Chloro-2-oxo-4-phenylazetidin-1-yl)-2-(naphthalen-1-yloxy) acetamide (4a)
Yield: 0.71 g (38%); mp: 80–82°C; IR (KBr, cm−1): 3353 and 3211(NH str.), 3070 (CH str. aromatic), 2970 (CH str. aliphatic), 1717 (C=O str. in β lactam), 1658 (C=O str. in amide), 1339 (C-N str.), 1078 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.95 (s, 1H, NH), 8.74-6.85 (m, 12H, ArH), 5.33 (d, 1H, CHCl), 4.90 (s, 2H, CH2), 4.68 (s, 1H, CH).
N-(3-Chloro-2-(4-nitrophenyl)-4-oxoazetidin-1-yl)-2-(naphthalen-1-yloxy) acetamide (4b)
Yield: 0.82 g (38 %); mp: 92-94°C; IR (KBr, cm−1): 3325 and 3205 (NH str.), 3082 (CH str. aromatic), 2952 (CH str. aliphatic), 1696 (C=O str. in β lactam), 1647 (C=O str. in amide), 1339 (C-N str.), 1079 (C–O–C str.); 1H NMR (DMSO-d6, δ ppm): 11.65 (s, 1H, NH), 8.32-6.20 (m, 11H, ArH), 5.28 (d, 1H, CHCl), 4.80 (s, 2H, CH2 ), 4.72 (s, 1H, CH).
Antimicrobial Activity
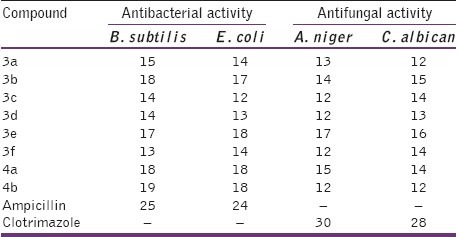
The antibacterial and antifungal activity was performed by the cup diffusion method. The synthesized compounds, as 1 mg/ ml solutions in dimethylformamide (DMF), were evaluated in vitro for activity against E. coli (ESS 2231), B. subtilis (MTCC 441), A. niger (NCIM 618), and C. albican (NCIM 3557). Compounds showing inhibitory zones of at least 18 mm were considered active and were further evaluated for their minimal inhibitory concentration (MIC) using the twofold serial dilution method.[12] Sterile nutrient agar was inoculated with the test organisms (each 100 ml of the medium received 1 ml of 24 h broth culture), and then seeded agar was poured into sterile petri dishes. Cups (8 mm in diameter) were cut in the agar, and each cup received 0.1 ml of the test compound solution. The plates were then incubated at 37°C for 24 h. The activities were estimated as zones of inhibition in mm diameter [Table 1]. An ampicillin solution (5 μg/ml) as antibacterial and a clotrimazole solution (0.01%) as antifungal were used as reference standards. Dimethylformamide was used as a control and it did not show any inhibition zones.
Table 1.
Zone of inhibition (in mm) against the microbes by the title compounds (3a-f and 4a-b)
Minimum inhibitory concentration measurement
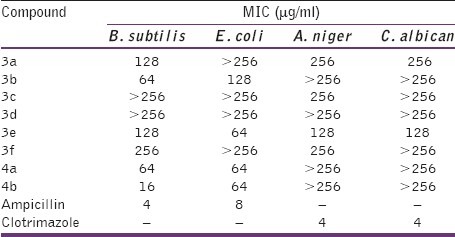
Using the twofold serial dilution method, the test organisms were grown in suitable broth for 24 h for bacteria and 48 h for fungi at 37°C. Twofold serial dilutions of the test compound solutions were prepared using the suitable broth to obtain concentrations between 1000 and 16 μg/ml. The tubes were then inoculated with the test organism (each 5 ml received 0.1 ml of the above inoculums) and were incubated at 37°C for 48 h. The tubes were then observed for the presence or absence of microbial growth. The lowest concentration showing no growth was taken as the minimum inhibitory concentration. The Minimum inhibitory concentration (MIC) values of the prepared compounds are listed in Table 2.
Table 2.
Minimum inhibitory concentration of title compounds (3a-f and 4a-b) against microbes
Results and Discussion
Chemistry
The synthetic route is outlined in Scheme 1. Ethyl-2-(naphthalen-1-yloxy) acetate (1) was synthesized by refluxing 1-naphthol with ethylchloroacetate in dry acetone. Compound 1 on reacting with hydrazine hydrate gave 2-(naphthalen-1-yloxy)-acetohydrazide (2). Condensation of 2 with various aromatic aldehydes afforded the Schiff bases N’-(substituted benzylidene) -2-(naphthalen-1-yloxy) acetohydrazides (3a-f), which on cyclocondensation with chloroacetylchloride in the presence of triethylamine in dioxane yielded N-[3-chloro-2-oxo-4-(substituted phenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamides (4a-b). The purity of the compounds was established by their sharp melting point and single spot in TLC whereas the structures of all compounds were characterized by spectral analysis. The absence of –OH stretching in the IR spectra of 1 and appearance of peaks at 1643, assigned to CO stretching and 1043 cm−1 assigned to C–O–C stretching. In 1H NMR spectra, the presence of absorbance peaks at 4.73, 4.16, and 1.35 δ ppm attributed to –OCH2CO, –CH2, and –CH3 protons respectively, indicated the formation of the compound 1. The presence of peaks at 1254 and 1518 cm−1 assigned to –CN stretching in amide and –NH bending of –NH2 in the IR spectra, and presence of absorbance peaks at 8.43 and 4.69 δ ppm attributed to the amide –NH, and –NH2 protons, respectively, in the 1H NMR spectra of compound 2 confirmed its structure. The presence of singlet absorbance peaks in the range of 11.96–11.38 and 5.39–5.30 δ ppm attributed to –NH, =CH indicated the formation of various N’-(substituted benzylidene)-2-(naphthalen-1-yloxy)-acetohydrazides (3a-f). The singlet absorbance peak in the range of 11.95–11.65 and 5.33–5.28 δ ppm attributed to –NH and –CHCl in 1H NMR spectra of (4a-b) confirmed their structures.
Scheme 1.
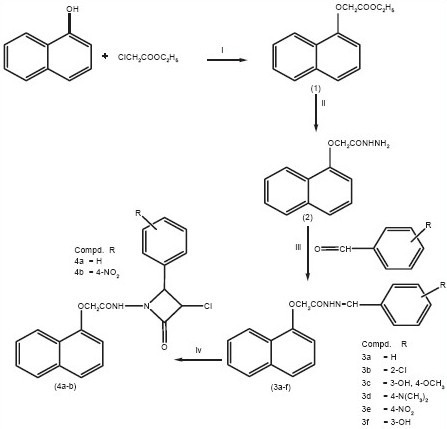
Synthesis of the target compounds. Reagents and condition: (i) Anhydrous K2CO3, CH3COCH3, Reflux (ii) NH2NH2, CH3OH, Reflux (iii) CH3OH, Glacial acetic acid, Reflux (iv) Dioxane, ClCH2COCl, Triethylamine, Reflux
In vitro antimicrobial activity
In the present study, a total of eight derivatives (3a-f and 4a-b) were evaluated for their in vitro antibacterial and antifungal activity against microbial strains. The results in Tables 1 and 2 revealed that the majority of the synthesized compounds showed variable inhibition activities against the tested bacterial and fungal strains. Compound N-[3-chloro-2-oxo-4-(4-nitrophenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamide (4b) displayed the highest antibacterial activity with a MIC value of 16 μg/ml against Gram positive bacteria B. subtilis and MIC value of 64 μg/ml against the Gram negative E. coil. The antibacterial potency shown by the most potent compound in the series synthesized is however not comparable with that of the standard drug ampicillin. Since the antibacterial activity ranged from moderate to low further study is necessary to elucidate the mechanism and structure activity relationship.
From Table 2, it could be observed that in general, N-[3-chloro-2-oxo-4-(substituted phenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamides exhibited better antibacterial activity than N’-(substituted benzylidene)-2-(naphthalen-1-yloxy)-acetohydrazides, indicating the importance of beta-lactam moiety in enhancing the antibacterial activity. It was interesting to point out that the compounds 3b and 3e bearing chloro and nitro moiety in the phenyl ring were mostly endowed with higher antimicrobial activities with respect to other substituents, proving that these are more favorable for the antimicrobial activities. However, introduction of group such as hydroxy and the dimethylamino to the phenyl ring (3c, 3f, and 3d) caused the appreciable decrease in inhibitory activity, which suggested that the polar (hydroxyl) and dimethylamino groups on the phenyl ring were unfavorable for antibacterial activity. The antifungal evaluation of the synthesized compounds showed that the activities were relatively weak compared to the antibacterial activities. Unfortunately, most of the compounds showed poor or no obvious activities (MIC>128 μg/ml) against the tested antifungal strains. Compound N′-(3-hydroxy-4-methoxybenzylidene)-2-(naphthalen-1-yloxy)-acetohydrazide (3e) showed moderate antifungal activity with MIC values of 128 μg/ml against A. niger and C. albican respectively.
Conclusion
A series of 1-naphthol derivatives have been synthesized and evaluated for their antibacterial and antifungal activity. All new compounds were characterized by physicochemical and spectral methods. In general, the results revealed that N-[3-chloro-2-oxo-4-(4-substitutedphenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy) acetamides (4a-b) exhibited better antibacterial activity than the synthesized N’-(substituted benzylidene)-2-(naphthalen-1-yloxy)-acetohydrazides (3a-f). Compound N-[3-chloro-2-oxo-4-(4-nitrophenyl)-azetidin-1-yl]-2-(naphthalen-1-yloxy)acetamide (4b) was showed most potent antibacterial activity (MIC values of 16–64 μg/ml). The antifungal activity of the synthesized compounds against the tested fungal strains was relatively weak, most of the compounds showed poor activities (MIC>128 μg/ml).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tandon VK, Maurya HK, Mishra NN, Shukla PK. Design, synthesis and biological evaluation of novel nitrogen and sulfur containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. Eur J Med Chem. 2009;44:3130–7. doi: 10.1016/j.ejmech.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Biot C, François N, Maciejewski L, Brocard J, Poulain D. Synthesis and antifungal activity of a ferrocene-flucanazole analogue. Bioorg Med Chem Lett. 2000;10:839–41. doi: 10.1016/s0960-894x(00)00120-7. [DOI] [PubMed] [Google Scholar]
- 3.Bairagi S, Bhosale A, Deodhar M. Design, synthesis and evaluation of Schiff's bases of 4-chlro-3- coumarin aldehyde as antimicrobial agents. E-Journal of Chemistry. 2009;6:759–62. [Google Scholar]
- 4.Naik B, Desai KR. Novel approach for the rapid and efficient synthesis of heterocyclic Schiff bases and azetidinones under microwave irradiation. Indian J Chem. 2006;45:267–71. [Google Scholar]
- 5.Chavan AA, Pai NR. Synthesis and biological activities of N-substituted-3-chloro-2-azetidinones. Molecules. 2007;12:2467–77. doi: 10.3390/12112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Chandna M, Gupta M. Synthesis and antimicrobial activity of Schiff bases and azetidinones of 7-hydroxy-4-methyl-chromen-2-one. J Pharm Res. 2010;3:3010–2. [Google Scholar]
- 7.Moussa HH, Abdel Mequid S, EI- Hawaary S. Novel antimicrobial compounds among naphthalene and methylenedioxyphenyl derivatives. Pharmazie. 1981;36:805–7. [PubMed] [Google Scholar]
- 8.Ingarsal N, Saravanan G, Amutha P, Nagarajan S. Synthesis, in vitro antibacterial and antifungal evaluations of 2-amino-4-(1-naphthyl)-6-arylpyrimidines. Eur J Med Chem. 2007;42:517–20. doi: 10.1016/j.ejmech.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Chang KR, Dong HK, Hee JK, Sae YC. The antimicrobial activities of some 1, 4-naphthalenediones (IV) Arch Pharm Res. 1993;16:327–30. [Google Scholar]
- 10.Bansal E, Srivastava V, Kumar A. Synthesis and anti-inflammatory activity of 1-acetyl-5-substituted aryl-3-(beta-aminonaphthyl)-2-pyrazolines and beta-(substituted aminoethyl) amidonaphthalenes. Eur J Med Chem. 2001;36:81–2. doi: 10.1016/s0223-5234(00)01179-x. [DOI] [PubMed] [Google Scholar]
- 11.Rokade YB, Sayyed RZ. Naphthalene derivatives: A new range of antimicrobials with high therapeutic value. Rasayan J Chem. 2009;2:972–80. [Google Scholar]
- 12.Collee JG, Duguid JP, Fraser AG, Marmion BP. Mackie and Mc. Cartney Practical Medicinal Microbiology. 13th ed. London: Churchill Livingstone; 1989. [Google Scholar]


