Abstract
Purpose of the study was to examine the antihyperglycemic and hepato-renal protective effects of Emblica officinalis (Eo) fruit as a food supplement in fluoride induced toxicity. Eo fruit powder was incorporated into the diet (2.5, 5 and 10 gm %) of fluoride exposed animals for a duration of 30 days. Fluoride exposure caused significant elevation in plasma glucose, serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), acid phosphatase (ACP), alkaline phosphatase (ALP) activities, hepatic glucose-6-phosphatase (G-6-Pase) and decreased hepatic glycogen content, hexokinase activity and antioxidant profiles (hepatic and renal). An inclusion of Eo fruit powder significantly reduced plasma glucose levels, SGOT, SGPT, ACP and ALP activities, hepatic G-6-Pase activity and increased hepatic glycogen content and hexokinase activity. Hepatic and renal antioxidant status of fluoride exposed animals improved upon feeding Eo fruit powder. We, therefore, conclude that E. officinalis fruit could be useful in regulating hyperglycemia and enhances antioxidant status of fluoride exposed animals.
KEY WORDS: Antioxidant, Emblica officinalis fruit, fluoride toxicity, glucose
Across the world, high levels of fluoride in potable water and natural resources have been recognized as potential health hazard. An estimated 66.62 million people in India alone are at risk facing fluorosis due to high water-borne fluoride concentrations. The Indian state of Gujarat on the west coast has at least 24 districts with high fluoride content in their water resources.[1] Fluoride in excess amounts cause several ailments viz, metabolic disturbances, endocrine dysfunctions and physiological alterations in the body.[2] Chronic exposure to fluoride results in hyperglycemia besides development of classical symptoms of fluorosis, indicating the diabetogenic effect of fluoride as fluoride is known to inhibit the key enzymes involved in glycolysis and TCA cycle.[3–7] A recent study also indicated that an exposure to fluoride lowers insulin secretion and that it could be one of the reasons for increased blood glucose levels in fluoride intoxicated animals.[8] Long term exposure to fluoride causes hepatic damage in terms of significant increases in the activities of serum glutamate oxalate-, glutamate pyruvate-, serum aspartate- and serum alanine transaminases.[9,10] Further, fluoride consumption in the early developmental stages was shown to enhance oxidative stress in blood and decrease the liver antioxidant profiles.[11,12] Other than defluoridation techniques, there are no viable options to reduce fluoride content from water. Defluoridation techniques are not always feasible in underprivileged communities in endemic areas throughout the world. Thus, it becomes necessary to search for a renewable and affordable alternative/s, which can be suggested and propagated in the areas of endemic fluorosis. In this regard, certain nutrients have been shown to be useful as remedial measures to tackle fluoride toxicity. These include protein, calcium, vitamin C, vitamin E, and other antioxidants.[2,13] Herbal or natural products are being increasingly investigated for their role in reducing the effects of fluoride toxicity.[14–25] The present investigation is an effort in this direction and deals with the antihyperglycemic, hepato- and renal protective effects of E. officinalis in fluoride induced toxicity in laboratory rats.
Emblica officinalis G., (F: Euphorbiaceae; common name: Amla) fruit is reported to possess hypoglycemic, hypolipidemic, hepatoprotective, anti-cancerous, anti-microbial, anti-inflammatory, anti-pyretic and analgesic activities.[26] Although the effects of aqueous extracts of fruit of Emblica officinalis in combination with Tamarindus indicus fruit pulp have been recently reported,[27] the potential effects of amla fruit powder as a dietary supplement in fluoride toxicity has not been investigated. The present work was aimed at (i) evaluating the antihyperglycemic properties and (ii) determining the hepato-renal protective effects of E. officinalis fruit as a dietary supplement in fluoride induced toxicity in laboratory albino rats.
Materials and Methods
Emblica officinalis fruit powder preparation and analysis
Emblica officinalis fruits were purchased from the local market and authenticated by our faculty taxonomist Dr. A. S. Reddy, and voucher specimen was retained in the departmental herbarium (RAV # 01). The pulp was extracted, air dried, ground to powder and stored in an air tight container. A quantitative phytochemical analysis for phytosterols, saponins, polyphenols, flavanoids, total ascorbic acid and fiber was carried out using standard methods.[28–31]
Animals
Colony bred adult male albino rats (Charles Foster; 200 - 250 gm bw) were provided standard diet (Pranav Agro Industries, Vadodara, India), water ad libitum and were housed individually in well-ventilated animal unit (26±2°C, humidity 62%, and 12-h light/dark cycle). The care and procedure for the present experiment were in accordance with Institutional Animal Ethics Committee (MoEF/CPCSEA/Reg.337).
Experimental design
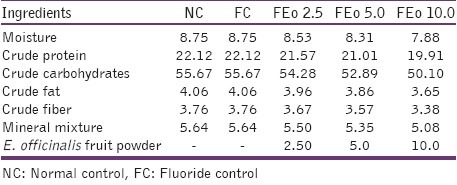
After 10-day adaptation period, 30 animals were randomly segregated into 5 groups of 6 animals each: Normal control (NC)- normal animals without any treatment; Fluoride control (FC)- 100 ppm sodium fluoride (NaF) through drinking water; FEo 2.5 - 100 ppm NaF exposed animals administered with 2.5 gm % Eo fruit powder in diet; FEo 5.0 - 100 ppm NaF exposed animals administered with 5 gm % Eo fruit powder in diet; FEo 10.0 - 100 ppm NaF exposed animals administered with 10 gm % Eo fruit powder in diet. The composition of the diet for experimental animals is given in Table 1.
Table 1.
Composition of the experimental diet (gm %)
At the end of the 4 week period, animals were fasted overnight and sacrificed under mild ether anesthesia. Blood was collected by cardiac puncture, and plasma was separated by centrifugation. Liver and kidney tissues were excised, and both plasma and tissues were kept frozen until analyzed.
Biochemical analysis
Plasma glucose levels were measured by standard kits (Eve's Inn Diagnostics, India). Hepatic glycogen was extracted with 30% potassium hydroxide, and the yield was determined by anthrone-sulfuric acid method.[32] The hepatic hexokinase (EC 2.7.1.1) and glucose-6-phosphatase (EC 3.1.3.9) activities were determined using the methods of Brandstrup et al., (1957) and Baginsky et al. (1974), respectively.[33,34]
Serum glutamate oxalocetate (SGOT) and pyruvate (SGPT) transaminases, acid and alkaline phosphatases (ACP, ALP) levels were determined using standard kits (Eve's Inn Diagnostics, Baroda, India).
Catalase (CAT; EC 1.11.1.6) and superoxide dismutase (SOD; EC 1.15.1.1) were measured according to the methods of Aebi (1974) and Kakkar et al., (1984), respectively.[35,36] Total ascorbic acid (TAA) was estimated using 2, 4-dinitrophenyl hydrazine reagent.[31] Glutathione peroxidase (GPx; EC 1.11.1.9) and reduced glutathione (GSH) were analyzed by the methods of Flohe and Gunzler (1984) and Jollow et al. (1974), respectively.[37,38]
Data are presented as mean ± SEM. One-way analysis of variance (ANOVA) with Tukey's significant difference post hoc test was used to compare differences among groups. Data were statistically handled by Graph Pad Prism 3.0 statistical software. P values < 0.05 were considered statistically significant.
Results and Discussion
The present study provides an evidence for the positive influence of Emblica officinalis on body carbohydrate and antioxidant metabolism in fluoride intoxicated animals. The effects of E. officinalis in fluoride exposed rats were found to be dose dependent: 10 gm % dose was maximally effective compared to 2.5 and 5.0 gm % doses.
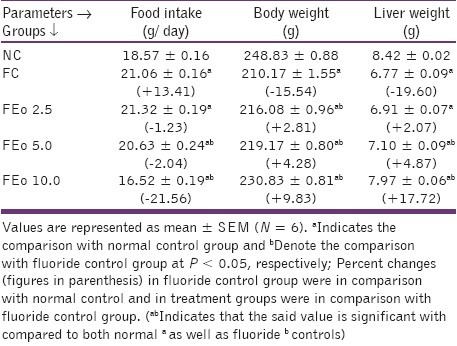
Significant loss in the body and liver weights were observed in fluoride exposed animals with an increase in food intake. Addition of E. officinalis fruit powder to the diet increased the body and liver weights (9%, 17%, respectively) with a reduction in food intake (22%) in fluoride exposed animals. The reduction in body weight in fluoride exposed rats could be because of an unavailability of carbohydrate for energy utilization though food intake increased. It is possible that fluoride may have suppressed the hunger centers of central nervous system, resulting in increased food intake without enhancing energy assimilation, prompting a decline in body and liver weights. A reverse phenomenon may have been caused by the Eo fruit powder i.e., E. officinalis fruit powder could have regulated the appetite and caused an increase in body and liver weights in fluoride exposed animals [Table 2].
Table 2.
Effects of Emblica officinalis on food intake, body and liver weights
Fluoride administration is known to cause hyperglycemia, simulating diabetic conditions.[5–7] In the present context too, a significant increase in plasma glucose levels, hepatic G-6-Pase activity and reduction in hepatic glycogen content and hepatic hexokinase activities were noted. An inclusion of E. officinalis fruit powder to the diet of fluoride exposed animals resulted in significantly decreased blood glucose levels and G-6-Pase activity with increased hepatic glycogen content and hexokinase activity [Table 3]. These observations clearly indicate the potential of E. officinalis to counter the effects of possibly lowered levels of insulin in fluoride intoxicated rats. While fluoride exposure significantly increased the SGOT, SGPT, ACP and ALP levels, Eo fruit powder supplementation reversed this trend in a dose-dependent manner, indicating the hepatic restoratory effect of Eo fruit [Table 4].
Table 3.
Effects of Emblica officinalis on plasma glucose, hepatic glycogen content, hepatic hexokinase and glucose-6- phosphatase
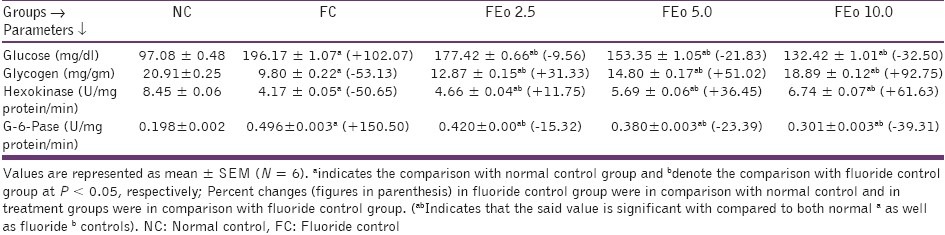
Table 4.
Effects of Emblica officinalis on the activities of SGOT, SGPT, ACP and ALP

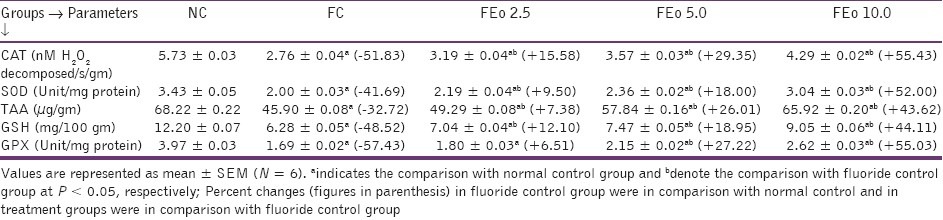
Chronic fluoride toxicity is reported to enhance the oxidative stress as evidenced by a significant increase in malondialdehyde (MDA) content and a reduction in body antioxidant status.[2,7] An exposure to fluoride in the present context also resulted in decreased levels of antioxidants - TAA, SOD, CAT, GSH and GPX contents. E. officinalis fruit powder addition at 10 gm % dose level exhibited significant increase in TAA, GSH content and SOD, CAT, GPX activities when compared to FC and as well as F Eo 2.5 and F Eo 5 animals [Tables 5 and 6].
Table 5.
Effects of Emblica officinalis on hepatic antioxidant profiles
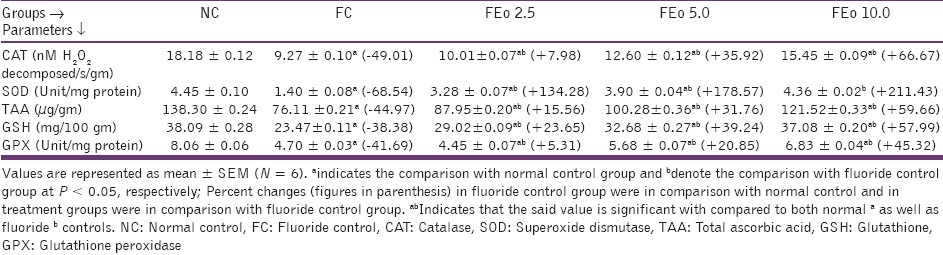
Table 6.
Effects of Emblica officinalis on renal antioxidant profiles
The Eo fruit contained 3.2 gm % fiber, 8.65 gm % phytosterol, 0.05 gm % saponins, 19.70 gm % polyphenols, 0.342 gm % flavonoids and 0.425 gm % ascorbic acid content. Thus, the presently observed anti-hyperglycemic, hepato-renal protective and antioxidant effects could be due to additive / individual components’ ability as polyphenols, and flavonoids are known to be hepato- and gastro-protective, anti-carcinogenic, ant-idiabetic, ameliorative agents for insulin resistance by protecting pancreatic islet β cells, antioxidative and anti-hyperlipemic in nature.[39–41] Dietary saponins, phytosterols and ascorbic acid play an important role as antihyperglycemic and improve glycemic index, lowering both fasting blood glucose and glycosylated hemoglobin, modulating the insulin's action.[42–44]
Thus, results of present study and our earlier report[45] clearly suggest that the fruits of E. officinalis exhibit anti-hyperglycemic, anti-hyperlipemic, anti-peroxidative and antioxidant properties in fluoride exposed animals. It is, therefore, concluded that fruit of E. officinalis are useful in regulating fluoride induced hyperglycemia and improve the antioxidant status.
Acknowledgement
Author Rupal A. Vasant gratefully acknowledges the financial assistance from UGC, New Delhi Funding source: UGC, New Delhi in the form of a research fellowship
Footnotes
Source of Support: UGC, New Delhi
Conflict of Interest: None declared.
References
- 1.Susheela AK. A Treatise on Fluorosis. 3rd ed. New Delhi: Fluorosis Research and Rural Development Foundation; 2007. [Google Scholar]
- 2.Strunecka A, Patocka J, Blaylock RL, Chinoy NJ. Fluoride interactions: From molecules to disease. Curr Signal Transduct Ther. 2007;2:190–213. [Google Scholar]
- 3.Dousset JC, Rioufol C, Philibert C, Bourbon P. Effects of inhaled hydrofluoric acid on cholesterol, carbohydrate and tricarboxylic acid metabolism in guinea pigs. Fluoride. 1987;20:137–41. [Google Scholar]
- 4.Hordyjewska A, Pasternek K. Influence of fluoride on organism of human. J Elementol. 2004;9:883–97. [Google Scholar]
- 5.Chlubek D, Grucka-Mamczar E, Birkner E, Polaniak R, Stawiarska- Pieta B, Duliban H. Activity of pancreatic antioxidative enzymes and malondialdehyde concentrations in rats with hyperglycemia caused by fluoride intoxication. J Trace Elem Med Biol. 2003;17:57–60. doi: 10.1016/S0946-672X(03)80047-0. [DOI] [PubMed] [Google Scholar]
- 6.Grucka-Mamczar E, Birkner E, Zalejska-Fiolka J, Machoy Z, Kasperczyk S, Blaszczyk I. Influence of extended exposure to sodium fluoride and caffeine on the activity of carbohydrate metabolism enzymes in rat blood serum and liver. Fluoride. 2007;40:62–6. [Google Scholar]
- 7.Rupal AV, Dhrutigna RK, Krutika LB, Narasimhacharya AVRL. Therapeutic benefits of glibenclamide in fluoride intoxicated diabetic rats. Fluoride. 2010;43:141–9. [Google Scholar]
- 8.Garcia-Montalvo EA, Reyes-Perez H, Del-Razo LM. Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology. 2009;263:75–83. doi: 10.1016/j.tox.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Guo XY, Sun GF, Sun YC. Oxidative stress from fluoride-induced hepatotoxicity in rats. Fluoride. 2003;36:25–9. [Google Scholar]
- 10.Bouaziz H, Ketata S, Jammoussi K, Boudawara T, Ayedi F, Ellouze F, et al. Effects of sodium fluoride on hepatic toxicity in adult mice and their suckling pups. Pestic Biochem Physiol. 2006;86:124–30. [Google Scholar]
- 11.Shivarajashankara YM, Shivashankara AR, Bhat PG, Rao HS. Lipid peroxidation and antioxidant systems in the blood of young rats subjected to chronic fluoride toxicity. Indian J Exp Biol. 2003;41:857–60. [PubMed] [Google Scholar]
- 12.Shantakumari D, Srinivasalu S, Subramanian S. Effect of fluoride intoxication on lipid peroxidation and antioxidant status in experimental rats. Toxicology. 2004;204:219–28. doi: 10.1016/j.tox.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 13.Blaszczyk I, Grucka-Mamczar E, Kasperczyk S, Birkner E. Influence of fluoride on rat kidney antioxidant system: Effects of methionine and vitamin E. Biol Trace Elem Res. 2008;121:51–9. doi: 10.1007/s12011-007-8030-6. [DOI] [PubMed] [Google Scholar]
- 14.Khandare AL, Udaykumar P, Lakshmaiah N. Beneficial effect of tamarind ingestion on fluoride toxicity in dogs. Fluoride. 2000;33:33–8. [Google Scholar]
- 15.Khandare AL, Rao GS, Lakshmaiah N. Effects of tamarind ingestion on fluoride excretion in humans. Eur J Clin Nutr. 2002;56:82–5. doi: 10.1038/sj.ejcn.1601287. [DOI] [PubMed] [Google Scholar]
- 16.Khandare AL, Udaykumar P, Shanker RJ, Venkaiah K, Lakshmaiah N. Additional beneficial effect of tamarind ingestion over defluoridated water supply to adolescent boys in a fluorotic area. Nutrition. 2004;20:433–6. doi: 10.1016/j.nut.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Ekambaram P, Namitha T, Bhuvneswari S, Aruljothi S, Vasanth D, Sarvanakumar M. Therapeutic efficacy of Tamarindus indica (L) to protect against fluoride induced oxidative stress in the liver of female rats. Fluoride. 2010;43:134–40. [Google Scholar]
- 18.Stanely VA, Kumar T, Lal AAS, Pillai KS, Murthy PBK. Moringa oleifera (radish tree) seed extract as an antidote for fluoride toxicity. Fluoride. 2002;35:251. [abstract] [Google Scholar]
- 19.Ranjan R, Swarup D, Patra RC, Chandar V. Tamarindus indica L and Moringa oleifera M. extract administration ameliorates fluoride toxicity in rabbits. Indian J Exp Biol. 2009;47:900–5. [PubMed] [Google Scholar]
- 20.Sinha M, Manna P, Sil PC. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride induced hepatic and renal oxidative stress. J Nat Med. 2007;61:251–60. [Google Scholar]
- 21.Ghosh J, Das J, Manna P, Sil PC. Cytoprotective effect of arjunolic acid in response to sodium fluoride mediated oxidative stress and cell death via necrotic pathway. Toxicol In Vitro. 2008;22:1918–26. doi: 10.1016/j.tiv.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Manna P, Sinha M, Sil PC. A 43 kD protein isolated from the herb Cajanus indicus L attenuates sodium fluoride induced hepatic and renal disorders in vivo. J Biochem Mol Biol. 2007;40:382–95. doi: 10.5483/bmbrep.2007.40.3.382. [DOI] [PubMed] [Google Scholar]
- 23.Verma RJ, Trivedi MH, Chinoy NJ. Black tea amelioration of sodium- fluoride induced alterations of DNA, RNA, and protein contents in the cerebral hemisphere, cerebellum, and medulla oblongata regions of mouse brain. Fluoride. 2007;40:7–12. [PubMed] [Google Scholar]
- 24.Hassan HA, Yousef MI. Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol. 2009;47:2332–7. doi: 10.1016/j.fct.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Rupal AV, Narasimhacharya AVRL. Alleviation of fluoride induced hepatic and renal oxidative stress in rats by the fruit of Limonia acidissima. Fluoride. 2011;44:14–20. [Google Scholar]
- 26.Khan KH. Roles of Emblica officinalis in medicine- a review. Bot Res Int. 2009;2:218–28. [Google Scholar]
- 27.Chaudhary S, Singh PK, Gupta AK, Singh AP. Combined effect of Tamarindus indica and Emblica officinalis on enzyme profile and lipid profile after fluoride intoxication in albino rats. J Ecophysiol Occup Health. 2010;10:77–83. [Google Scholar]
- 28.Goad LJ, Akihisa T. Analysis of Sterols, Blackie Academic and Professional. 1st ed. London: 1997. pp. 423–5. [Google Scholar]
- 29.Ebrahimzadeh H, Niknam V. A revised spectrophotometric method for determination of triterpenoid saponins. Indian Drugs. 1998;35:379–81. [Google Scholar]
- 30.Thimmaiah SK. Standard methods of biochemical analysis. New Delhi, India: Kalyani Publishers; 1999. p. 64. (293-8). [Google Scholar]
- 31.Schaffert RR, Kingsley GR. A rapid, simple method for the determination of reduced, dehydro- and total ascorbic acid in biological material. J Biol Chem. 1955;212:59–68. [PubMed] [Google Scholar]
- 32.Seifter S, Dayton S, Novic B, Muntwyler E. Estimation of glycogen with anthrone reagent. Arch Biochem. 1950;25:191–200. [PubMed] [Google Scholar]
- 33.Brandstrup N, Kirk JE, Bruni C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol. 1957;12:166–71. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- 34.Baginsky ES, Foa PP, Zad B. Glucose- 6- phosphatase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 788–92. [Google Scholar]
- 35.Aebi H. Catalase. In: Bergmeyer, editor. Methods of Enzymatic Analysis. Second ed. Vol. 2. New York: Academic Press; 1974. pp. 673–84. [Google Scholar]
- 36.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 37.Flohe L, Gunzler WA. Methods in Enzymology. New York: Academic Press; 1984. Assay of glutathione peroxidase; pp. 114–21. [DOI] [PubMed] [Google Scholar]
- 38.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene induced liver necrosis.Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 39.Yao LH, Jiang YM, Shi J, Tomás-Barberán FA, Datta N, Singanusong R, et al. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59:113–22. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 40.Zunino ZS, David HS, Stephensen CB. Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in non-obese diabetic mice. J Nutr. 2007;137:1216–21. doi: 10.1093/jn/137.5.1216. [DOI] [PubMed] [Google Scholar]
- 41.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2:737–51. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: A review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 43.Kritchevsky D, Chen SC. Phytosterols- health benefits and potential concerns: A review. Nutr Res. 2005;25:413–28. [Google Scholar]
- 44.Oguntibeju OO. The biochemical, physiological and therapeutic roles of ascorbic acid. Afr J Biotechnol. 2008;7:4700–5. [Google Scholar]
- 45.Rupal AV, Narasimhacharya AVRL. Alleviatory effects of Emblica officinalis G. as a food supplement in fluoride induced hyperlipemia and oxidative stress. Int J Pharm Pharm Sci. 2012;4:404–8. [Google Scholar]


