Abstract
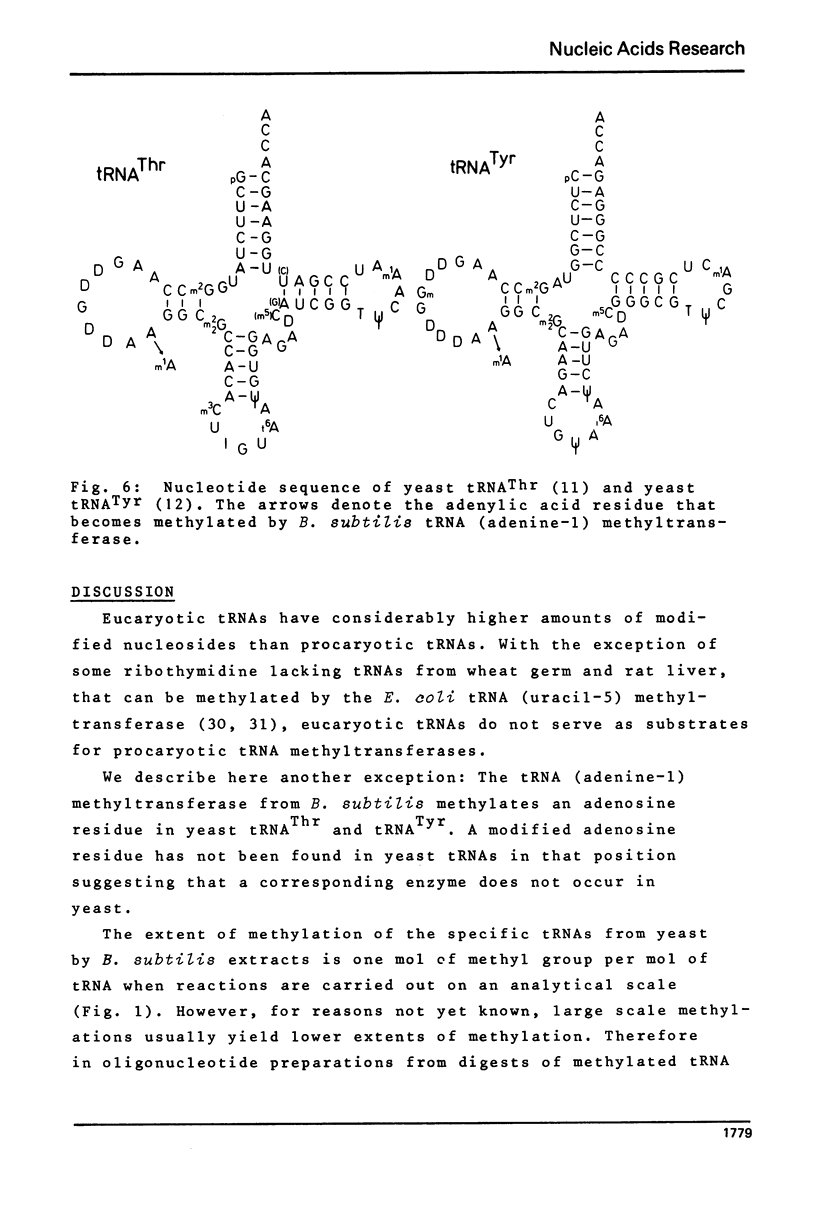
tRNA (adenine-1) methyltransferase occurs in Bacillus subtilis. Eucaryotic tRNAThr and tRNATyr from yeast in which 1-methyladenosine (m1A) is already present in the TpsiC loop, can be methylated in vitro with S-adenosylmethionine and B. subtilis extracts. Each of the specific tRNAs accepts 1 mol of methyl groups per mol tRNA. The enzyme transforms into m1A the 3'-terminal adenylic acid residue of the dihydrouridine loop, a new position for a modified adenosine residue in tRNA. Both tRNAs have the sequence Py-A-A-G-G-C-m2(2)G in the D-loop and D-stem region. Other tRNAs with the same sequence in this region also serve as substrates for the tRNA (adenine-1) methyltransferase.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Agris P. F., Spremulli L. L., Brown G. M. tRNA methylases from HeLa cells: purification and properties of an adenine-1-methylase and a guanine-N2-methylase. Arch Biochem Biophys. 1974 May;162(1):38–47. doi: 10.1016/0003-9861(74)90102-7. [DOI] [PubMed] [Google Scholar]
- Arnold H. H., Schmidt W., Raettig R., Sandig L., Domdey H., Kersten H. S-Adenosylmethionine and tetrahydrofolate-dependent methylation of tRNA in Bacillus subtilis. Incomplete methylations caused by trimethoprim, pactamycin, or chloramphenicol. Arch Biochem Biophys. 1976 Sep;176(1):12–20. doi: 10.1016/0003-9861(76)90135-1. [DOI] [PubMed] [Google Scholar]
- Arnold H., Kersten H. The occurrence of ribothymidine, 1-methyladenosine, methylated guanosines and the corresponding methyltransferases in E. coli and Bacillus subtilis. FEBS Lett. 1973 Oct 1;36(1):34–38. doi: 10.1016/0014-5793(73)80331-x. [DOI] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P., Bonnet J., Gangloff J., Keith G., Krebs B., Kuntzel B., Roy A., Weissenbach J., Werner C. Structure primaire des tRN. Biochimie. 1972;54(2):127–144. doi: 10.1016/s0300-9084(72)80097-x. [DOI] [PubMed] [Google Scholar]
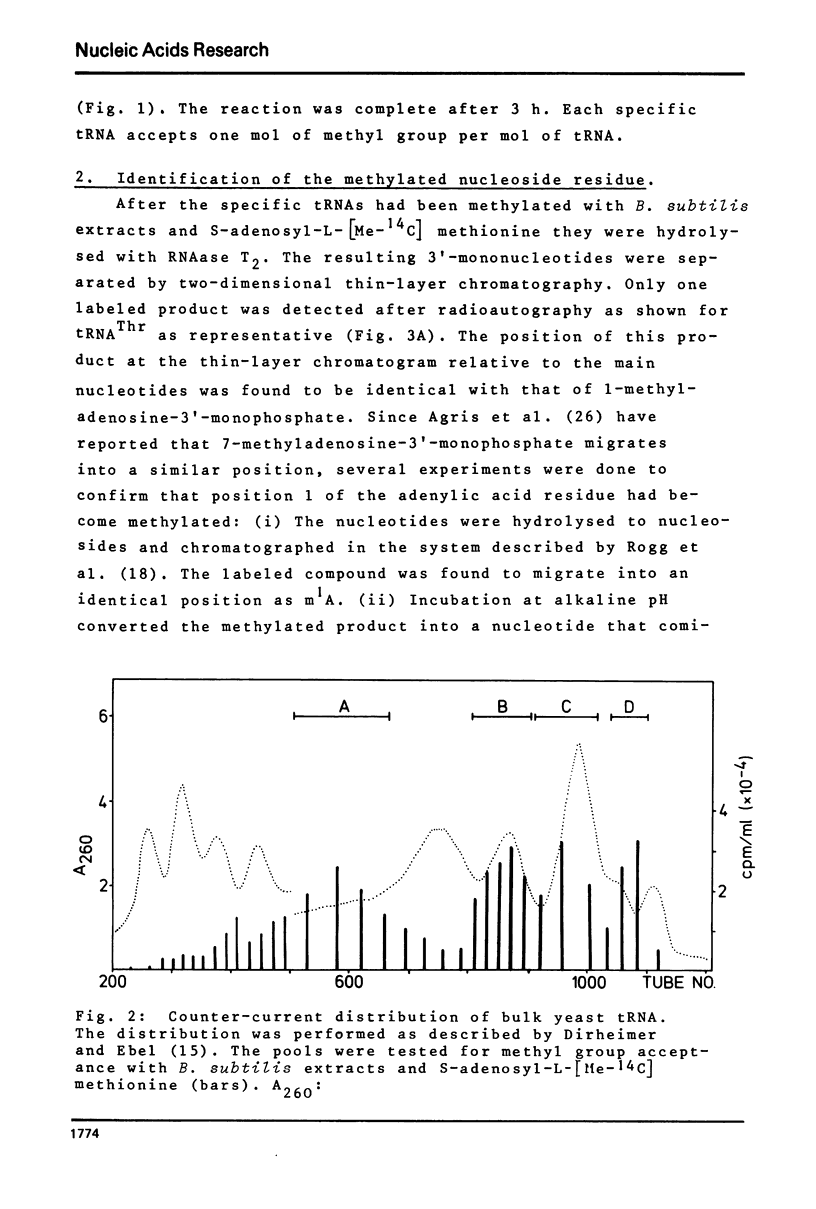
- Dirheimer G., Ebel J. P. Fractionnement des tRNA de levure de bière par distribution en contre-courant. Bull Soc Chim Biol (Paris) 1967;49(12):1679–1687. [PubMed] [Google Scholar]
- Dubois E. G., Dirheimer G., Weil J. H. Methylation of yeast tRNA Asp by enzymes from cytoplasm, chloroplasts and mitochondria of phaseolus vulgaris. Biochim Biophys Acta. 1974 Dec 20;374(3):332–341. doi: 10.1016/0005-2787(74)90254-8. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Hildesheim J., Blanchard P., Michelot R. A base-pairing hypothesis for t-RNA methylation. Experientia. 1974 Aug 15;30(8):884–886. doi: 10.1007/BF01938339. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Keith G., Ebel J. P., Dirheimer G. The primary structure of two mammalian tRNAs Phe: identity of calf liver and rabbit liver tRNAs Phe. FEBS Lett. 1974 Nov 1;48(1):50–52. doi: 10.1016/0014-5793(74)81059-8. [DOI] [PubMed] [Google Scholar]
- Keith G., Rogg H., Dirheimer G., Menichi B., Heyham T. Post-transcriptional modification of tyrosine tRNA as a function of growth in Bacillus subtilis. FEBS Lett. 1976 Jan 15;61(2):120–123. doi: 10.1016/0014-5793(76)81017-4. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. An evolutionary study of the methylation of transfer and ribosomal ribonucleic acid in prokaryote and eukaryote organisms. J Biol Chem. 1973 Apr 10;248(7):2612–2620. [PubMed] [Google Scholar]
- Kuntzel B., Weissenbach J., Dirheimer G. Structure primaire des tRNA Arg/III de levure de biére. I. Hydrolyse totale par les ribonucléases pancréatique et T1. Biochimie. 1974;56(8):1053–1067. doi: 10.1016/s0300-9084(74)80095-7. [DOI] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. Chromatographic studies of nucleic acids; a technique for the identification and estimation of purine and pyrimidine bases, nucleosides and related substances. Biochem J. 1949;45(3):294–298. doi: 10.1042/bj0450294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macon J. B., Wolfenden R. 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry. 1968 Oct;7(10):3453–3458. doi: 10.1021/bi00850a021. [DOI] [PubMed] [Google Scholar]
- Madison J. T., Everett G. A., Kung H. K. Oligonucleoides from yeast tyrosine transfer ribonucleic acid. J Biol Chem. 1967 Mar 25;242(6):1318–1323. [PubMed] [Google Scholar]
- Madison J. T., Kung H. K. Large oligonucleotides isolated from yeast tyrosine transfer ribonucleic acid after partial digestion with ribonuclease T1. J Biol Chem. 1967 Mar 25;242(6):1324–1330. [PubMed] [Google Scholar]
- Marcu K., Mignery R., Reszelbach R., Roe B., Sirover M., Dudock B. The absence of ribothymidine in specific eukaryotic transfer RNAs. I. Glycine and threonine tRNAs of wheat embryo. Biochem Biophys Res Commun. 1973 Nov 16;55(2):477–483. doi: 10.1016/0006-291x(73)91111-x. [DOI] [PubMed] [Google Scholar]
- Munns T. W., Podratz K. C., Katzman P. A. A method for determination of the methylated constituents of transfer ribonucleic acid. Biochemistry. 1974 Oct 8;13(21):4409–4416. doi: 10.1021/bi00718a026. [DOI] [PubMed] [Google Scholar]
- Raettig R., Schmidt W., Mahal G., Kersten H., Arnold H. H. Purification and characterization of tRNAMet-f, tRNAPhe and tRNATyr2 from Baccillus subtilis. Biochim Biophys Acta. 1976 Jun 18;435(2):109–118. doi: 10.1016/0005-2787(76)90241-0. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Chen E. Y., Tsen H. Y. Studies on the ribothymidine content of specific rat and human tRNAs: a postulated role for 5-methyl cytosine in the regulation of ribothymidine biosynthesis. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1339–1347. doi: 10.1016/0006-291x(76)90343-0. [DOI] [PubMed] [Google Scholar]
- Rogg H., Brambilla R., Keith G., Staehelin M. An improved method for the separation and quantitation of the modified nucleosides of transfer RNA. Nucleic Acids Res. 1976 Jan;3(1):285–295. doi: 10.1093/nar/3.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal R. P., Vold B. Changes in transfer ribonucleic acids of Bacillus subtilis during different growth phases. Nucleic Acids Res. 1976 May;3(5):1249–1262. doi: 10.1093/nar/3.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Király I., Dirheimer G. The nucleotide sequences of two threonine tRNAs from brewer's yeast. FEBS Lett. 1976 Nov 15;72(1):6–8. doi: 10.1016/0014-5793(76)80885-x. [DOI] [PubMed] [Google Scholar]



