Abstract
Salt-induced fluxes of H+, Na+, K+, and Ca2+ were investigated in ectomycorrhizal (EM) associations formed by Paxillus involutus (strains MAJ and NAU) with the salt-sensitive poplar hybrid Populus × canescens. A scanning ion-selective electrode technique was used to measure flux profiles in non-EM roots and axenically grown EM cultures of the two P. involutus isolates to identify whether the major alterations detected in EM roots were promoted by the fungal partner. EM plants exhibited a more pronounced ability to maintain K+/Na+ homeostasis under salt stress. The influx of Na+ was reduced after short-term (50 mm NaCl, 24 h) and long-term (50 mm NaCl, 7 d) exposure to salt stress in mycorrhizal roots, especially in NAU associations. Flux data for P. involutus and susceptibility to Na+-transport inhibitors indicated that fungal colonization contributed to active Na+ extrusion and H+ uptake in the salinized roots of P. × canescens. Moreover, EM plants retained the ability to reduce the salt-induced K+ efflux, especially under long-term salinity. Our study suggests that P. involutus assists in maintaining K+ homeostasis by delivering this nutrient to host plants and slowing the loss of K+ under salt stress. EM P. × canescens plants exhibited an enhanced Ca2+ uptake ability, whereas short-term and long-term treatments caused a marked Ca2+ efflux from mycorrhizal roots, especially from NAU-colonized roots. We suggest that the release of additional Ca2+ mediated K+/Na+ homeostasis in EM plants under salt stress.
Soil salinization is a serious factor restricting the expansion of agriculture and forestry around the world. Among the novel biotechnological tools that can enhance salt resistance, inoculation with ectomycorrhizal (EM) fungi has been suggested to be an important measure for enhancing the performance of and ensuring biomass production by woody species in saline environments (Luo et al., 2009). In general, EM fungi enhance the growth of host plants by increasing mineral nutrition and reducing the uptake of sodium ions (Na+) under salt stress (Hall, 2002; Polle and Schützendübel, 2003; Smith and Read, 2008). For example, Scleroderma bermudense significantly increases phosphorus and K+ levels but decreases Na+ and Cl− concentrations in Coccoloba uvifera plants (Bandou et al., 2006). Similarly, Hebeloma crustuliniforme and Laccaria bicolor reduce tissue Na+ and Cl− concentrations and alleviate salt injury in white spruce (Picea glauca), black spruce (Picea mariana), and jack pine (Pinus banksiana) seedlings (Muhsin and Zwiazek, 2002; Nguyen et al., 2006). In contrast to conifers, EM associations did not decrease tissue concentrations of Na+ and Cl− in NaCl-treated trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera; Yi et al., 2008), showing that the fungal effect on salt accumulation varies with EM fungus and host plant species.
Paxillus involutus strains MAJ and NAU have been identified as highly salt-tolerant fungi (Gafur et al., 2004; Langenfeld-Heyser et al., 2007; Zhang et al., 2008). Colonization with P. involutus strain MAJ reduces the buildup of Na+ but enhances K+ accumulation in the leaves of a salt-sensitive hybrid poplar, Populus × canescens (Langenfeld-Heyser et al., 2007, Luo et al., 2011). The maintenance of a high K+/Na+ ratio is critical for salt tolerance in herbaceous plants (Shabala, 2000; Tester and Davenport, 2003; Chen et al., 2007; Shabala and Cuin, 2008) and woody species, including Populus species (Chen et al., 2001, 2002a, 2002b, 2003; Sun et al., 2009a, 2009b, 2010, 2012). At the cellular level, salinized plants avoid Na+ toxicity in the cytosol by compartmentalizing Na+ into the vacuole and excreting Na+ into the external environment or the apoplast (Blumwald et al., 2000; Hasegawa et al., 2000; Zhu, 2001, 2003; Ottow et al., 2005; Apse and Blumwald, 2007; Munns and Tester, 2008; Chen and Polle, 2010). The driving force for Na+/H+ antiporters is provided by H+-ATPases, which make an important contribution to the maintenance of low Na+ levels in the cytosol (Chen and Polle, 2010). In addition, NaCl-induced K+ deficiency in plants is regulated by depolarization-activated (DA) outward-rectifying K+ channels (KORCs) and DA nonselective cation channels (NSCCs; Shabala et al., 2005, 2006a; Demidchik and Maathuis, 2007; Shabala and Cuin, 2008). Moreover, salinity is known to cause oxidative stress (Zhu, 2003; Demidchik et al., 2010), and a large proportion of NSCCs are reactive oxygen species activated (Demidchik et al., 2002, 2003). Roots of EM plants accumulate more, and leaves less, Na+ than the respective tissues of non-EM plants, probably due to decreased xylem loading (Langenfeld-Heyser et al., 2007). EM roots increase the supply of K+ under salt stress (Langenfeld-Heyser et al., 2007). However, how EM fungi assist plants by improving Na+ and K+ relationships after exposure to salinity is not yet clear.
It has been shown that different strains of P. involutus differ in their abilities to form typical mycorrhizal structures with P. × canescens roots (Gafur et al., 2004). While the fungal strain MAJ forms a typical hyphal mantle and Hartig net with roots of P. × canescens, NAU induces defense reactions, such as cell wall thickening, and is unable to intrude between the host cells (Gafur et al., 2004). Clarifying whether and how the incompatible fungal isolate affects the salt tolerance of host plants is necessary.
P. × canescens roots exhibit increased Ca2+ enrichment during mycorrhizal symbiosis with P. involutus strain MAJ (Langenfeld-Heyser et al., 2007). Ramos et al. (2009) found that EM roots are more efficient than non-EM roots in taking up Ca2+ from the external medium. Under NaCl stress, Ca2+ regulates K+/Na+ homeostasis in a salt-overly-sensitive3 mutant and wild-type Arabidopsis (Arabidopsis thaliana; Liu and Zhu, 1997). Ca2+ has been suggested to restrict Na+ uptake via voltage-independent-NSCCs (Demidchik and Tester, 2002; Tester and Davenport, 2003) and to restrain K+ loss through DA-KORCs and DA-NSCCs (Shabala et al., 2006a; Sun et al., 2009b; Chen and Polle, 2010). Although Ca2+ is well known to ameliorate salt stress, how Ca2+ impacts K+/Na+ homeostasis via EM associations is unclear.
Using ion-selective vibrating microelectrodes, significant correlations between anion, Ca2+, and H+ fluxes were found on root surfaces, with increased fluxes after colonization of different hosts with either ectomycorrhizae or arbuscular mycorrhizae (Ramos et al., 2008, 2009). The goal of this study was to examine the role of ectomycorrhizae in ion homeostasis under salt stress. We used the scanning ion-elective electrode technique (SIET) to measure steady and transient profiles of ion fluxes (Na+, H+, K+, and Ca2+) in P. × canescens-P. involutus associations, non-EM roots, and fungal mycelia of the two P. involutus isolates MAJ and NAU. We also examined the effects of Ca2+ on K+ and Na+ fluxes in P. × canescens roots; P. × canescens exhibited Ca2+ enrichment upon colonization with the EM fungus P. involutus.
RESULTS
Na+, K+, and Ca2+ Concentrations in Roots and Leaves
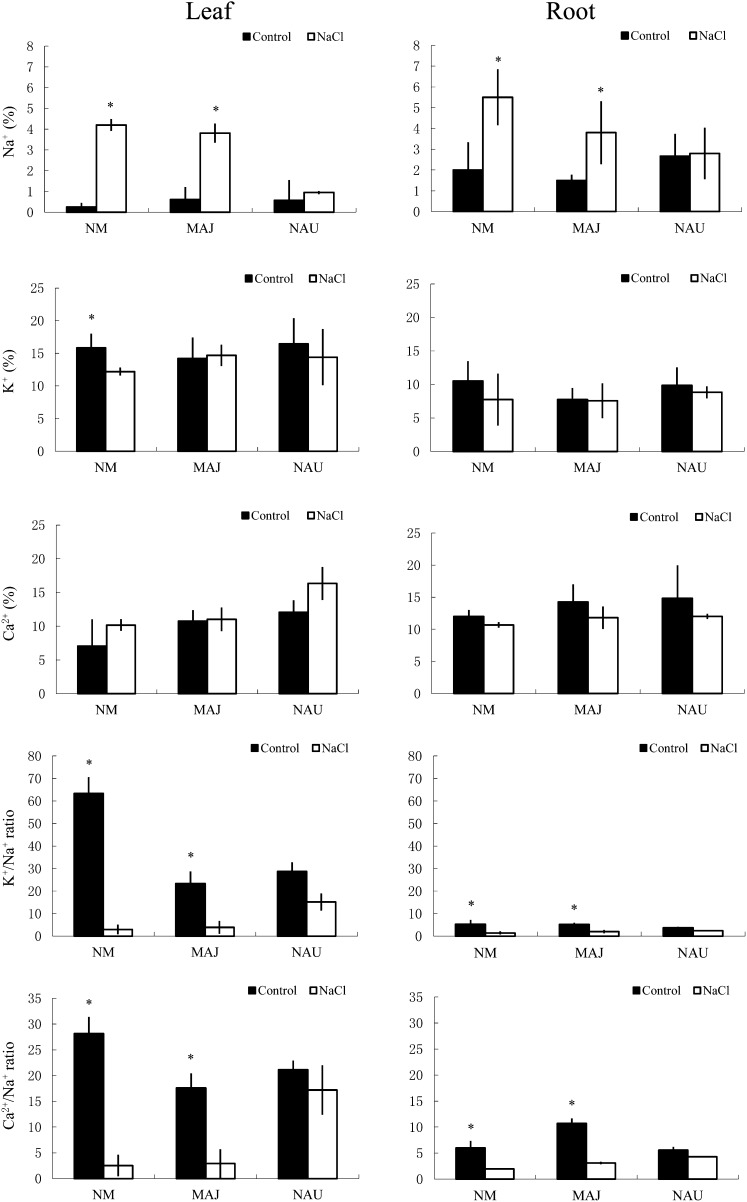
Scanning electron microscopy-energy dispersive x-ray spectrometry (SEM-EDX) was used to measure relative changes in the Na+, K+, and Ca2+ concentrations in the cross-sections of roots and leaves. NaCl treatment (50 mm) for 1 week significantly increased Na+ in the roots and leaves, with the exception of plants colonized with P. involutus strain NAU (Fig. 1). In contrast to Na+, root and leaf K+ concentrations decreased in salt-treated plants, especially nonmycorrhizal (NM) plants (Fig. 1). As a result, the K+/Na+ ratio in roots and leaves was markedly reduced by salt stress, with a more pronounced effect in NM plants compared with EM plants (Fig. 1). P. involutus mycorrhization increased Ca2+ by 19% to 24% in the roots and by 53% to 72% in the leaves of nonstressed plants (Fig. 1). Salinized P. × canescens had a reduced Ca2+/Na+ ratio in the roots and leaves; however, the salt effect was less evident in NAU-mycorrhizal plants compared with NM and MAJ-mycorrhizal plants (Fig. 1).
Figure 1.
Effects of NaCl (50 mm, 1 week) on Na+, K+, Ca2+, K+/Na+, and Ca2+/Na+ in the leaves and roots of mycorrhizal (MAJ and NAU) and NM P. × canescens plants. Amounts of K+, Na+, and Ca2+ are expressed as atomic mass fraction (%). Each column is the mean of three to four individual plants, and error bars represent se. Columns labeled with asterisks indicate significant differences at P < 0.05 between control and NaCl treatments.
Steady and Transient Ion Fluxes in Roots and EM Fungus
Na+ Flux
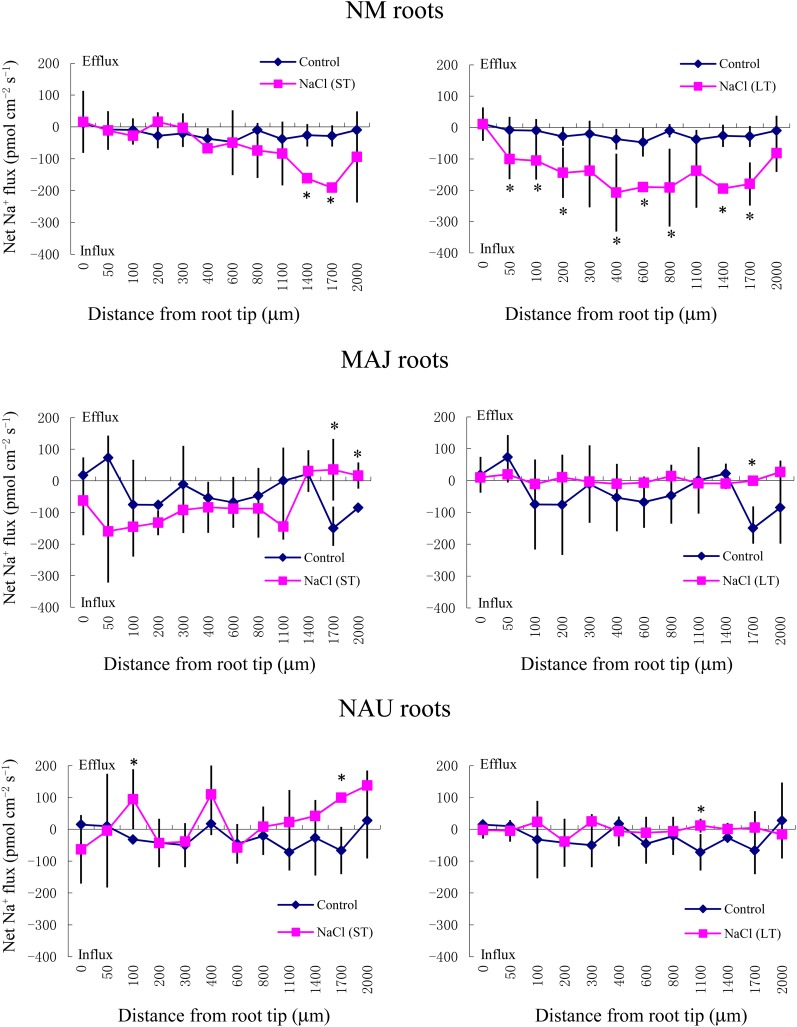
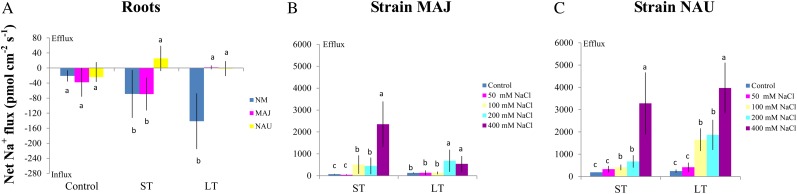
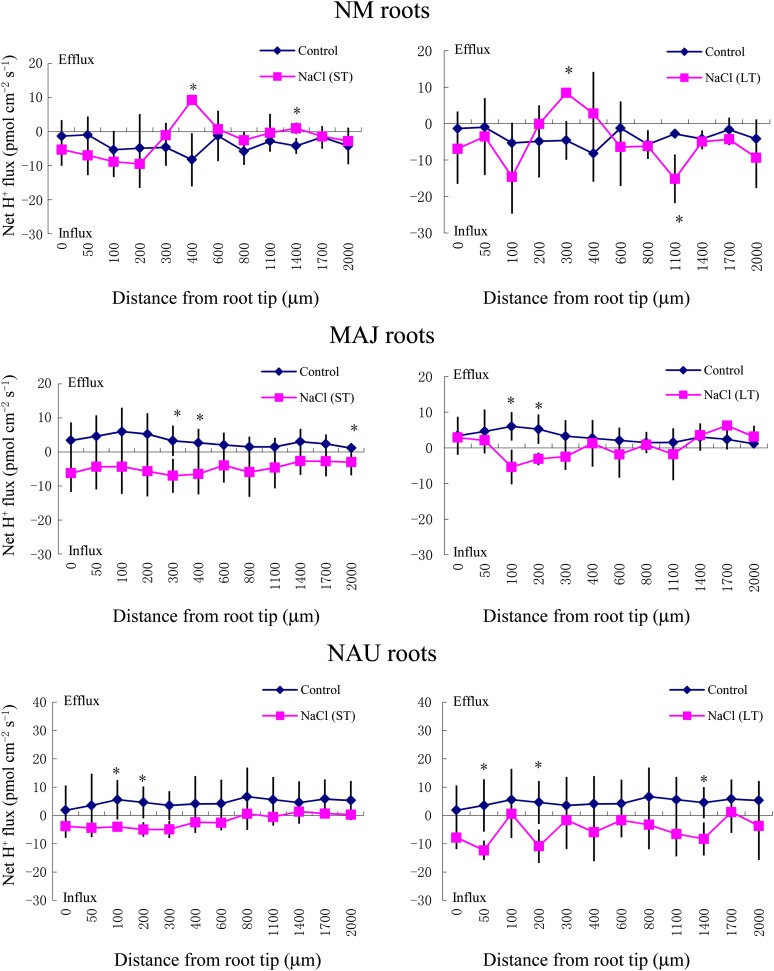
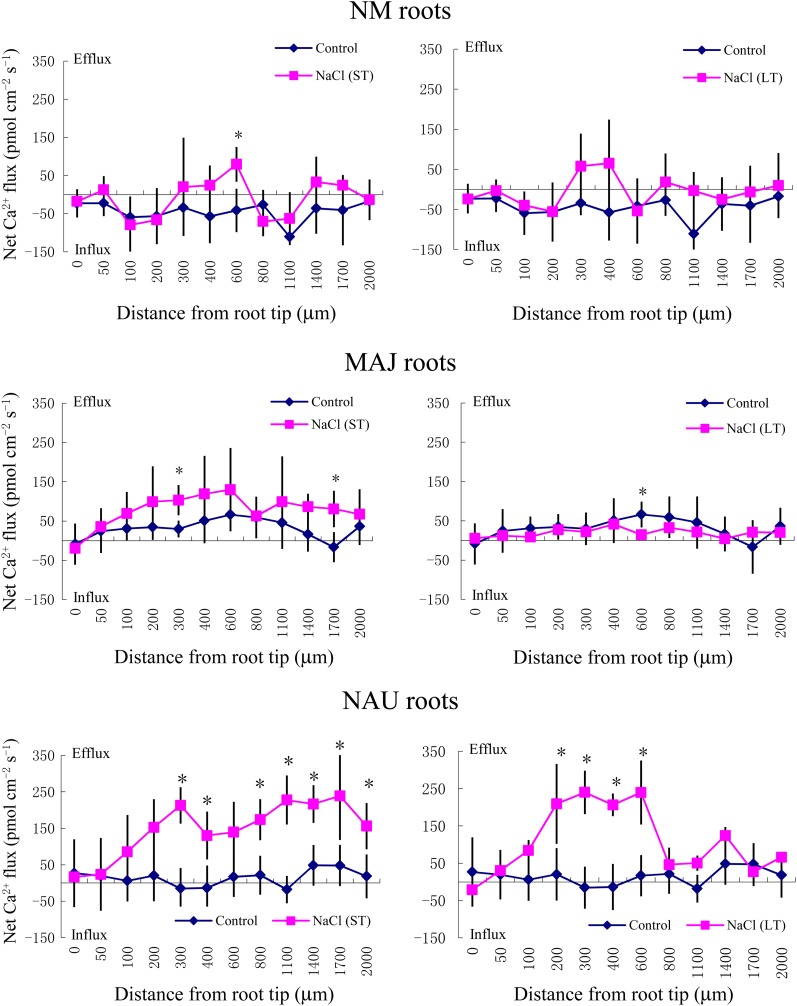
SIET analyses showed that the pattern of Na+ fluxes in EM roots differed from that of NM roots after short-term (ST; 24 h) and long-term (LT; 7 d) exposure to 50 mm NaCl (Fig. 2). In NM roots, ST stress caused a net Na+ influx at the region 1,400 to 2,000 μm from the apex, whereas LT salinity resulted in a stable and constant influx along the whole measured distance from 50 to 2,000 μm (Fig. 2). Compared with NM roots, the salt-induced entry of Na+ was less pronounced in MAJ- and NAU-mycorrhizal roots when considering both spatially resolved values along the scanned surface (Fig. 2) and mean values (Fig. 3A). A salt-induced efflux of Na+ was detected in some regions along these mycorrhizal roots (Fig. 2).
Figure 2.
Effects of ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d) on the net Na+ flux in the mycorrhizal (MAJ and NAU) and NM roots of P. × canescens plants. Control roots were treated without NaCl. Na+ fluxes were measured along root axes (0–2,000 μm from the apex) at intervals of 50 to 300 μm. Each point is the mean of five to six individual plants, and error bars represent se. *P < 0.05 between treatments. [See online article for color version of this figure.]
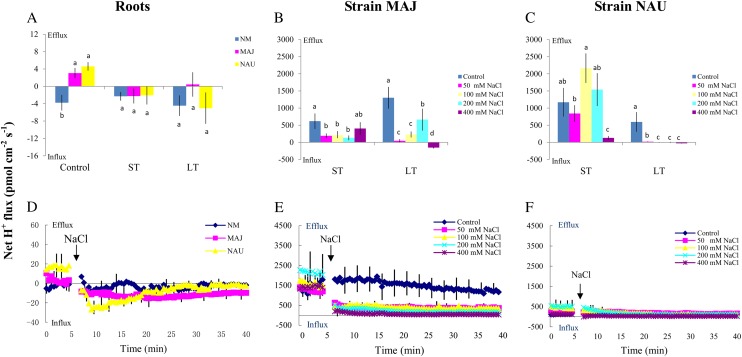
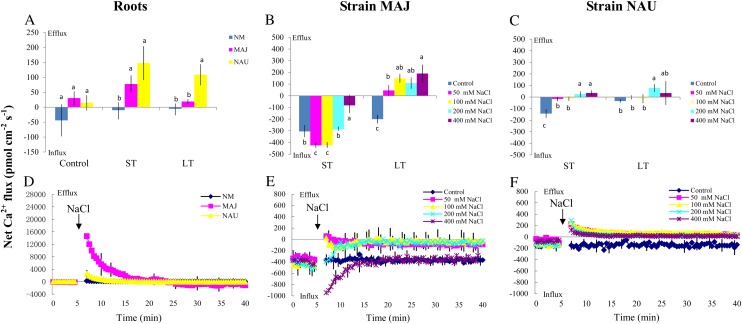
Figure 3.
Effects of NaCl on steady Na+ fluxes in P. × canescens roots (NM, MAJ, NAU) and P. involutus strains MAJ and NAU. A, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d), respectively. Control roots were treated without NaCl. For each plant, Na+ fluxes were measured along root axes (0–2,000 μm from the apex at intervals of 50–300 μm), and mean values are given. Each column is the mean of five to six individual plants, and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between NM and EM roots. B and C, P. involutus isolates MAJ and NAU were subjected to ST salinity (50, 100, 200, or 400 mm NaCl for 24 h) and LT salinity (50, 100, 200, or 400 mm NaCl for 7 d), respectively. Control axenic mycelia were treated without NaCl. Na+ fluxes of fungal hyphae were measured over a recording period of 30 to 40 min, and mean values are given. Each column is the mean of five to six axenic EM cultures (pelleted hyphae), and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between salinity levels. [See online article for color version of this figure.]
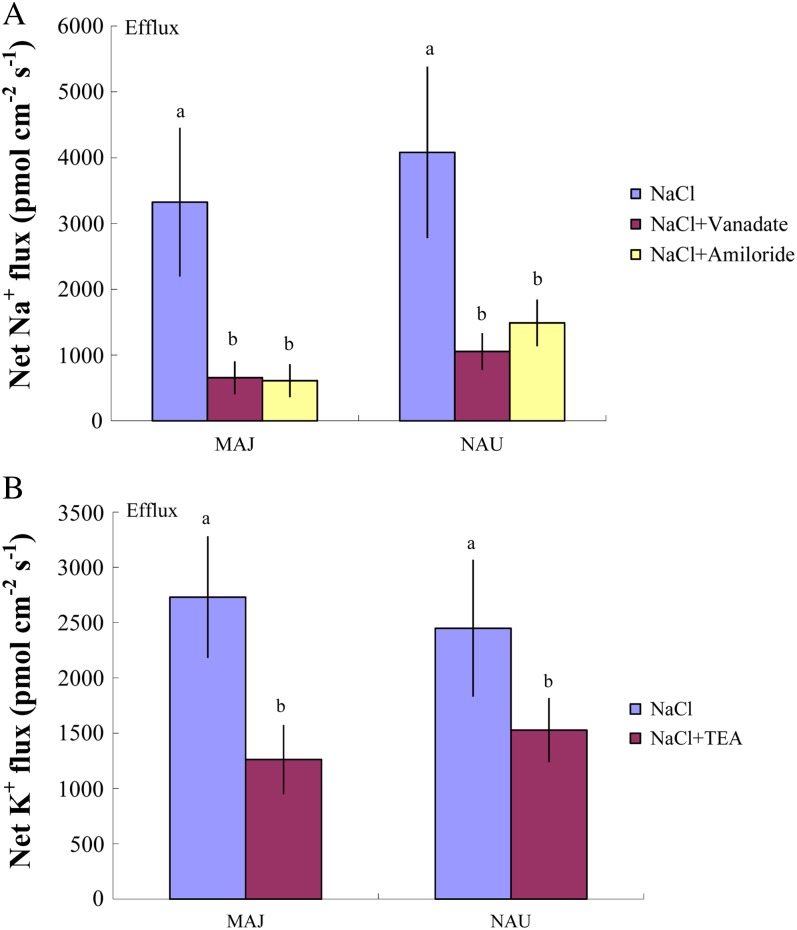
The mycelia of the two P. involutus strains, MAJ and NAU, exhibited a marked Na+ efflux under ST and LT stress (Fig. 3, B and C). The NaCl-induced Na+ efflux was typically higher under conditions of high salinity (400 mm NaCl) compared with lower salinity (50, 100, and 200 mm NaCl; Fig. 3, B and C). However, the two P. involutus strains displayed different capacities to sustain the Na+ efflux at high salt concentrations (400 mm NaCl). LT-treated NAU exhibited a flux rate similar to that of ST-treated hyphae, whereas a reduced Na+ efflux was detected in MAJ under LT salinity (Fig. 3, B and C). Pharmacological experiments showed that the high-salt-induced Na+ efflux in the two fungal strains was significantly reduced by the Na+/H+ antiporter inhibitor amiloride or the plasma membrane H+-ATPase inhibitor sodium orthovanadate (Fig. 4A).
Figure 4.
Effects of pharmacological agents on net Na+ and K+ fluxes in ST-treated P. involutus isolates MAJ and NAU. A, ST-treated (400 mm NaCl, 24 h) axenic mycelia were pretreated with 500 μm sodium orthovanadate or 50 μm amiloride for 30 min prior to measuring Na+ flux. Measuring solutions containing sodium orthovanadate were removed slowly with a pipette, and 10 mL of fresh solution was then slowly added to the measuring chamber. B, ST-treated (400 mm NaCl, 24 h) axenic mycelia were pretreated with 50 μm TEA for 30 min prior to measuring K+ flux. Each column is the mean of five to six axenic EM cultures (pelleted hyphae), and error bars represent se. Na+ and K+ fluxes of fungal hyphae were measured over a recording period of 30 to 40 min, and mean values are given. Columns labeled with different letters indicate significant differences at P < 0.05 between treatments. [See online article for color version of this figure.]
H+ Flux
SIET measurements of root apices revealed a net H+ influx into NM roots in the absence of salt stress (Fig. 5; mean values are shown in Fig. 6A). In contrast, EM roots were characterized by stable and constant H+ effluxes along the measured regions (Figs. 5 and 6A). ST and LT salinity caused a typical shift of H+ efflux toward an influx in EM roots (Figs. 5 and 6A). In the absence of fungal colonization, NaCl did not significantly change the H+ flux profile along the root axis after ST or LT exposure, although the H+ flux oscillated in the measured regions (Fig. 5).
Figure 5.
Effects of ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d) on the net H+ flux in roots of mycorrhizal (MAJ and NAU) and NM P. × canescens plants. Control roots were treated without NaCl. H+ flux was measured along root axes (0–2,000 μm from the apex) at intervals of 50 to 300 μm. Each point is the mean of five to six individual plants, and error bars represent se. *P < 0.05 between treatments. [See online article for color version of this figure.]
Figure 6.
Effects of NaCl on steady and transient H+ fluxes in P. × canescens roots (NM, MAJ, NAU) and P. involutus strains MAJ and NAU. A, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d), respectively. Control roots were treated without NaCl. For each plant, H+ fluxes were measured along root axes (0–2,000 μm from the apex at intervals of 50–300 μm), and mean values are given. Each column is the mean of five to six individual plants, and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between NM and EM roots. B and C, P. involutus isolates MAJ and NAU were subjected to ST salinity (50, 100, 200, or 400 mm NaCl for 24 h) and LT salinity (50, 100, 200, or 400 mm NaCl for 7 d), respectively. Control axenic mycelia were treated without NaCl. H+ fluxes of fungal hyphae were measured over a recording period of 30 to 40 min, and mean values are given. Each column is the mean of five to six axenic EM cultures (pelleted hyphae), and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between salinity levels. D, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to salt shock with 50 mm NaCl. H+ kinetics were recorded at the apex (the measuring site was approximately 500 μm from the root tip) after the required amount of 200 mm NaCl stock was introduced into the measuring chamber. E and F, P. involutus isolates MAJ and NAU were subjected to salt shock with 50 to 400 mm NaCl. H+ kinetics of axenic mycelia were recorded after the required amount of 200 to 800 mm NaCl stock was introduced into the measuring chamber. Before the salt shock, steady H+ fluxes were monitored for approximately 5 min. In D to F, each point is the mean of four individual plants or axenic EM cultures (pelleted hyphae), and error bars represent se. [See online article for color version of this figure.]
MAJ and NAU mycelia exhibited a net H+ efflux under control conditions similar to that of EM roots (Fig. 6, A–C). ST and LT salinity reduced the efflux of H+ from strain MAJ (Fig. 6B). A similar trend was observed in the salinized hyphae of strain NAU, although H+ fluxes in ST-treated NAU varied with salt concentration (Fig. 6C). The salinized fungus NAU exhibited a temporary influx (approximately 5–10 min) during the period of recording (30 min), especially under LT treatment, although the mean value indicated outward rectification (data not shown).
The salt-induced transient H+ kinetics in roots and fungal mycelia were also examined. In the absence of salt stress, H+ efflux was detected in EM roots instead of H+ influx in NM roots (Fig. 6D). Salt shock (50 mm NaCl) caused a pronounced shift in the H+ efflux toward an influx in EM roots (Fig. 6D), but no significant changes in the H+ kinetics of NM roots were observed during the recording period (Fig. 6D). The responses of the transient H+ kinetics to salt shock were compared between the two P. involutus strains. After exposure to NaCl (50 to 400 mm), hyphae exhibited an instantaneous decrease in the H+ efflux, which then remained constant during the period of recording (40 min; Fig. 6, E and F). Compared with strain NAU, the shock-induced reduction in H+ efflux was more pronounced in strain MAJ over the concentration range of 50 to 400 mm NaCl (Fig. 6, E and F).
K+ Flux
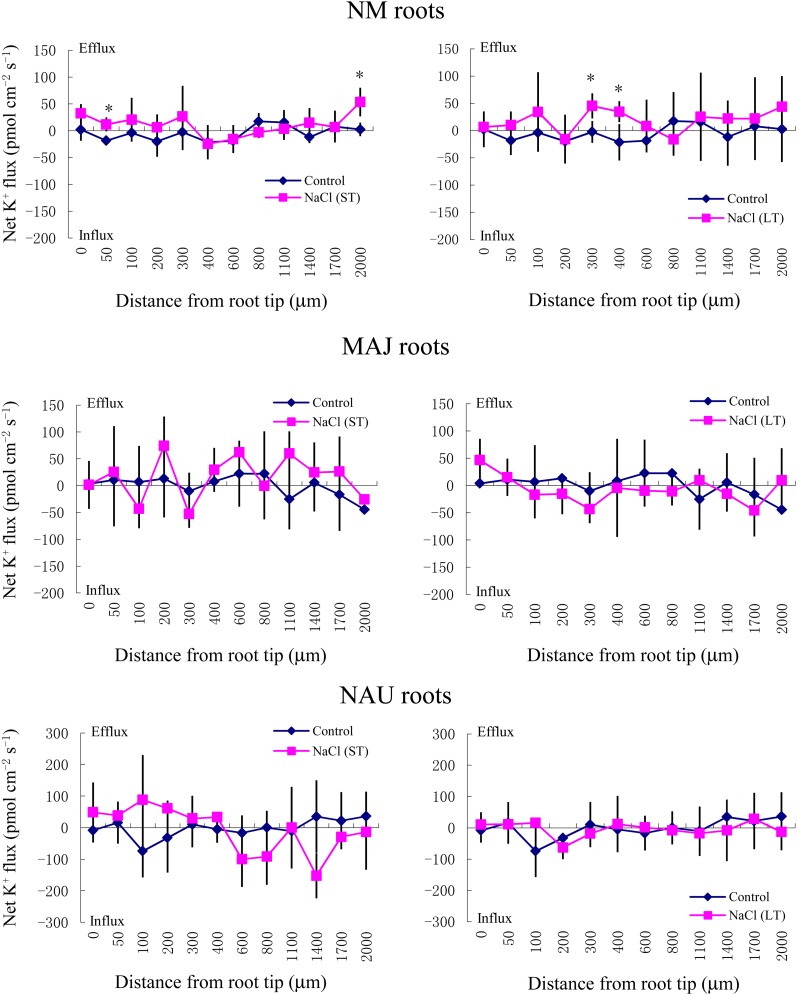
Although the K+ flux varied along the root axis (Fig. 7), ST and LT salt treatments resulted in an overall net K+ efflux from NM roots (Fig. 8A). Mycorrhization of P. × canescens roots with P. involutus reduced the K+ efflux under ST and LT treatments, with the exception of ST-stressed MAJ-mycorrhizal roots (Figs. 7 and 8A). ST and LT treatment caused a net K+ efflux from the mycelia of both NAU and MAJ, but the effect was more pronounced in MAJ, especially under ST salinity (Fig. 8, B and C). Tetraethylammonium chloride (TEA), a K+ channel blocker, significantly decreased the salt-induced K+ efflux from the hyphae of the two strains (Fig. 4B).
Figure 7.
Effects of ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d) on net K+ flux in roots of mycorrhizal (MAJ and NAU) and NM P. × canescens plants. Control roots were treated without NaCl. The K+ flux was measured along root axes (0–2,000 μm from the apex) at intervals of 50 to 300 μm. Each point is the mean of five to six individual plants, and error bars represent se. *P < 0.05 between treatments. [See online article for color version of this figure.]
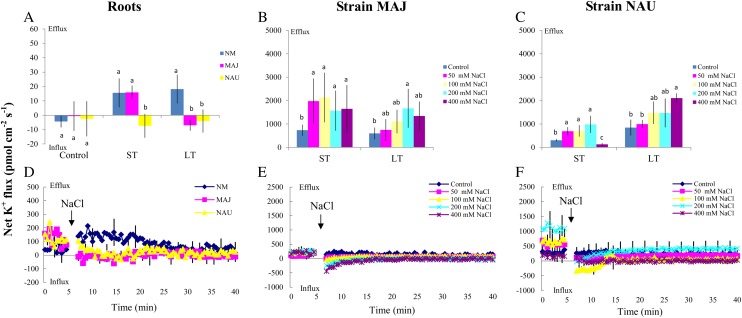
Figure 8.
Effects of NaCl on steady and transient K+ fluxes in P. × canescens roots (NM, MAJ, NAU) and P. involutus strains MAJ and NAU. A, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d), respectively. Control roots were treated without NaCl. For each plant, K+ fluxes were measured along root axes (0–2,000 μm from the apex at intervals of 50–300 μm), and mean values are given. Each column is the mean of five to six individual plants, and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between NM and EM roots. B and C, P. involutus isolates MAJ and NAU were subjected to ST salinity (50, 100, 200, or 400 mm NaCl for 24 h) and LT salinity (50, 100, 200, or 400 mm NaCl for 7 d), respectively. Control axenic mycelia were treated without NaCl. K+ fluxes of fungal hyphae were measured over a recording period of 30 to 40 min, and mean values are given. Each column is the mean of five to six axenic EM cultures (pelleted hyphae), and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between salinity levels. D, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to salt shock with 50 mm NaCl. K+ kinetics were recorded at the apex (the measuring site was approximately 500 μm from the root tip) after the required amount of 200 mm NaCl stock was introduced into the measuring chamber. E and F, P. involutus isolates MAJ and NAU were subjected to salt shock with 50 to 400 mm NaCl. K+ kinetics of axenic mycelia were recorded after the required amount of 200 to 800 mm NaCl stock was introduced into the measuring chamber. Before the salt shock, steady K+ fluxes were monitored for approximately 5 min. In D to F, each point is the mean of four individual plants or axenic EM cultures (pelleted hyphae), and error bars represent se. [See online article for color version of this figure.]
In salinized P. × canescens roots, the transient K+ kinetics in response to salt shock followed a trend similar to the steady-state measurements (Fig. 8, A and D). Salt shock caused an evident K+ efflux in NM roots, but the flux rate was lower in MAJ- and NAU-mycorrhizal roots (Fig. 8D). In the hyphae of the two strains, the rate of K+ efflux was reduced after the addition of NaCl (50–400 mm; Fig. 8, E and F). An instantaneous increase in the K+ influx was detected in MAJ and NAU after the onset of salt shock (Fig. 8, E and F).
Ca2+ Flux
Steady-state flux measurements showed that ST and LT treatment accelerated Ca2+ efflux along mycorrhizal roots, especially in NAU-mycorrhizal plants (Fig. 9; mean values are shown in Fig. 10A). In the absence of salt stress, the hyphae of the two strains exhibited Ca2+ influx, with a higher flux rate in MAJ than in NAU (Fig. 10, B and C). ST-treated MAJ maintained the Ca2+ influx, but the flux rate decreased with increasing NaCl concentrations (Fig. 10B). In strain NAU, ST salinity reduced the influx under low-salt conditions (50 and 100 mm NaCl) and shifted toward an efflux at high salinity (200 and 400 mm NaCl; Fig. 10C). Under LT stress, the pattern of Ca2+ flux was similar in the two strains. LT salinity reduced the influx or reversed the rectification toward an efflux (Fig. 10, B and C).
Figure 9.
Effects of ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d) on the net Ca2+ flux in the roots of mycorrhizal (MAJ and NAU) and NM P. × canescens plants. Control roots were treated without NaCl. The Ca2+ flux was measured along root axes (0–2,000 μm from the apex) at intervals of 50 to 300 μm. Each point is the mean of five to six individual plants, and error bars represent se. *P < 0.05 between treatments. [See online article for color version of this figure.]
Figure 10.
Effects of NaCl on steady and transient Ca2+ fluxes in P. × canescens roots (NM, MAJ, NAU) and P. involutus strains MAJ and NAU. A, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to ST salinity (50 mm NaCl for 24 h) and LT salinity (50 mm NaCl for 7 d), respectively. Control roots were treated without NaCl. For each plant, Ca2+ fluxes were measured along root axes (0–2,000 μm from the apex at intervals of 50–300 μm), and mean values are given. Each column is the mean of five to six individual plants, and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between NM and EM roots. B and C, P. involutus isolates MAJ and NAU were subjected to ST salinity (50, 100, 200, or 400 mm NaCl for 24 h) and LT salinity (50, 100, 200, or 400 mm NaCl for 7 d), respectively. Control axenic mycelia were treated without NaCl. Ca2+ fluxes of fungal hyphae were measured over a recording period of 30 to 40 min, and mean values are given. Each column is the mean of five to six axenic EM cultures (pelleted hyphae), and error bars represent se. Columns labeled with different letters indicate significant differences at P < 0.05 between salinity levels. D, Mycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to salt shock with 50 mm NaCl. Ca2+ kinetics were recorded at the apex (the measuring site was approximately 500 μm from the root tip) after the required amount of 200 mm NaCl stock was introduced into the measuring chamber. E and F, P. involutus isolates MAJ and NAU were subjected to salt shock with 50 to 400 mm NaCl. Ca2+ kinetics of axenic mycelia were recorded after the required amount of 200 to 800 mm NaCl stock was introduced into the measuring chamber. Before the salt shock, steady Ca2+ fluxes were monitored for approximately 5 min. In d to F, each point is the mean of four individual plants or axenic EM cultures (pelleted hyphae), and error bars represent se. [See online article for color version of this figure.]
When subjected to salt shock, EM roots exhibited a transient increase in Ca2+ efflux, but no corresponding changes were observed in NM roots (Fig. 10D). The shock-induced Ca2+ efflux was more pronounced in MAJ-mycorrhizal roots than NAU-colonized roots (Fig. 10D). LaCl3, an inhibitor of Ca2+-permeable channels, did not markedly restrict the high rate of Ca2+ efflux from salt-shocked MAJ and NAU roots (Supplemental Fig. S1). In the absence of salt stress, the mycelia of the two strains exhibited a stable and steady influx of Ca2+, typically with higher flux rates in MAJ than in NAU (Fig. 10, E and F). NAU hyphae exhibited a Ca2+ efflux immediately after the addition of NaCl (50–400 mm; Fig. 10F). Similarly, salt shock reduced the Ca2+ influx in strain MAJ, although a transient increase in Ca2+ influx was observed in the presence of 400 mm NaCl (Fig. 10E).
Effects of Ca2+ on Salt-Induced K+ and Na+ Fluxes in P. × canescens Roots
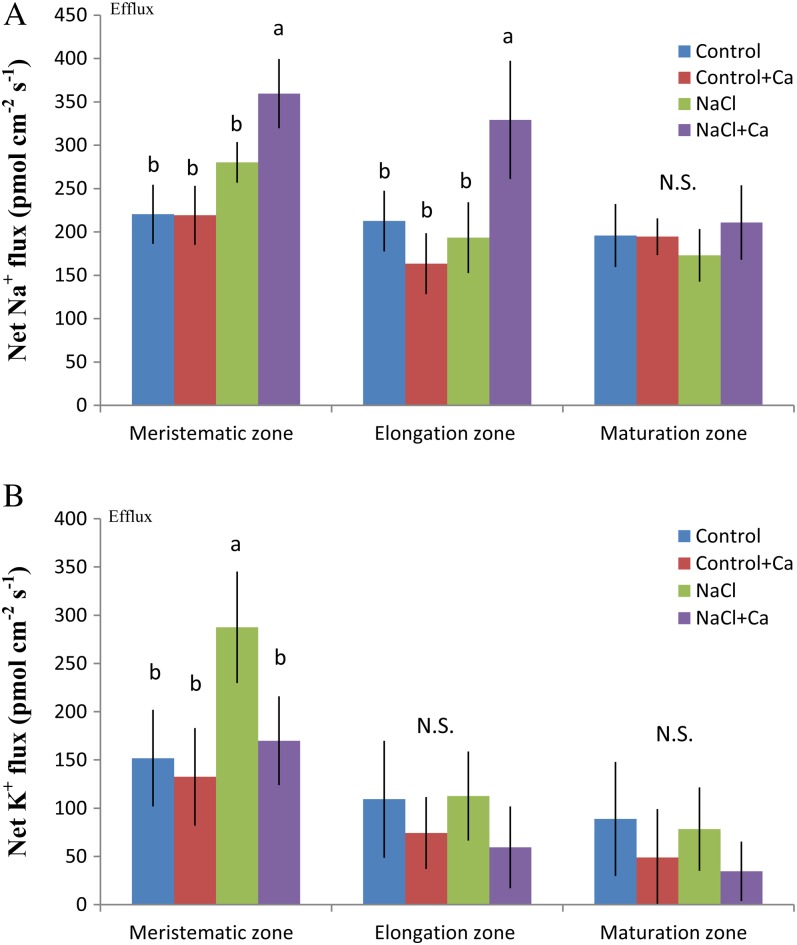
The effect of Ca2+ on K+ and Na+ fluxes was examined in salinized roots. The aim was to investigate whether Ca2+ released from salt-treated mycorrhizal associations benefits root cells in the control of K+/Na+ homeostasis. Ca2+ application (10 mm) markedly limited the salt-induced K+ efflux and enhanced the apparent Na+ efflux in P. × canescens roots (Fig. 11). More profound effects were found in the meristematic zone (K+ and Na+) and elongation region (Na+) than in other parts of the root.
Figure 11.
Effects of NaCl and Ca2+ supplementation on Na+ (A) and K+ (B) flux at the meristematic, elongation, and maturation zones of P. × canescens roots. Plants were subjected to 1 week of NaCl stress (50 mm) supplemented with or without 10 mm CaCl2. Control plants were well fertilized but treated without additional NaCl. Roots were sampled at 24 h and 7 d, and mean flux values are given. Steady fluxes at the meristematic, elongation, and maturation regions were measured along the root axes at intervals of 30 to 50 μm. Each column is the mean of six to eight individual plants, and error bars represent se (for each plant, the average fluxes at the meristematic, elongation, and maturation zones were calibrated from measuring points in the measured region). Columns labeled with different letters indicate significant differences at P < 0.05 between treatments. N.S., No significant difference. [See online article for color version of this figure.]
DISCUSSION
EM Fungal Colonization Ameliorated K+/Na+ Homeostasis under Salt Stress
Colonization of the salt-sensitive P. × canescens with the EM fungus P. involutus (strain MAJ) was previously found to improve growth, prime for increased stress tolerance, and increase nutrition under salt stress (Langenfeld-Heyser et al., 2007; Luo et al., 2009, 2011). Similar findings were observed in this study. NM plants abscised old leaves from the lower shoots, and upper leaves displayed salt damage after 7 d of NaCl treatment (50 mm; Supplemental Fig. S2D). However, the salt injury was alleviated by EM colonization (Supplemental Fig. S2, D–F). Salinized P. × canescens exhibited an enhanced capacity to maintain K+/Na+ homeostasis in the presence of fungal colonization (strains MAJ and NAU). The maintenance of K+/Na+ homeostasis is crucial for P. × canescens to tolerate saline conditions (Chen et al., 2001, 2002a, 2002b, 2003; Sun et al., 2009a, 2009b, 2010; Chen and Polle, 2010). SEM-EDX microanalysis data indicate that the high K+/Na+ ratio in mycorrhizal plants was the result of less Na+ accumulation and K+ reduction under NaCl stress than in NM plants (Fig. 1). This finding is in accordance with our previous finding that MAJ-mycorrhizal P. × canescens had diminished leaf Na+ but increased the K+ supply under salt stress (Langenfeld-Heyser et al., 2007; Luo et al., 2009). Unexpectedly, we found that NAU-colonized plants exhibited a higher ability to maintain K+/Na+ homeostasis than MAJ, although NAU does form a Hartig net with P. × canescens and ensheathes the root tip with a loose mycelial network (Supplemental Fig. S2; Gafur et al., 2004). Thus, the formation of a physical barrier by the mantel formed by MAJ is likely not involved in modifying salt uptake. The increased capacity for ion balance control in the salt-sensitive host plant, which was promoted by fungal colonization, was probably due to the modulation of transport systems.
Ectomycorrhizae Enhance Na+ Extrusion
The reduction in shoot Na+ uptake has been suggested to be an important resistance mechanism in EM plants growing in salinized soil (Muhsin and Zwiazek, 2002; Nguyen et al., 2006). Leaf Na+ in mycorrhizal P. × canescens was 6% (MAJ) and 76% (NAU) lower than in NM plants after 1 week of exposure to 50 mm NaCl (Fig. 1), which is in agreement with our previous findings (Langenfeld-Heyser et al., 2007). Similarly, the EM fungi H. crustuliniforme and L. bicolor have been shown to reduce tissue Na+ concentrations and alleviate salt injury in several conifers (Muhsin and Zwiazek, 2002; Nguyen et al., 2006). The diminished buildup of salt in shoots is likely the result of salt uptake and transport restriction in roots (Chen et al., 2002a, 2003). Our SIET data show that the salt-induced entry of Na+ was markedly impeded in EM plants, especially in NAU-colonized roots (Figs. 2 and 3). The same trend was shown in fungal hyphae, which exhibited a steady Na+ efflux under ST and LT stress. As amiloride, a Na+/H+ antiporter inhibitor, reduced Na+ efflux in the salt-treated mycelia of both strains (Fig. 4), our data suggest that P. involutus contributed to active Na+ extrusion in the salinized roots of P. × canescens.
Notably, the salt-enhanced Na+ efflux was always associated with an H+ influx into EM roots (Figs. 2, 3, 5, and 6), whereas EM roots exhibited a net H+ efflux in the absence of salt stress (Figs. 5 and 6). The H+ efflux in EM roots apparently resulted from the root-ensheathing fungus, as MAJ and NAU hyphae exhibited an evident H+ efflux (Fig. 6). Our data agree with the findings of Ramos et al. (2009), who reported significant H+ efflux from Eucalyptus globulus roots (the apex, meristematic, and elongation zones) colonized with Pisolithus species. The mycorrhiza-stimulated H+ efflux is due to the activity of plasma membrane H+-ATPase in the EM associations. In the roots of Pinus sylvestris-Laccaria laccata, Lei and Dexheimer (1988) showed the localization of ATPase activity along the Hartig net hyphal plasma membranes and the plasma membranes of contiguous living cortical cells. Arbuscular mycorrhizal symbioses induced the expression of two genes (pma2 and pma4) responsible for de novo H+-ATPase activity in the periarbuscular membrane of invaded cells (Gianinazzi-Pearson et al., 2000). Some host plasma membrane H+-ATPase isoforms show high activity in arbuscular mycorrhizal associations (Ramos et al., 2005; Rosewarne et al., 2007). In addition, three plasma membrane H+-ATPase genes (LHA1, LHA2, and LHA4) were found to be regulated by arbuscular mycorrhiza in tomato (Solanum lycopersicum) plants (Ferrol et al., 2002). The molecular analysis of ATPases in EM roots appears to be missing. In our study, salt exposure reversed the rectification of H+ from efflux to influx in the EM roots of P. × canescens (Figs. 5 and 6). In accordance, the H+ efflux from hyphae was diminished by various salt treatments (salt shock, ST, and LT treatments, with a few exceptions) or shifted to a net influx during the period of recording. In the presence of an inhibitor of the plasma membrane proton pump, sodium orthovanadate, the salt-enhanced Na+ efflux was reduced in the two strains (Fig. 4). Taken together, these data suggest that colonization with P. involutus stimulates the H+-ATPase activity in P. × canescens-ectomycorrhizae associations, which pumps protons to promote the secondary active Na+/H+ antiport at the plasma membrane (Blumwald et al., 2000; Zhu, 2003). We have noticed that under salt exposure, the influx of H+ was not equivalent to the efflux of Na+. The flux inconsistency of Na+ and H+ is mainly due to the superimposition of two effects: salt-induced H+ excretion, as salinity stress is usually associated with increased H+ efflux, and at the same time, higher SALT-OVERLY-SENSITIVE1 Na+/H+ exchanger activity of EM plants, which leads to an accelerated H+ uptake. As such, the net H+ flux will not be changed.
For Na+ flux measurements, special attention needs to be paid to Na+ microelectrodes because of the nonideal selectivity of the commercially available Na+ liquid ion exchanger (LIX; Chen et al., 2005). Na+ electrodes produced signals when we calibrated Na+ LIX in a range of K+ or Ca2+ standards, in accordance with the finding by Chen et al. (2005). To reduce interfering effects of K+ on Na+ flux, K+ was omitted from the bathing medium (Cuin et al., 2011). In our study, Ca2+ and K+ concentrations in the measuring solution were set to low concentrations, 0.1 and 0.5 mm, respectively. To estimate the interference of K+ and Ca2+ on Na+ flux in plant materials, we measured root Na+ flux in control and salinized P. × canescens plants (50 mm NaCl for 24 h) in the presence and absence of interfering ions (K+ and Ca2+). We found that 0.5 mm K+ and 0.1 mm Ca2+ had no significant effects on root Na+ flux in no-salt controls (data not shown). However, in salt-treated roots, the absence of K+ and Ca2+ in the measuring buffer resulted in higher signals but did not change the tendency of root flux (data not shown). We found that the detected Na+ signals in salinized roots were unstable and fluctuated greatly during the period of recording. This is presumably the plant response to nutrient deficiency in the root medium. In our study, the presence of K+ and Ca2+ may not affect the accuracy of our conclusions relating to Na+ fluxes in mycorrhizal roots and axenic EM cultures. The experimental evidence and explanations are briefly listed here. (1) Na+ electrodes exhibited much higher selectivity for Na+ relative to K+ and Ca2+ in the presence of both Na+ and interfering ions (Supplemental Table S1). Moreover, the released Na+ from salinized mycorrhizal roots and hyphae would increase the ratio of Na+ to K+ and Ca2+ near the electrode, thus increasing the selectivity of the Na+ electrode during the period of recording. (2) Treatment with amiloride (an inhibitor of the Na+/H+ antiporter) significantly decreased the Na+ efflux in axenic mycelia (Fig. 4), suggesting that the detected signals were largely carried by Na+ across the plasma membrane. (3) The different trends of Na+, K+, and Ca2+ fluxes suggest that the selectivity of Na+ LIX was sufficient for Na+/K+ and Na+/Ca2+ discrimination. Salinized axenic mycelia exhibited an outward rectification of Na+ and K+, but with different patterns with increasing NaCl concentrations (Figs. 3 and 8). Salt treatment caused an evident Na+ efflux in EM cultures, while the detected Ca2+ efflux was low and an inward rectification was usually seen in salinized mycelia (Figs. 3 and 10). Salinity induced an evident Ca2+ efflux in mycorrhizal roots, whereas there was no equivalent Na+ flux corresponding to the Ca2+ efflux in these roots (Figs. 2, 3, 9, and 10).
EM Ameliorates K+ Homeostasis
In our study, K+ concentrations in the roots and leaves were reduced by salt treatment to a lesser extent in mycorrhizal plants than in NM plants. This finding agrees with the results of Langenfeld-Heyser et al. (2007), who found that MAJ-mycorrhizal plants contained higher leaf K+ levels than non-EM plants. Our studies show that P. involutus fungi assist host plants in the maintenance of K+ homeostasis by delivering the nutrient to the plant and slowing the loss of K+ under NaCl stress. Zhang et al. (2008) reported that P. involutus mycelium, especially strain MAJ, increased the uptake of K+ after exposure to salt treatment. Salt shock caused an instantaneous influx of K+ into the fungal mycelium; however, ST- and LT-stressed hyphae exhibited a K+ efflux (Fig. 8). Our data indicate that prolonged NaCl treatment resulted in K+ loss from P. involutus. Currently, we cannot conclude that K+ efflux is mediated by cation channels, although the K+ efflux caused by NaCl (400 mm) was significantly reduced by the K+ channel blocker (TEA) in the two tested strains (Fig. 4). The high rate of K+ efflux detected in the control and salinized mycelia (ST and LT treatments) relates, to some extent, to the concentrations of the K+ sources (Fig. 8). The enriched K+ in EM hyphae is thought to be transferred to the host during the period of salt treatment, which caused a lesser reduction of K+ in the roots and leaves of EM plants (Fig. 1). Rygiewicz and Bledsoe (1984) reported that external hyphae in EM symbiosis have a high capacity to take up K+ and deliver the nutrient to the host plant. In addition to stimulating K+ uptake, P. involutus colonization benefits the salinized hosts by slowing down the rate of K+ loss. Steady and transient flux data for P. × canescens roots indicate that the salt-induced K+ efflux was decreased by P. involutus colonization (Figs. 7 and 8). The reduced rate of K+ efflux in ST- and LT-treated EM roots is partly the result of a delayed K+ loss from inner host cells. In the fungus-ensheathed roots, K+ in the compatible (MAJ) or incompatible (NAU) hyphae was replaced by Na+ before K+ exchange with the root cells of the host was expected. Moreover, the released Ca2+ ions that are replaced by Na+ can assist plants in maintaining K+ homeostasis under salt stress.
Mediation of Ca2+ in K+/Na+ Homeostasis in EM Plants
In our study, EM P. × canescens plants exhibited an enhanced Ca2+ uptake ability in the absence of salinity stress (Fig. 1). The Ca2+ enrichment was caused by colonization with P. involutus, because the mycelium also displayed a stable Ca2+ influx under these conditions (Fig. 10). Similarly, the EM roots of E. globulus colonized by Pisolithus species exhibit a high uptake capacity for Ca2+ from the external medium (Ramos et al., 2009). Transient and steady flux measurements of mycorrhizal roots revealed a significant Ca2+ efflux under salt stress, suggesting that the Ca2+ accumulated in mycorrhizal roots could be replaced by Na+ (Figs. 9 and 10). The salt-induced Ca2+ flux may originate from the cell wall (Arif et al., 1995; Shabala and Newman, 2000); LaCl3, an inhibitor of Ca2+-permeable channels, did not markedly restrict the high rate of Ca2+ efflux from salt-shocked MAJ and NAU roots (Supplemental Fig. S1). A large amount of cell wall Ca2+ has been shown to be exchangeable in mycorrhizal roots (Peterson and Enstone, 1996; Kuhn et al., 2000; Bücking et al., 2002). The increase in leaf Ca2+ as a consequence of NaCl treatment indicates that the root-derived element could be transported to the shoots in addition to being released from the root surface. Thus, freed Ca2+ is able to assist plants in ameliorating the K+/Na+ ratio under salt stress. Ca2+ inhibits DA-KORCs and DA-NSCCs to reduce K+ efflux in Arabidopsis under saline conditions (Shabala et al., 2006a; Sun et al., 2009b). Exogenous Ca2+ improved the K+/Na+ balance by inhibiting K+ efflux and increasing the apparent Na+ efflux in P. × canescens roots (Fig. 11). These results are consistent with our previous findings in NM roots of another salt-sensitive poplar, Populus popularis (Sun et al., 2009b). Therefore, we conclude that the increased availability of free Ca2+, which was released by Ca2+/Na+ exchange from EM roots, favored the establishment of K+/Na+ homeostasis in P. × canescens under salt treatment.
Differences in K+/Na+ maintenance between MAJ- and NAU-colonized roots are clear (Fig. 1), which might have resulted from differences in the release of exchangeable Ca2+ from mycorrhizal associations. MAJ-mycorrhizal roots lost a large amount of Ca2+ after being subjected to salt shock (Fig. 10), whereas NAU-colonized roots exhibited a long-sustained release of free Ca2+ during ST and LT salt treatments (Figs. 9 and 10). Consequently, the Ca2+ ions released from the mycorrhizal associations would benefit inner root cells controlling K+/Na+ homeostasis in terms of the effects of Ca2+ on K+ and Na+ fluxes in P. × canescens roots. We noticed that MAJ-mycorrhizal roots displayed a significant Ca2+ efflux under salt stress; however, a net Ca2+ influx was usually recorded in the fungal mycelium of this strain after exposure to salt shock and ST treatment (Figs. 9 and 10). Therefore, the salt-induced Ca2+ efflux that we detected from MAJ-mycorrhizal roots likely came from inner host cells, at least in part, as a result of Na+/Ca2+ exchange. Compared with NAU, the inner root cells might have been more accessible to Na+ because MAJ forms a typical Hartig net with the roots of P. × canescens, whereas NAU exhibits cell wall thickening (Supplemental Fig. S2; Gafur et al., 2004).
MATERIALS AND METHODS
Plant and Fungal Cultures for EM Colonization
The EM fungi used in this study were the Paxillus involutus isolates MAJ and NAU. Plants, fungal cultures, and the technique for synthesizing ectomycorrhizae followed the procedures described by Gafur et al. (2004). In brief, the two isolates were grown on 2% modified Melin Norkrans (MMN) agar medium [the medium contains the following components in g L−1: 0.5 KH2PO4, 0.25 (NH4)2SO4, 0.15 MgSO4·7H2O, 0.05 CaCl2·2H2O, 0.025 NaCl, 0.01 FeCl3·6H2O, 0.0001 thiamine-HCl, 10 Glc, and 3 malt extract, pH 5.0; Gafur et al., 2004]. After fungal inoculation, the petri dishes (diameter, 90 mm) were sealed with a strip of Parafilm and kept in permanent darkness at 23°C.
In this study, we used a petri dish culture system for the colonization of Populus × canescens with the P. involutus strains MAJ and NAU (Gafur et al., 2004). In brief, regenerated plantlets of P. × canescens were grown for 2 to 3 weeks on Murashige and Skoog rooting medium. The fungi were pregrown on the agar culture medium for 1 week prior to colonization. Vigorous plantlets with sufficient roots were used for ectomycorrhization. Rooted plantlets from sterile culture were carefully freed from agar particles. Rooted plantlets were placed on the MMN agar medium in the presence or absence of EM mycelium. During the period of incubation, the room temperature was 24°C ± 1°C with a light period of 14 h (6:00 am to 8:00 pm). Photosynthetic active radiation of 200 μmol m−2 s−1 was supplied by cool-white fluorescent lamps. Root-fungus associations were formed during 1 month after colonization. All plantlets exhibited well-developed shoots and roots (Supplemental Fig. S2, A–C). Some leaves at lower shoots became dry at the tips (Supplemental Fig. S2, A–C). Samples of EM and NM root tips for anatomical investigations were embedded, stained, and photographed as described previously (Gafur et al., 2004). Anatomical analyses of mycorrhizal roots showed that the hyphae of strain MAJ penetrated into the cell walls of the cortex, whereas NAU hyphae were only detected on the outer epidermal cell walls (Supplemental Fig. S2, G–I). Uniform mycorrhizal and NM plants were used for acclimation and salt treatment.
Liquid Culture of Fungi
Liquid culture of P. involutus was grown at the College of Biological Sciences and Technology, Beijing Forestry University. Strains MAJ and NAU were obtained from the Büsgen Institute of Forest Botany and Tree Physiology, Göttingen University. For liquid culture, agar was absent and the medium was buffered with citrate (Ott et al., 2002). Mycelium from the agar plate was homogenized, transferred into 150 mL of liquid medium in flasks, and incubated on a rotary shaker in darkness (150 rpm, 23°C; Langenfeld-Heyser et al., 2007). P. involutus in submerged culture grew in the form of compact spherical masses of mycelium (pellets). For salt treatment, sterile filtered NaCl solutions were added to achieve final concentrations of 0, 50, 100, 200, and 400 mm. After ST (24 h) or LT (7 d) treatment, axenic cultures of MAJ and NAU were used for steady flux measurements of Na+, H+, K+, and Ca2+.
Plant Acclimation and Salt Treatments
Prior to salt treatment, mycorrhizal and NM plants were carefully removed from MMN agar medium, planted in individual pots containing fine sand, and grown in a greenhouse at the College of Biological Sciences and Technology, Beijing Forestry University. The room temperature was 24°C ± 1°C with a light period of 14 h (6:00 am to 8:00 pm), and photosynthetic active radiation of 200 μmol m−2 s−1 was supplied by cool-white fluorescent lamps. Mycorrhizal and NM plantlets were exposed to 50 mm NaCl for ST (24 h) or LT (7 d) treatments. The required NaCl concentrations were added to the LN (Matzner et al., 1982) nutrient solution (Langenfeld-Heyser et al., 2007). Control plantlets received LN solution without NaCl. On days 1 and 7, roots with apices of 1 to 2 cm were sampled from mycorrhizal and NM plants and used for steady-state measurements of the net Na+, H+, K+, and Ca2+ fluxes. At the final harvest, leaf segments (2–3 mm long and 1–2 mm wide) and root segments with 1.0-cm apices were sampled from mycorrhizal and NM plantlets, freeze dried, and used for microanalysis by SEM-EDX.
CaCl2 Treatment
Plantlets of P. × canescens were multiplied by micropropagation as described by Leplé et al. (1992). Rooted plantlets were cultivated in hydroponic LN nutrient solutions with low nitrogen supply (Gafur et al., 2004). To acclimate the plants to ambient conditions, plantlets were covered with plastic bags and illuminated with low light (50 μmol m−2 s−1) supplied by cool-white fluorescent lamps. The plastic bags were gradually opened over 1 week. Acclimated plants were subjected to NaCl (50 mm) or NaCl (50 mm) plus CaCl2 (10 mm). The required amounts of NaCl and CaCl2 were added to the nutrient solution. Control plants were treated in the same manner without the addition of NaCl. Plants were continuously aerated by passing air to hydroponic LN nutrient solution, which was regularly renewed. Steady fluxes of K+ and Na+ in meristematic, elongation, and maturation zones were measured after 24 h and 7 d of treatment.
Inhibitor Treatments
ST-treated (400 mm NaCl) P. involutus isolates MAJ and NAU were subjected to sodium orthovanadate (500 μm), amiloride (50 μm), or TEA (50 μm) for 30 min in the measuring solutions. Prior to recording Na+ flux, the measuring solutions containing sodium orthovanadate were replaced with 10 mL of fresh solution, but the measuring solution with amiloride and TEA was not renewed (amiloride and TEA had no clear effect on the Nernstian slopes of Na+ and K+ electrodes; Sun et al., 2009a, 2009b). Steady-state fluxes of Na+ (orthovanadate or amiloride treatment) and K+ (TEA treatment) were measured in axenic mycelia (pelleted hyphae) pretreated with or without inhibitors at pH 6.0.
Steady-State Measurements of Net Na+, H+, K+, and Ca2+ Fluxes
The net fluxes of Na+, H+, K+, and Ca2+ in roots and mycelia were measured using a noninvasive SIET (BIO-001A; Younger USA Science and Technology; Xu et al., 2006; Sun et al., 2009a, 2009b). The concentrations of the ions and concentration gradients were measured by moving the ion-selective microelectrode between two positions close to the materials in a preset excursion (30 μm) at a programmable frequency in the range of 0.3 to 0.5 Hz.
Ion-selective microelectrodes for the target ions were calibrated prior to flux measurements: (1) Na+, 0.1, 0.5, 1.0 mm (Na+ concentration was usually 0.1 mm in the measuring buffer for roots and axenic mycelia); (2) H+, pH 4.0, 5.0, 6.0 (pH was 5.0 in the measuring buffer); (3) K+, 0.1, 0.5, 1.0 mm (K+ was 0.5 mm in the measuring buffer); and (4) Ca2+, 0.1, 0.5, 1.0 mm (Ca2+ was 0.1 mm in the measuring buffer). All electrodes used for steady and transient recordings were usually corrected two to three times by calibrations during the experiments. The ion flux rate was calculated using Fick’s law of diffusion:
where J is the ion flux in the x direction, dc represents the ion concentration difference, dx is the microelectrode movement between two positions, dc/dx is the ion concentration gradient, and D represents the ion diffusion coefficient in a particular medium.
Flux Oscillations
Rhythmic (ultradian) flux oscillations are ubiquitous in the measured plant species (Souda et al., 1990; Toko et al., 1990; Shabala et al., 1997, 2003, 2006b; Shabala and Knowles, 2002). H+ flux oscillations have been widely reported in a variety of plant species. In our study, oscillations in the H+ flux in P. × canescens roots were not as noticeable as in herbaceous species. The H+ oscillations were more like fluctuations, and oscillations in the Na+, K+, and Ca2+ fluxes displayed similar trends as H+ (Supplemental Fig. S3). This finding is presumably due to a lower growth rate of woody roots compared with crop species. Toko et al. (1990) found that roots exhibit no oscillations when they have a slow growth speed. Salinity caused a significant decrease in the period of H+ flux oscillations (Shabala, 2003). Our data show that the oscillatory periods of measured ions (H+, Na+, K+, and Ca2+) in P. × canescens roots are usually in the range of several minutes. In our scanning studies, fluxes were recorded for 8 to 10 min at each point, which is long enough to ensure the absence of oscillations.
Ion Selectivity of Na+ Electrodes
We found that Na+ microelectrodes were not able to record Na+ flux in a measuring buffer containing high Na+ due to the low signal/noise ratio of Na+ LIX (Fluka 71178; Sun et al., 2009a). Na+-selective microelectrodes were also found to be unsuitable for screening Na+ fluxes, because of the nonideal selectivity of the commercially available Na+ LIX (Chen et al., 2005). K+ was omitted from the bathing medium to reduce the interfering effects of K+ on Na+ flux (Cuin et al., 2011). To determine the interfering effects of K+ and Ca2+ on Na+-selective electrodes, Na+-selective microelectrodes were calibrated in Na+ solution (0.1, 0.5, 1.0 mm) in the presence or absence of K+ (0.1, 0.5, 1.0 mm) and Ca2+ (0.1, 0.5, 1.0 mm). The calibration characteristics (Nernst slope) of the Na+ electrode were not altered by the interfering K+ ion (concentration range of 0.1–1.0 mm; Supplemental Table S1). In the presence of Ca2+, the Nernst slope of the Na+ electrodes was reduced up to 24% at 1.0 mm Ca2+ (Supplemental Table S1). To reduce the interfering effects of Ca2+ on Na+ electrodes, the Ca2+ concentration in the measuring solution was set to 0.1 mm. The Nernst slope and intercept of the Na+ electrodes in the measuring solution (0.5 mm KCl, 0.1 mm NaCl, 0.1 mm CaCl2, 0.1 mm MgCl2) were 50.0031 ± 1.9249 and 73.1593 ± 0.6543, similar to the values in the absence of K+ and Ca2+ (Nernst slope, 55.7648 ± 1.9751; Nernst intercept, 75.9074 ± 1.8814).
Experimental Protocols for SIET Measurements
Roots sampled from mycorrhizal and NM plants and mycelial hyphae collected from the liquid culture medium were rinsed with distilled water and incubated in the basic measuring solution (0.5 mm KCl, 0.1 mm NaCl, 0.1 mm CaCl2, and 0.1 mm MgCl2) to equilibrate for 30 min. To record the Na+ flux in mycelial hyphae, the axenic EM cultures (pelleted form) were equilibrated for 60 min to reach a stable flux rate (Supplemental Fig. S4). The roots or hyphae were transferred to petri dishes containing 10 mL of fresh measuring solution. Prior to recording the flux, root and fungal samples were immobilized on the bottom. The Na+, H+, K+, and Ca2+ fluxes in the roots were measured along the root apex (0–2,000 μm from the tip) at intervals of 50 to 300 μm. Ion fluxes in the mycelium were measured over a recording period of 30 to 40 min. Real-time flux measurements of NM roots, EM roots, and axenic mycelia (pelleted hyphae) are shown in Supplemental Figure S5.
Transient Flux Kinetics
Mycorrhizal and NM roots were sampled from nonsalinized plants. After equilibration to the basic measuring solution, the steady-state fluxes of H+, K+, and Ca2+ in the apical region (500 μm from the root apex) were recorded (5–6 min) prior to salt shock. NaCl (200 mm) was slowly added to the measuring solution until the final NaCl concentration in the buffer reached 50 mm. Ion flux recording was continued for 30 to 40 min. The effect of lanthanum chloride (200 μm) on salt shock-induced transient Ca2+ kinetics was examined in the roots of mycorrhizal (MAJ and NAU) and NM P. × canescens plants.
Fungal mycelia were exposed to 0, 50, 100, 200, or 400 mm NaCl to induce salt shock. H+, K+, and Ca2+ fluxes were monitored over a continuous recording period of 30 to 40 min. For transient flux kinetics, the data measured during the first 2 to 3 min were discarded due to the diffusion effects of stock addition.
X-Ray Microanalysis
The samples from control and stressed plantlets were rapidly frozen in liquid nitrogen and vacuum freeze dried at −100°C for 7 d. The freeze-dried samples were gold coated in a high-vacuum sputter coater and analyzed using a Hitachi S-3400N scanning electron microscope equipped with an energy-dispersive x-ray spectrometer (EX-250; Horiba). Probe measurements of roots and leaves were made with a broad electron beam covering the whole cross-section. The relative amounts of K+, Na+, and Ca2+ were expressed as atomic mass fractions (%).
Data Analysis
Three-dimensional ionic fluxes were calculated using MageFlux, developed by Xu Yue (http://xuyue.net/mageflux). All mean data were subjected to ANOVA. Significant differences between means were determined by Duncan’s multiple range test. Unless otherwise stated, differences were considered significant at P < 0.05.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Effects of lanthanum chloride (200 μm) on salt shock-induced transient Ca2+ kinetics in the roots of mycorrhizal (MAJ and NAU) and NM P. × canescens plants.
Supplemental Figure S2. EM colonization of P. × canescens and performance of mycorrhizal (MAJ and NAU) and NM plants under salt stress.
Supplemental Figure S3. Fluctuations and oscillations in the net H+ flux in the elongation zone of P. × canescens roots.
Supplemental Figure S4. Kinetics of the net Na+ efflux in axenic mycelia of P. involutus strains MAJ and NAU after being exposed to 400 mm NaCl for 24 h.
Supplemental Figure S5. Representative images showing real-time flux measurements of NM roots, EM roots, and axenically grown EM cultures.
Supplemental Table S1. Interfering effects of K+ and Ca2+ on Na+-selective electrodes.
Supplementary Material
Acknowledgments
The Platform of Large Instruments and Equipment at Beijing Forestry University is acknowledged for offering the SEM-EDX apparatus. We thank Ms. Hui Zhang for her assistance in preparing samples for microanalysis and Ms. Christine Kettner for plant cultivation and the provision of EM P. × canescens.
Glossary
- EM
ectomycorrhizal
- DA
depolarization-activated
- KORCs
outward-rectifying K+ channels
- NSCCs
nonselective cation channels
- SIET
scanning ion-elective electrode technique
- SEM-EDX
scanning electron microscopy-energy dispersive x-ray spectrometry
- NM
nonmycorrhizal
- ST
short-term
- LT
long-term
- TEA
tetraethylammonium chloride
- MMN
modified Melin Norkrans
References
- Apse MP, Blumwald E. (2007) Na+ transport in plants. FEBS Lett 581: 2247–2254 [DOI] [PubMed] [Google Scholar]
- Arif I, Newman IA, Keenlyside N. (1995) Proton flux measurements from tissues in buffered solution. Plant Cell Environ 18: 1319–1324 [Google Scholar]
- Bandou E, Lebailly F, Muller F, Dulormne M, Toribio A, Chabrol J, Courtecuisse R, Plenchette C, Prin Y, Duponnois R, et al. (2006) The ectomycorrhizal fungus Scleroderma bermudense alleviates salt stress in seagrape (Coccoloba uvifera L.) seedlings. Mycorrhiza 16: 559–565 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP. (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465: 140–151 [DOI] [PubMed] [Google Scholar]
- Bücking H, Kuhn AJ, Schröder WH, Heyser W. (2002) The fungal sheath of ectomycorrhizal pine roots: an apoplastic barrier for the entry of calcium, magnesium, and potassium into the root cortex? J Exp Bot 53: 1659–1669 [DOI] [PubMed] [Google Scholar]
- Chen S, Li J, Wang S, Hüttermann A, Altman A. (2001) Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees 15: 186–194 [Google Scholar]
- Chen S, Li J, Fritz E, Wang S, Hüttermann A. (2002a) Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress. For Ecol Manage 168: 217–230 [Google Scholar]
- Chen S, Li J, Wang S, Fritz E, Hüttermann A, Altman A. (2003) Effects of NaCl on shoot growth, transpiration, ion compartmentation and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can J Res 33: 967–975 [Google Scholar]
- Chen S, Li J, Yin W, Wang S, Fritz E, Polle A, Hüttermann A. (2002b) Tissue and cellular K+, Ca2+ and Mg2+ of poplar under saline conditions. J Beijing For Univ 24: 84–88 [Google Scholar]
- Chen S, Polle A. (2010) Salinity tolerance of Populus. Plant Biol (Stuttg) 12: 317–333 [DOI] [PubMed] [Google Scholar]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ 28: 1230–1246 [Google Scholar]
- Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, et al (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol 145: 1714–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S. (2011) Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant Cell Environ 34: 947–961 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. (2010) Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J Cell Sci 123: 1468–1479 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53: 67–107 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175: 387–404 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128: 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrol N, Pozo MJ, Antelo M, Azcón-Aguilar C. (2002) Arbuscular mycorrhizal symbiosis regulates plasma membrane H+-ATPase gene expression in tomato plants. J Exp Bot 53: 1683–1687 [DOI] [PubMed] [Google Scholar]
- Gafur A, Schützendübel A, Langenfeld-Heyser R, Fritz E, Polle A. (2004) Compatible and incompetent Paxillus involutus isolates for ectomycorrhiza formation in vitro with poplar (Populus x canescens) differ in H2O2 production. Plant Biol (Stuttg) 6: 91–99 [DOI] [PubMed] [Google Scholar]
- Gianinazzi-Pearson V, Arnould C, Oufattole M, Arango M, Gianinazzi S. (2000) Differential activation of H+-ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco. Planta 211: 609–613 [DOI] [PubMed] [Google Scholar]
- Hall JL. (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53: 1–11 [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Kuhn AJ, Schröder WH, Bauch J. (2000) The kinetics of calcium and magnesium entry into mycorrhizal spruce roots. Planta 210: 488–496 [DOI] [PubMed] [Google Scholar]
- Langenfeld-Heyser R, Gao J, Ducic T, Tachd P, Lu CF, Fritz E, Gafur A, Polle A. (2007) Paxillus involutus mycorrhiza attenuate NaCl-stress responses in the salt-sensitive hybrid poplar Populus x canescens. Mycorrhiza 17: 121–131 [DOI] [PubMed] [Google Scholar]
- Lei J, Dexheimer J. (1988) Ultrastructural localization of ATPase activity in the Pinus sylvestris/Laccaria laccata ectomycorrhizal association. New Phytol 108: 329–334 [DOI] [PubMed] [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L. (1992) Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11: 137–141 [DOI] [PubMed] [Google Scholar]
- Liu JP, Zhu JK. (1997) An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc Natl Acad Sci USA 94: 14960–14964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Li K, Gai Y, Göbel C, Wildhagen H, Jiang X, Feußner I, Rennenberg H, Polle A. (2011) The ectomycorrhizal fungus (Paxillus involutus) modulates leaf physiology of poplar towards improved salt tolerance. Environ Exp Bot 72: 304–311 [Google Scholar]
- Luo ZB, Janz D, Jiang X, Göbel C, Wildhagen H, Tan Y, Rennenberg H, Feussner I, Polle A. (2009) Upgrading root physiology for stress tolerance by ectomycorrhizas: insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol 151: 1902–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner E, Khanna P, Meiwes K, Lindheim M, Prenzel J, Ulrich B. (1982) Elementflüsse in Waldökosystemen im Solling: Datendokumentation. Göttingen Bodenkundliche Berichte 71: 1–27 [Google Scholar]
- Muhsin TM, Zwiazek JJ. (2002) Colonization with Hebeloma crustuliniforme increases water conductance and limits shoot sodium uptake in white spruce (Picea glauca) seedlings. Plant Soil 238: 217–225 [Google Scholar]
- Munns R, Tester M. (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Nguyen H, Calvo Polanco M, Zwiazek JJ. (2006) Gas exchange and growth responses of ectomycorrhizal Picea mariana, Picea glauca, and Pinus banksiana seedlings to NaCl and Na2SO4. Plant Biol (Stuttg) 8: 646–652 [DOI] [PubMed] [Google Scholar]
- Ott T, Fritz E, Polle A, Schützendübel A. (2002) Characterisation of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol Ecol 42: 359–366 [DOI] [PubMed] [Google Scholar]
- Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M, Kangasjärvi J, Jiang X, Polle A. (2005) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139: 1762–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CA, Enstone DE. (1996) Functions of passage cells in the endodermis and exodermis of roots. Physiol Plant 97: 592–598 [Google Scholar]
- Polle A, Schützendübel A. (2003) Heavy metal signalling in plants: linking cellular and organismic responses. In H Hirt, K Shinozaki, eds, Plant Responses to Abiotic Stresses, Vol 4, Topics in Current Genetics. Springer-Verlag, Berlin, pp 167–215
- Ramos AC, Façanha AR, Feijó JA. (2008) Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol 178: 177–188 [DOI] [PubMed] [Google Scholar]
- Ramos AC, Lima PT, Dias PN, Kasuya MCM, Feijó JA. (2009) A pH signaling mechanism involved in the spatial distribution of calcium and anion fluxes in ectomycorrhizal roots. New Phytol 181: 448–462 [DOI] [PubMed] [Google Scholar]
- Ramos AC, Martins MA, Façanha AR. (2005) ATPase and pyrophosphatase activities in corn root microsomes colonized with arbuscular mycorrhizal fungi. Braz J Plant Physiol 29: 207–213 [Google Scholar]
- Rosewarne GM, Smith FA, Schachtman DP, Smith SE. (2007) Localization of proton-ATPase genes expressed in arbuscular mycorrhizal tomato plants. Mycorrhiza 17: 249–258 [DOI] [PubMed] [Google Scholar]
- Rygiewicz PT, Bledsoe CS. (1984) Mycorrhizal effects on potassium fluxes by northwest coniferous seedlings. Plant Physiol 76: 918–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala L, Cuin TA, Newman IA, Shabala S. (2005) Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 222: 1041–1050 [DOI] [PubMed] [Google Scholar]
- Shabala S. (2000) Ionic and osmotic components of salt stress specifically modulate net ion fluxes from bean leaf mesophyll. Plant Cell Environ 23: 825–837 [Google Scholar]
- Shabala S. (2003) Physiological implications of ultradian oscillations in plant roots. Plant Soil 255: 217–226 [Google Scholar]
- Shabala S, Cuin TA. (2008) Potassium transport and plant salt tolerance. Physiol Plant 133: 651–669 [DOI] [PubMed] [Google Scholar]
- Shabala S, Demidchik V, Shabala L, Cuin TA, Smith SJ, Miller AJ, Davies JM, Newman IA. (2006a) Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol 141: 1653–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Knowles A. (2002) Rhythmic patterns of nutrient acquisition by wheat roots. Funct Plant Biol 29: 595–605 [DOI] [PubMed] [Google Scholar]
- Shabala S, Newman IA. (2000) Salinity effects on the activity of plasma membrane H+ and Ca2+ transporters in bean leaf mesophyll: masking role of the cell wall. Ann Bot (Lond) 85: 681–686 [Google Scholar]
- Shabala S, Shabala L, Gradmann D, Chen Z, Newman I, Mancuso S. (2006b) Oscillations in plant membrane transport: model predictions, experimental validation, and physiological implications. J Exp Bot 57: 171–184 [DOI] [PubMed] [Google Scholar]
- Shabala S, Shabala L, Van Volkenburgh E. (2003) Effect of calcium on root development and root ion fluxes in salinized barley seedlings. Funct Plant Biol 30: 507–514 [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J. (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ. (2008) Mycorrhizal Synthesis, Ed 3. San Diego, Academic Press
- Souda M, Toko K, Hayashi K, Fujiyoshi T, Ezaki S, Yamafuji K. (1990) Relationship between growth and electric oscillations in bean roots. Plant Physiol 93: 532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, et al. (2009a) NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149: 1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Dai S, Wang R, Chen S, Li N, Zhou X, Lu C, Shen X, Zheng X, Hu Z, et al. (2009b) Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol 29: 1175–1186 [DOI] [PubMed] [Google Scholar]
- Sun J, Wang MJ, Ding MQ, Deng SR, Liu MQ, Lu CF, Zhou XY, Shen X, Zheng XJ, Zhang ZK, et al. (2010) H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ 33: 943–958 [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang CL, Deng SR, Lu CF, Shen X, Zhou XY, Zheng XJ, Hu ZM, Chen SL. (2012) An ATP signalling pathway in plant cells: extracellular ATP triggers programmed cell death in Populus euphratica. Plant Cell Environ 35: 893–916 [DOI] [PubMed] [Google Scholar]
- Tester M, Davenport R. (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot (Lond) 91: 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toko K, Souda M, Matsuno T, Yamafuji K. (1990) Oscillations of electrical potential along a root of a higher plant. Biophys J 57: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sun T, Yin LP. (2006) Application of non-invasive microsensing system to simultaneously measure both H+ and O2 fluxes around the pollen tube. J Integr Plant Biol 48: 823–831 [Google Scholar]
- Yi H, Calvo-Polanco M, MacKinnon MD, Zwiazek JJ. (2008) Responses of ectomycorrhizal Populus tremuloides and Betula papyrifera seedlings to salinity. Environ Exp Bot 62: 357–363 [Google Scholar]
- Zhang H, Li J, Chen S, Lu C, Wang R, Dai S, Zhu H, Zhang Y, Shi Y, Wang M, et al. (2008) Effect of NaCl on growth and ion relations in two salt-tolerant strains of Paxillus involutus. For Stud China 10: 95–100 [Google Scholar]
- Zhu JK. (2001) Plant salt tolerance. Trends Plant Sci 6: 66–71 [DOI] [PubMed] [Google Scholar]
- Zhu JK. (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6: 441–445 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.