Abstract
Plants utilize a large number of immune receptors to recognize pathogens and activate defense responses. A small number of these receptors belong to the receptor-like protein family. Previously, we showed that a gain-of-function mutation in the receptor-like protein SNC2 (for Suppressor of NPR1, Constitutive2) leads to constitutive activation of defense responses in snc2-1D mutant plants. To identify defense signaling components downstream of SNC2, we carried out a suppressor screen in the snc2-1D mutant background of Arabidopsis (Arabidopsis thaliana). Map-based cloning of one of the suppressor genes, BDA1 (for bian da; “becoming big” in Chinese), showed that it encodes a protein with amino-terminal ankyrin repeats and carboxyl-terminal transmembrane domains. Loss-of-function mutations in BDA1 suppress the dwarf morphology and constitutive defense responses in snc2-1D npr1-1 (for nonexpressor of pathogenesis-related genes1,1) and also result in enhanced susceptibility to bacterial pathogens. In contrast, a gain-of-function allele of bda1 isolated from a separate genetic screen to search for mutants with enhanced pathogen resistance was found to constitutively activate cell death and defense responses. These data suggest that BDA1 is a critical signaling component that functions downstream of SNC2 to regulate plant immunity.
Plants have evolved two types of immune receptors to recognize different spectra of pathogen molecules and initiate downstream defense responses. Pathogen-associated molecular pattern (PAMP) receptors recognize conserved microbial molecules collectively known as PAMPs (Boller and Felix, 2009). Most of the known PAMP receptors, such as FLS2, EFR, and CERK1, belong to the receptor-like kinase family (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006; Miya et al., 2007; Wan et al., 2008). The receptor-like kinase BAK1 functions as a coreceptor of FLS2 and EFR in the perception of bacterial flagellin and translation elongation factor EF-Tu (Chinchilla et al., 2007; Heese et al., 2007; Schulze et al., 2010; Roux et al., 2011). In rice (Oryza sativa), CERK1 functions together with the lysin motif (LysM) domain protein CEBiP to recognize the fungal cell wall component chitin (Shimizu et al., 2010). Together with two other LysM domain proteins, LYM1 and LYM3, Arabidopsis (Arabidopsis thaliana) CERK1 is also involved in the perception of bacterial peptidoglycan (Willmann et al., 2011). The other class of immune receptors is composed of plant Resistance (R) proteins, which have evolved to detect pathogen-specific effector molecules secreted to suppress host defense and promote virulence. The majority of plant R genes encode nucleotide-binding site Leu-rich-repeats proteins (Rafiqi et al., 2009).
Receptor-like proteins (RLPs) containing extracellular Leu-rich repeats, a single transmembrane motif, and a short cytoplasmic tail form one of the largest protein families in plants. A small number of RLPs, such as the tomato (Solanum lycopersicum) Cf and Ve proteins and the apple (Malus domestica) HcrVf2 protein, were shown to function as R proteins (Jones et al., 1994; Kawchuk et al., 2001; Belfanti et al., 2004; Fradin et al., 2009). Interestingly, Ve1-mediated resistance to Verticillium requires BAK1 (Fradin et al., 2009, 2011). Two other RLPs, tomato Eix1 and Eix2, function as receptors for the fungal ethylene-inducing xylanase (Ron and Avni, 2004). The Arabidopsis genome encodes 57 RLPs (Wang et al., 2008). The best studied ones include CLAVATA2 and TOO MANY MOUTHS, both functioning as critical regulators of plant development (Jeong et al., 1999; Nadeau and Sack, 2002). Recently, the RLP SNC2 was identified as a critical defense regulator in Arabidopsis (Zhang et al., 2010). Loss-of-function mutations in SNC2 result in enhanced susceptibility to the bacterial pathogen Pseudomonas syringae pv tomato (P.s.t.) DC3000.
snc2-1D (for suppressor of npr1, constitutive2-1D) is a semidominant gain-of-function mutation conferring constitutively activated defense responses in Arabidopsis (Zhang et al., 2010). The snc2-1D mutant plants exhibit dwarf morphology, accumulate high levels of salicylic acid (SA), express high levels of PR (Pathogenesis-Related) genes, and display enhanced resistance to pathogens. This mutant was identified from a screen searching for mutants with constitutively activated defense responses that are independent of NPR1 (for Nonexpressor of Pathogenesis-Related Genes1), an essential component of SA-induced gene expression and pathogen resistance (Dong, 2004). Because very little is known about how RLP-activated defense signals are transduced intracellularly, the identification of snc2-1D provides a very useful tool for dissecting defense pathways downstream of the RLP.
To study signal transduction pathways downstream of SNC2, a suppressor screen was performed in the snc2-1D mutant background and a large number of suppressor mutants named bda (for bian da; “becoming big” in Chinese) were identified (Zhang et al., 2010). BDA2 encodes the WRKY transcription factor WRKY70. Mutations in WRKY70 suppress the mutant morphology and defense gene expression but not the increased accumulation of SA in snc2-1D npr1-1, suggesting that WRKY70 is a critical regulatory component of the SA-independent defense pathway in snc2-1D npr1-1.
In this study, we report the characterization and positional cloning of BDA1. BDA1 encodes a novel protein with an N-terminal ankyrin-repeat domain and a C-terminal transmembrane domain. It is structurally related to ACD6, a known positive regulator of plant defense (Lu et al., 2003). Loss-of-function mutations in BDA1 suppress the dwarf morphology and constitutive defense responses in snc2-1D npr1-1 and result in enhanced susceptibility to pathogens. By contrast, a gain-of-function allele of BDA1 constitutively activates cell death and defense responses, suggesting that BDA1 is a critical regulator of plant immunity.
RESULTS
Identification and Characterization of bda1-1 snc2-1D npr1-1
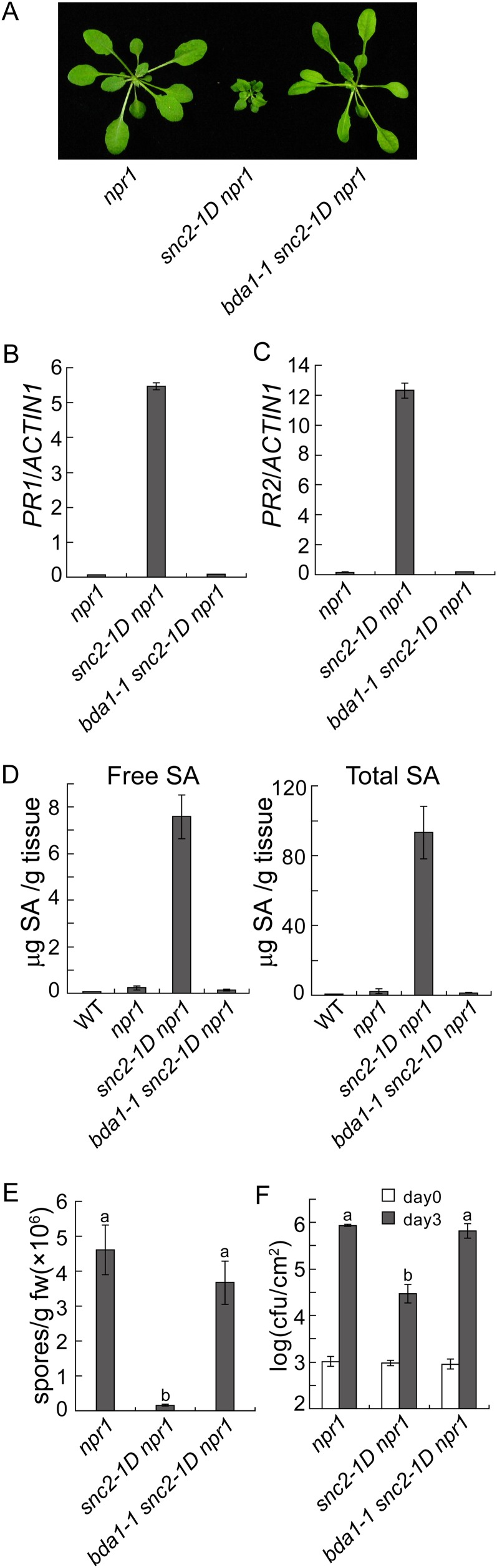
The bda1-1 snc2-1D npr1-1 triple mutant was identified from a suppressor screen of snc2-1D npr1-1 as described previously (Zhang et al., 2010). The dwarf morphology of snc2-1D npr1-1 was completely suppressed in the triple mutant (Fig. 1A). Real-time PCR (qPCR) analysis of the expression of defense marker genes PR1 (Fig. 1B) and PR2 (Fig. 1C) showed that the constitutive expression of both genes in snc2-1D npr1-1 is completely suppressed in the triple mutant. In addition, increased accumulation of SA and enhanced resistance to the virulent oomycete pathogen Hyaloperonospora arabidopsidis (H.a.) Noco2 and the bacterial pathogen P.s.t. DC3000 in snc2-1D npr1-1 are also suppressed in bda1-1 snc2-1D npr1-1 (Fig. 1, D–F). Taken together, bda1-1 completely suppresses the mutant morphology as well as constitutive defense responses in snc2-1D npr1-1.
Figure 1.
Characterization of the bda1-1 snc2-1D npr1-1 triple mutant. A, Morphology of npr1-1, snc2-1D npr1-1, and bda1-1 snc2-1D npr1-1. Plants were grown on soil and photographed approximately 4 weeks after planting. B and C, Expression of PR1 (B) and PR2 (C) in npr1-1, snc2-1D npr1-1, and bda1-1 snc2-1D npr1-1 as determined by qPCR. RNA was extracted from 12-d-old seedlings grown on one-half-strength Murashige and Skoog medium. Values were normalized to the expression of ACTIN1. Error bars represent sd from three measurements. D, Free and total SA levels in npr1-1, snc2-1D npr1-1, and bda1-1 snc2-1D npr1-1 plants. Error bars represent sd from four measurements. WT, Wild type. E, Growth of H.a. Noco2 on npr1-1, snc2-1D npr1-1, and bda1-1 snc2-1D npr1-1. Sixteen-day-old seedlings were sprayed with H.a. Noco2 spores (50,000 spores mL−1). Infection was scored 7 d after inoculation. The values presented are averages of three measurements ± sd. Statistical differences among the samples are labeled with different letters (P < 0.001). fw, Fresh weight. F, Growth of P.s.t. DC3000 on npr1-1, snc2-1D npr1-1, and bda1-1 snc2-1D npr1-1. Five-week-old plants were infiltrated with a bacterial suspension at an optical density at 600 nm of 0.0002. The values presented are averages of four replicates ± sd. Statistical differences among the samples are labeled with different letters (P < 0.001). cfu, Colony-forming units. [See online article for color version of this figure.]
Positional Cloning of BDA1
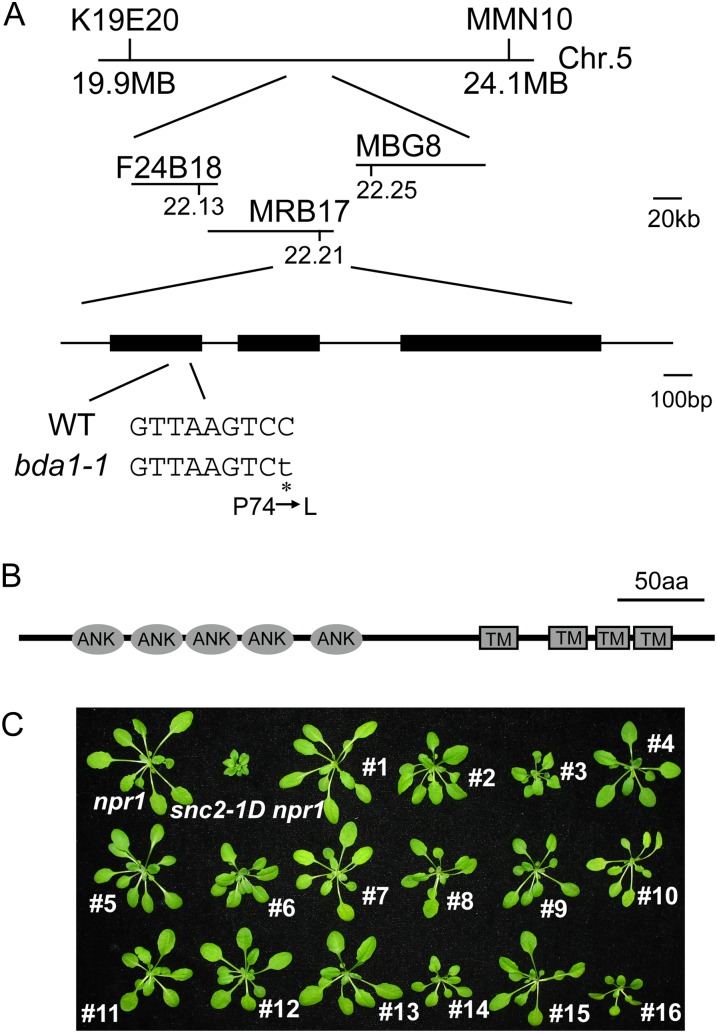
To map the bda1-1 mutation, bda1-1 snc2-1D npr1-1 (in the ecotype Columbia [Col-0] background) was crossed with Landsberg erecta (Ler) to generate a segregating mapping population. Crude mapping using the F2 progeny showed that the bda1-1 mutation is located between markers K19E20 and MMN10 on chromosome 5. Further fine-mapping narrowed the bda1-1 mutation to a region of approximately 80 kb between markers F24B18 and MRB17 (Fig. 2A). Sequence analysis of genes in this region in bda1-1 identified a single C-to-T mutation in At5g54610, which results in an amino acid change from Pro-74 to Leu. Sequence analysis using SMART (http://smart.embl-heidelberg.de/) showed that At5g54610 encodes a new protein with five ankyrin repeats at the N terminus and four transmembrane domains at its C terminus (Fig. 2B).
Figure 2.
Positional cloning of BDA1. A, Map position and mutation location in bda1-1. WT, Wild type. B, Predicted protein structure of BDA1. aa, Amino acids; ANK, ankyrin repeat; TM, transmembrane domain. C, Morphological phenotypes of additional bda1 snc2-1D npr1-1 mutant alleles. Plants were grown on soil and photographed approximately 4 weeks after planting. Plants 1 to 16, bda1-1 to bda1-16 in the snc2-1D npr1-1 background. [See online article for color version of this figure.]
To determine whether there are additional bda mutants that also contain mutations in the gene, At5g54610 was amplified from other bda mutants by PCR and sequenced. Fifteen additional mutant alleles of At5g54610 were identified (Table I; Fig. 2C), each containing a unique missense mutation. These results provide further evidence that BDA1 is At5g54610.
Table I. A list of bda1 mutant alleles.
| Allele | Mutationa | Amino Acid Change |
|---|---|---|
| bda1-1 | C221 to T | Pro-74 to Leu |
| bda1-2 | G650 to A | Gly-217 to Glu |
| bda1-3 | G157 to A | Ala-53 to Thr |
| bda1-4 | C220 to T | Pro-74 to Ser |
| bda1-5 | C386 to T | Pro-129 to Leu |
| bda1-6 | G415 to A | Gly-139 to Arg |
| bda1-7 | G418 to A | Glu-140 to Lys |
| bda1-8 | C437 to T | Thr-146 to Met |
| bda1-9 | G568 to A | Ala-190 to Thr |
| bda1-10 | C628 to T | Arg-210 to Trp |
| bda1-11 | G629 to A | Arg-210 to Gln |
| bda1-12 | G664 to A | Asp-222 to Asn |
| bda1-13 | G862 to A | Ala-288 to Thr |
| bda1-14 | C908 to T | Thr-303 to Ile |
| bda1-15 | G973 to A | Glu-325 to Lys |
| bda1-16 | G1162 to A | Gly-388 to Arg |
Location of the nucleotide change in the coding sequence.
BDA1 Is Required for Basal Resistance against P.s.t. DC3000
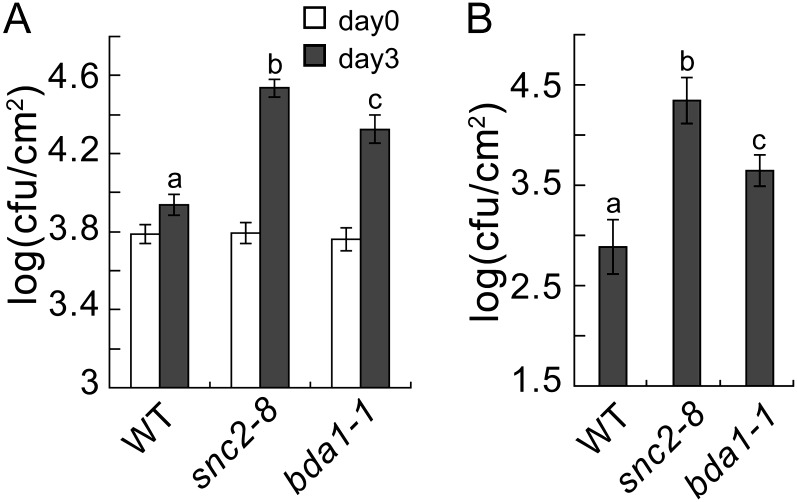
To determine whether BDA1 is required for pathogen resistance, we crossed bda1-1 snc2-1D npr1-1 with wild-type Col-0 and isolated the bda1-1 single mutant in the F2 progeny. We also obtained a transferred DNA insertion mutant, GABI_883C03, from the Nottingham Arabidopsis Stock Centre. Unfortunately, the transferred DNA insertion located in the second intron of BDA1 does not affect its expression. When bda1-1 and snc2-8, a loss-of-function snc2 mutant (Zhang et al., 2010), were inoculated with P.s.t. DC3000 by infiltration, both mutants supported modestly higher growth of the pathogen compared with the wild type (Fig. 3A). Spray inoculation of these mutants with P.s.t. DC3000 results in considerably higher bacterial growth than that observed in wild-type plants (Fig. 3B). These data suggest that, like SNC2, BDA1 is required for basal resistance against P.s.t. DC3000.
Figure 3.
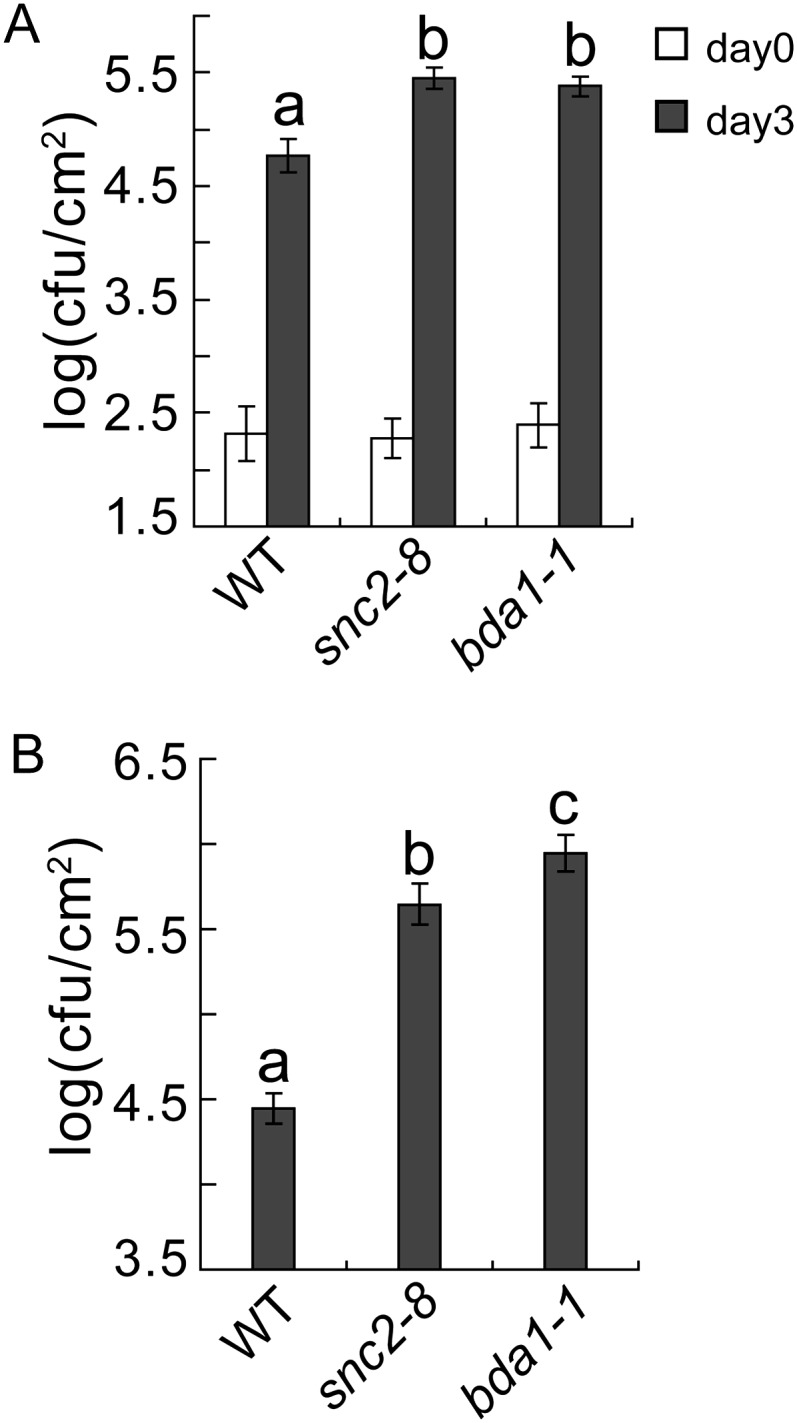
bda1-1 exhibits enhanced susceptibility to P.s.t. DC3000. A, Growth of P.s.t. DC3000 on wild-type (WT), snc2-8, and bda1-1 plants. Leaves of 5-week-old plants were infiltrated with a bacterial suspension at an optical density at 600 nm of 0.0002. The values presented are averages of four replicates ± sd. cfu, Colony-forming units. Statistical differences among the samples are labeled with different letters (P < 0.001). B, Growth of P.s.t. DC3000 on wild-type, snc2-8, and bda1-1 plants. Leaves were collected 2 d after spray inoculation with a bacterial suspension at an optical density at 600 nm of 0.2. The values presented are averages of six replicates ± sd. Statistical differences among the samples are labeled with different letters (P < 0.001).
BDA1 Is Required for Resistance against Nonpathogenic Bacteria
To determine whether SNC2 and BDA1 are required for PAMP-mediated resistance against nonpathogenic bacteria, we challenged snc2-8 and bda1-1 with P.s.t. DC3000 hrcC, a P.s.t. DC3000 mutant defective in type III protein secretion (Wei et al., 2000). In the absence of effector-triggered immunity, PAMP-triggered immunity plays a predominant role in resistance against the bacteria. As shown in Figure 4A, growth of P.s.t. DC3000 hrcC after bacterial infiltration was modestly higher in snc2-8 and bda1-1 compared with that in wild-type plants. Interestingly, both of the mutants supported much higher growth of the pathogen than the wild type when the plants were spray inoculated with P.s.t. DC3000 hrcC (Fig. 4B). Growth of P.s.t. DC3000 hrcC in snc2-8 was always higher than in bda1-1 regardless of the inoculation method. These data suggest that both SNC2 and BDA1 are required for resistance against nonpathogenic bacteria.
Figure 4.
PAMP-triggered immunity in snc2-8 and bda1-1. A, Growth of P.s.t. DC3000 hrcC on wild-type (WT), snc2-8, and bda1-1 plants. Leaves of 5-week-old plants were infiltrated with a bacterial suspension at an optical density at 600 nm of 0.002. The values presented are averages of four replicates ± sd. cfu, Colony-forming units. Statistical differences among the samples are labeled with different letters (P < 0.001). B, Growth of P.s.t. DC3000 hrcC on wild-type, snc2-8, and bda1-1 plants. Leaves were collected 2 d after spray inoculation with a bacterial suspension at an optical density at 600 nm of 0.2. The values presented are averages of six replicates ± sd. Statistical differences among the samples are labeled with different letters (P < 0.001).
A Gain-of-Function Allele of BDA1 Constitutively Activates Cell Death and Defense Responses
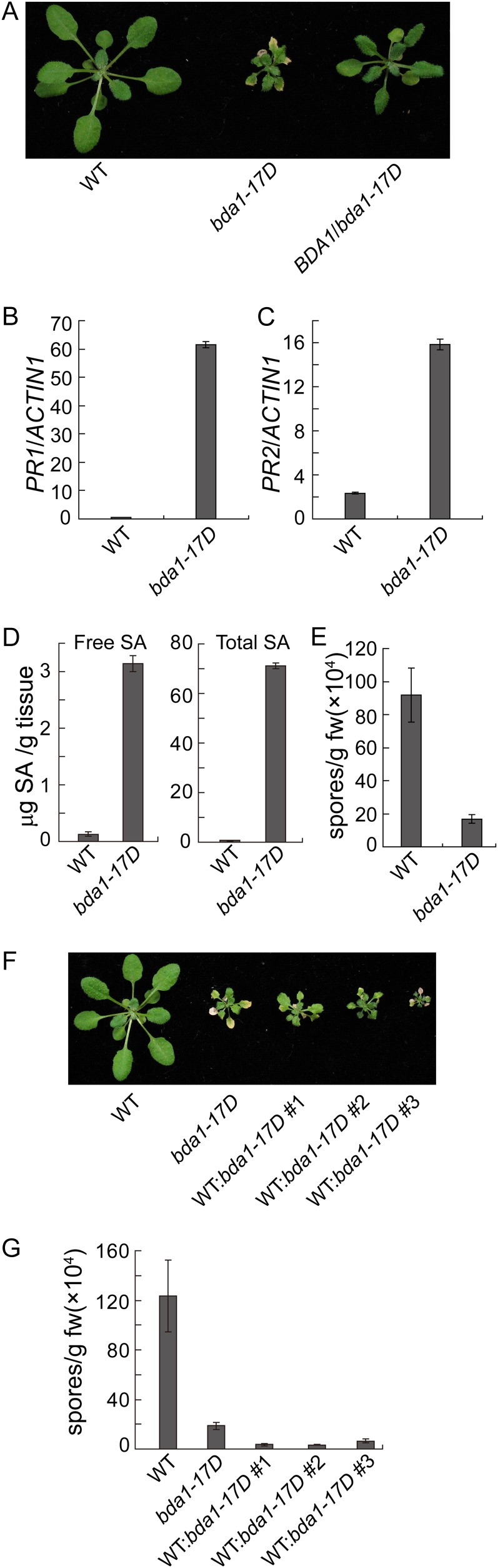
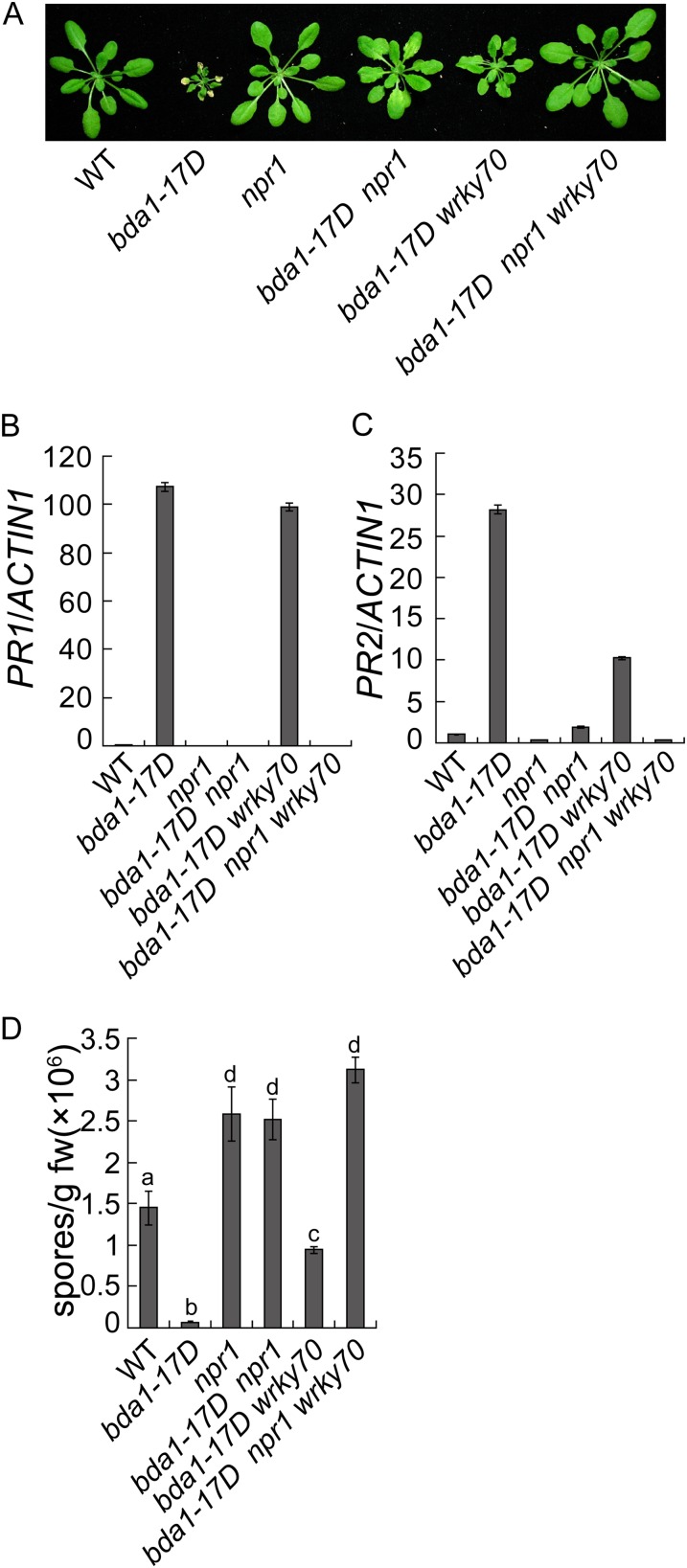
From a separate genetic screen to search for mutants with enhanced disease resistance against H.a. Noco2 (Bi et al., 2010), we obtained a semidominant mutant with a lesion-mimic phenotype (Fig. 5A). The mutant was mapped to the same region where BDA1 is located. Sequencing analysis revealed that it contains a mutation in BDA1 that changes Gly-330 to Glu in one of the transmembrane domains. As a result, we named this mutant bda1-17D. In bda1-17D, both PR1 and PR2 are constitutively expressed at high levels (Fig. 5, B and C). Analysis of SA levels in bda1-17D showed that it accumulates high levels of SA (Fig. 5D). When challenged with H.a. Noco2, bda1-17D displayed enhanced resistance to the pathogen (Fig. 5E). These data suggest that defense responses are constitutively activated in bda1-17D.
Figure 5.
bda1-17D constitutively activates cell death and defense responses. A, Phenotypes of wild-type (WT), bda1-17D, and BDA1/bda1-17D heterozygous plants. Plants were grown on soil and photographed approximately 4 weeks after planting. B and C, Expression of PR1 (B) and PR2 (C) in the wild type and bda1-17D determined by qPCR. RNA was extracted from 25-d-old soil-grown plants. Values were normalized to the expression of ACTIN1. Error bars represent sd from three measurements. D, Free and total SA in the wild type and bda1-17D. Error bars represent sd from four measurements. E, Growth of H.a. Noco2 on the wild type and bda1-17D. Sixteen-day-old seedlings were sprayed with H.a. Noco2 spores (50,000 spores mL−1). Infection was scored 7 d after inoculation. The values presented are averages of three measurements ± sd. fw, Fresh weight. F, Morphology of 4-week-old soil-grown wild-type, bda1-17D, and transgenic lines expressing the bda1-17D mutant gene in the wild-type background. G, Growth of H.a. Noco2 on wild-type and transgenic lines expressing the bda1-17D mutant gene. Sixteen-day-old seedlings were sprayed with H.a. Noco2 spores (50,000 spores mL−1). Infection was scored 7 d after inoculation. The values presented are averages of three measurements ± sd. [See online article for color version of this figure.]
To test whether the bda1-17D mutation causes the cell death and constitutive defense responses, a genomic clone containing the bda1-17 mutation was constructed and transformed into wild-type plants. About one-half of the transgenic lines with the mutant transgene exhibit bda1-17D-like morphology. We also made transgenic plants expressing wild-type BDA1 under the control of its native promoter. Among about 30 transgenic lines obtained, none of them display bda1-17D-like morphology. Three representative bda1-17D transgenic lines are shown in Figure 5F. When these transgenic lines were challenged with H.a. Noco2, they all displayed enhanced resistance to H.a. Noco2 (Fig. 5G), suggesting that the bda1-17D mutation is responsible for the constitutive defense responses in bda1-17D mutant plants.
BDA1 Functions Upstream of NPR1 and WRKY70
In snc2-1D, two parallel defense pathways were activated, one dependent on SA and NPR1 and the other dependent on WRKY70 (Zhang et al., 2010). To determine whether the constitutive defense responses in bda1-17D are dependent on NPR1 and WRKY70, we generated the bda1-17D npr1-1 and bda1-17D wrky70-1 double mutants and the bda1-17D npr1-1 wrky70-1 triple mutant. As shown in Figure 6A, the dwarf morphology of bda1-17D is partially suppressed in the double mutants and completely suppressed in the triple mutant. qPCR analysis showed that the constitutive PR1 expression in bda1-17D is mainly dependent on NPR1, with very little contribution from WRKY70 (Fig. 6B). In contrast, the expression of PR2 is reduced in both bda1-17D npr1-1 and bda1-17D wrky70-1 and completely blocked in bda1-17D npr1-1 wrky70-1 (Fig. 6C), suggesting that both NPR1 and WRKY70 contribute to the up-regulation of PR2 in bda1-17D. Analysis of resistance to H.a. Noco2 showed that the enhanced resistance in bda1-17D is dependent on both NPR1 and WRKY70, with greater contribution from NPR1 (Fig. 6D). Taken together, these data suggest that BDA1 functions upstream of NPR1 and WRKY70.
Figure 6.
bda1-17D activates both NPR1-dependent and WRKY70-dependent defense responses. A, Morphology of wild-type (WT), bda1-7D, npr1-1, bda1-17D npr1-1, bda1-17D wrky70-1, and bda1-17D npr1-1 wrky70-1 plants. Plants were grown on soil and photographed when they were approximately 4 weeks old. B and C, Expression of PR1 (B) and PR2 (C) in the indicated genotypes as determined by qPCR. Values were normalized to the expression of ACTIN1. RNA was extracted from 25-d-old soil-grown plants. Error bars represent sd from three measurements. D, Growth of H.a. Noco2 on the indicated genotypes. Sixteen-day-old seedlings were sprayed with H.a. Noco2 spores (10,000 spores mL−1). Infection was scored 7 d after inoculation. The values presented are averages of three measurements ± sd. Statistical differences among the samples are labeled with different letters (P < 0.05). fw, Fresh weight. [See online article for color version of this figure.]
DISCUSSION
From a suppressor screen of snc2-1D npr1-1, we identified BDA1 as an essential component of SNC2-mediated defense responses. Mutations in BDA1 suppress the dwarf morphology as well as defense responses in snc2-1D npr1-1. Loss of BDA1 function also results in enhanced susceptibility to P.s.t. DC3000. In addition, a gain-of-function allele of BDA1 was found to constitutively activate cell death and defense responses. These data suggest that BDA1 is a critical regulator of plant immunity.
When bda1-1 and snc2-8 loss-of-function mutants were challenged with the nonpathogenic bacterium P.s.t. DC3000 hrcC, bacterial growth in the mutants was significantly higher than in wild-type plants, suggesting that SNC2 and BDA1 play important roles in PAMP-triggered immunity. It is possible that SNC2 serves as a receptor or coreceptor of an unidentified bacterial PAMP signal and that BDA1 functions downstream of SNC2 to activate PAMP-triggered defense responses. Compared with inoculation by infiltration, greater differences in bacterial growth were found between wild-type and mutant plants upon spray inoculation with P.s.t. DC3000 or P.s.t. DC3000 hrcC. This indicates that SNC2 and BDA1 may also play critical roles in stomatal defense against bacterial pathogens.
BDA1 encodes a protein with ankyrin repeats and transmembrane domains. In Arabidopsis, there are 37 predicted ankyrin-repeat transmembrane proteins (Becerra et al., 2004). Among these proteins, only ACD6 and ITN1 have previously been characterized. ACD6 was shown to function as a positive regulator of SA signaling in local defense responses (Lu et al., 2003), whereas ITN1 is involved in salt stress tolerance (Sakamoto et al., 2008). How ACD6 and ITN1 are regulated is unknown. Our study suggests that BDA1 transduces signals perceived by the RLP SNC2 to activate downstream defense responses. It will be interesting to test whether ACD6 and ITN1 function as downstream components of other RLPs in Arabidopsis.
Ankyrin repeats are generally involved in protein-protein interactions (Sedgwick and Smerdon, 1999). One of the best studied plant ankyrin-repeat proteins is NPR1 (Cao et al., 1997; Ryals et al., 1997), which regulates SA-induced PR gene expression and pathogen resistance through its associations with TGA transcription factors (Zhang et al., 1999, 2003; Després et al., 2000). What the ankyrin-repeat domains of ACD6 and ITN1 interact with is unknown. We were not able to detect interactions between SNC2 and BDA1 by coimmunoprecipitation and bimolecular fluorescence complementation analysis. It is likely that other proteins are also involved in transducing the signal from SNC2 to BDA1. BDA1 and ACD6 share very low sequence identity in their ankyrin-repeat domains. Most likely, they regulate plant defense responses through interactions with distinct proteins. Identification of proteins interacting with BDA1 and ACD6 will lead to a better understanding of how they regulate plant defense responses.
In bda1-17D, a single amino acid change in the second transmembrane domain results in the activation of cell death and defense responses. bda1-17D has very similar mutant phenotypes to acd6-1, which also exhibits spontaneous cell death, expresses high levels of PR-1, and displays enhanced pathogen resistance (Rate et al., 1999). Interestingly, the gain-of-function mutation in acd6-1 also occurs in one of the transmembrane domains (Lu et al., 2003). These data suggest that the transmembrane domains of BDA1 and ACD6 play very important roles in the negative regulation of these proteins. One possibility is that these transmembrane domains interact with their negative regulators and that the mutations in bda1-17D and acd6-1 disrupt these interactions.
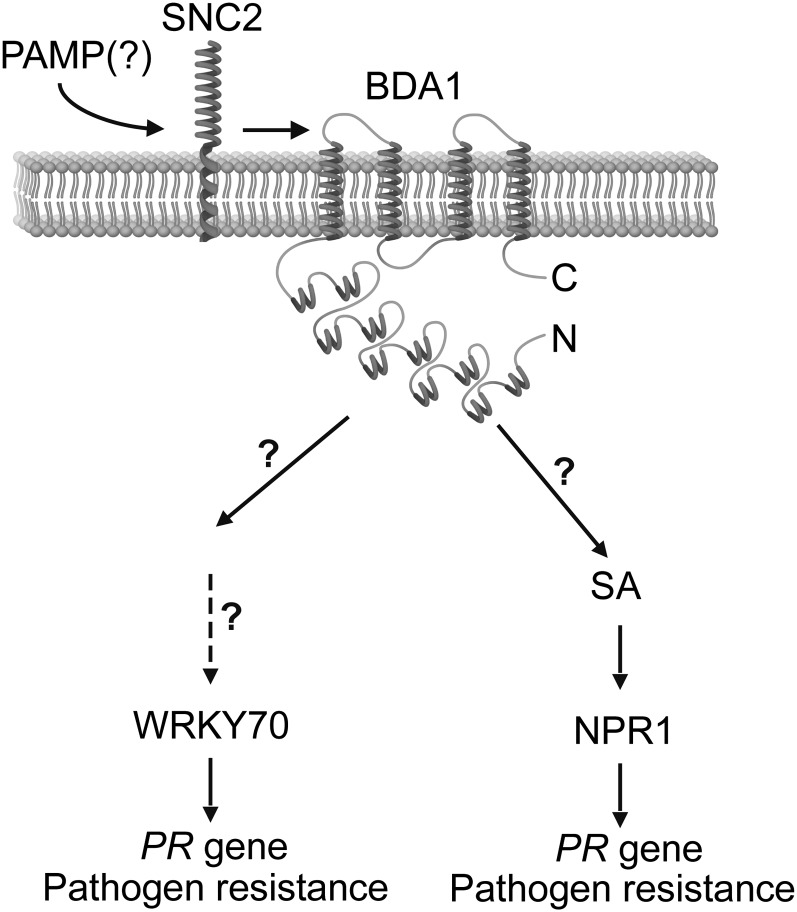
Our study identified BDA1 as a critical signaling component downstream of SNC2. A working model is proposed in Figure 7. When an unknown PAMP signal is perceived by SNC2, it activates the transmembrane ankyrin protein BDA1. Once it is activated, BDA1 probably recruits additional signaling components through its ankyrin-repeat domain to activate SA synthesis and WRKY70-dependent defense gene expression. It is unclear whether BDA1 also functions in regulating plant defense responses that are independent of SNC2. Future research on other BDA genes will provide us with more information on how SNC2 and BDA1 regulate plant defense responses.
Figure 7.
A working model for the role of BDA1 in SNC2-mediated immunity. BDA1 functions as a signaling component to transduce an unknown PAMP signal perceived by SNC2. Most likely through an association with other proteins, it activates two downstream defense pathways, one dependent on WRKY70 and the other dependent on SA and NPR1.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) npr1-1, snc2-1D npr1-1, snc2-8, and wrky70-1 were described previously (Cao et al., 1994; Ulker et al., 2007; Zhang et al., 2010). Loss-of-function alleles of bda1 were identified from an ethyl methanesulfonate-mutagenized snc2-1D npr1-1 population by screening for mutants of increased size. bda1-17D was identified from an ethyl methanesulfonate-mutagenized population in the Col-0 background by searching for mutants with increased resistance to Hyaloperonospora arabidopsidis (H.a.) Noco2 (Bi et al., 2010).
To obtain transgenic plants expressing the bda1-17 mutant gene, a genomic DNA fragment containing the bda1-17 mutant gene was amplified from bda1-17 mutant plant DNA by PCR using primers 5′-CGGGGTACCGTCGACCTCCACAAAATGCATGTCAAG-3′ and 5′-CGGAATTCGAGCTCTGTGGGGATTCAATACTATAGC-3′ and then cloned into pCAMBIA1305. The resulting plasmid was transformed into Agrobacterium tumefaciens and subsequently into wild-type Col-0 plants by floral dipping (Clough and Bent, 1998).
Mutant Characterization
To determine the expression levels of PR1 and PR2, RNA was extracted from 0.1 g of leaf tissue using the RNAiso reagent (Takara). Reverse transcription was carried out using the Moloney murine leukemia virus reverse transcriptase (Takara). qPCR was performed on the complementary DNA samples using the SYBR Premix Ex Taq II kit (Takara). Primers for amplification of PR1, PR2, and ACTIN1 were described previously (Zhang et al., 2003). SA was extracted from leaf tissues and measured by HPLC as described previously (Li et al., 1999).
H.a. Noco2 infection experiments were performed by spraying plant seedlings with spores of H.a. Noco2 at different concentrations. Plants were subsequently kept in a growth chamber with 95% humidity at 18°C under 12-h-light/12-h-dark cycles. Infections were scored 7 d later by counting the spores with a hemocytometer as described previously (Bi et al., 2010). For Pseudomonas syringae pv tomato DC3000 infections, plants were grown at 23°C under 12-h-light/12-h-dark cycles. Leaves of 5-week-old plants were inoculated by infiltrating or spraying with a bacterial suspension. Bacterial growth was determined by plating the bacteria on Kings B plates.
Positional Cloning of BDA1
To map bda1-1, the bda1-1 snc2-1D npr1-1 triple mutant in the Col-0 background was crossed with wild-type Ler. Plants homozygous for snc2-1D in the F2 population were identified by PCR and used for linkage analysis. For fine-mapping of the bda1-1 mutation, recombinants between markers K19E20 and MMN10 were identified by PCR and the phenotypes of the recombinants were determined by analyzing the segregation of the snc2-1D morphological phenotype in their progeny. For mapping bda1-17D, bda1-17D in the Col-0 background was crossed with wild-type Ler. Plants exhibiting bda1-17D-like morphology in the F2 population were used for linkage analysis. All markers used were designed according to the Monsanto Arabidopsis polymorphism and Ler sequence collection (Jander et al., 2002). Marker primers used include K19E20, 5′-GACAAGAACCACATGAGAGC-3′ and 5′-GTTATGTGTACACTTCAGGTC-3′; MMN10, 5′-AGCTGCAATAATGCCAAAGG-3′ and 5′-GAACCATCACCACTGGTGAG-3′; and MBG8, 5′-GCTAAGAAAGTAGAAAGCCG-3′ and 5′-ATGGTATCTCACCAATGGGG-3′. These markers were designed using insertion/deletion polymorphisms. Markers F24B18 and MRB17 are based on a single nucleotide polymorphism. The common, Col-specific, and Ler-specific primers for F24B18 are 5′-GGAAGGCAGAGATTATAGAC-3′, 5′-GAGCAAAGCTTTGATGTACCA-3′, and 5′-GAGCAAAGCTTTGATGTACCG-3′, respectively. The common, Col-specific, and Ler-specific primers for MRB17 are 5′-GAGTTCACAAGAGAAGACGT-3′, 5′-CTCTCACAAATCTGGGCATG-3′, and 5′-CTCTCACAAATCTGGGCATA-3′, respectively.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number NM_124842 (BDA1).
Acknowledgments
We thank Xiaoman Liu and Youjun Zhang for technical assistance.
Glossary
- PAMP
pathogen-associated molecular pattern
- RLP
receptor-like protein
- P.s.t.
Pseudomonas syringae pv tomato
- SA
salicylic acid
- qPCR
real-time PCR
- H.a.
Hyaloperonospora arabidopsidis
- Col-0
ecotype Columbia
- Ler
Landsberg erecta
References
- Becerra C, Jahrmann T, Puigdomènech P, Vicient CM. (2004) Ankyrin repeat-containing proteins in Arabidopsis: characterization of a novel and abundant group of genes coding ankyrin-transmembrane proteins. Gene 340: 111–121 [DOI] [PubMed] [Google Scholar]
- Belfanti E, Silfverberg-Dilworth E, Tartarini S, Patocchi A, Barbieri M, Zhu J, Vinatzer BA, Gianfranceschi L, Gessler C, Sansavini S. (2004) The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Proc Natl Acad Sci USA 101: 886–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi D, Cheng YT, Li X, Zhang Y. (2010) Activation of plant immune responses by a gain-of-function mutation in an atypical receptor-like kinase. Plant Physiol 153: 1771–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Després C, DeLong C, Glaze S, Liu E, Fobert PR. (2000) The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 12: 279–290 [PMC free article] [PubMed] [Google Scholar]
- Dong X. (2004) NPR1, all things considered. Curr Opin Plant Biol 7: 547–552 [DOI] [PubMed] [Google Scholar]
- Fradin EF, Abd-El-Haliem A, Masini L, van den Berg GC, Joosten MH, Thomma BP. (2011) Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol 156: 2255–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CD, Nazar RN, Robb J, Liu CM, Thomma BP. (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150: 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL. (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129: 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE. (1999) The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones JD. (1994) Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266: 789–793 [DOI] [PubMed] [Google Scholar]
- Kawchuk LM, Hachey J, Lynch DR, Kulcsar F, van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, et al. (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA 98: 6511–6515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Clarke JD, Li Y, Dong X. (1999) Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 98: 329–339 [DOI] [PubMed] [Google Scholar]
- Lu H, Rate DN, Song JT, Greenberg JT. (2003) ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell 15: 2408–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Rafiqi M, Bernoux M, Ellis JG, Dodds PN. (2009) In the trenches of plant pathogen recognition: role of NB-LRR proteins. Semin Cell Dev Biol 20: 1017–1024 [DOI] [PubMed] [Google Scholar]
- Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT. (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M, Avni A. (2004) The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 16: 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, et al. (1997) The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell 9: 425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Matsuda O, Iba K. (2008) ITN1, a novel gene encoding an ankyrin-repeat protein that affects the ABA-mediated production of reactive oxygen species and is involved in salt-stress tolerance in Arabidopsis thaliana. Plant J 56: 411–422 [DOI] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick SG, Smerdon SJ. (1999) The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci 24: 311–316 [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, Minami E, Okada K, Yamane H, Kaku H, et al. (2010) Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J 64: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B, Shahid Mukhtar M, Somssich IE. (2007) The WRKY70 transcription factor of Arabidopsis influences both the plant senescence and defense signaling pathways. Planta 226: 125–137 [DOI] [PubMed] [Google Scholar]
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G. (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ellendorff U, Kemp B, Mansfield JW, Forsyth A, Mitchell K, Bastas K, Liu CM, Woods-Tör A, Zipfel C, et al. (2008) A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol 147: 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Plovanich-Jones A, Deng WL, Jin QL, Collmer A, Huang HC, He SY. (2000) The gene coding for the Hrp pilus structural protein is required for type III secretion of Hrp and Avr proteins in Pseudomonas syringae pv. tomato. Proc Natl Acad Sci USA 97: 2247–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann R, Lajunen HM, Erbs G, Newman MA, Kolb D, Tsuda K, Katagiri F, Fliegmann J, Bono JJ, Cullimore JV, et al. (2011) Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA 108: 19824–19829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fan W, Kinkema M, Li X, Dong X. (1999) Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci USA 96: 6523–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tessaro MJ, Lassner M, Li X. (2003) Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15: 2647–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Fang B, Gannon P, Ding P, Li X, Zhang Y. (2010) Arabidopsis snc2-1D activates receptor-like protein-mediated immunity transduced through WRKY70. Plant Cell 22: 3153–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]