Abstract
Introduction:
The production of Metallo-β-lactamases (MBLs) is one of the resistance mechanisms of Pseudomonas aeruginosa and Acinetobacter species. There is not much Indian data on the prevalence of MBLs in burns and surgical wards.
Materials and Methods:
A total of 145 non-duplicate isolates of carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter species, isolated from pus/wound swabs and endotracheal secretions from burns and surgical wards, were tested for MBL production by modified ethylene diamine tetra acetic acid (EDTA) disc synergy and double disc synergy tests.
Results:
Prevalence of MBLs was 26.9% by both the above tests. All MBL-positive isolates were multidrug resistant. Only 6.06% (2/33) P.aeruginosa and 16.67% (1/06) Acinetobacter species were susceptible to piperacillin-tazobactam and netilmycin, respectively. These patients had multiple risk factors like >8 days hospital stay, catheterization, IV lines, previous antibiotic use, mechanical ventilation, etc. Graft application and surgical intervention were significant risk factors in MBL-positive patients. Overall mortality in MBL-positive patients was 34.21%.
Conclusion:
Emergence of MBL-producing Pseudomonas aeruginosa and Acinetobacter species in this hospital is alarming, which reflect excessive use of carbapenems and at the same time, pose a therapeutic challenge to clinicians as well as to microbiologists. Therefore, a strict antibiotic policy and implementation of proper infection control practices will go a long way to prevent further spread of MBLs. Detection of MBLs should also become mandatory in all hospitals.
Keywords: Burns and surgical wards, metallo-β-lactamase producers, risk factors
INTRODUCTION
Metallo-β- lactamases (MBLs) are metalloenzymes of Ambler Class B and are clavulanic acid resistant enzymes. They require divalent cations of zinc as co-factors for enzymatic activity and are universally inhibited by ethylenediamine tetra acetic acid (EDTA), as well as other chelating agents of divalent cations.[1] The first plasmid-mediated MBL was reported in Pseudomonas aeruginosa in Japan in 1991.[2] Since then, many countries like Asia, Europe, Australia, and America have reported MBLs.[3–8] In India, the prevalence of MBLs range from 7.5% to 71%,[9–11] but there are very few documented reports from India from burns and surgical wards. The present study was undertaken to determine the prevalence of MBLs in Pseudomonas aeruginosa and Acinetobacter species in burns and surgical wards and also to find out the risk factors for the acquisition of MBLs.
MATERIALS AND METHODS
A total of 145 non-duplicate isolates of carbapenem-resistant Pseudomonas aeruginosa (103) and Acinetobacter species (42) were prospectively recovered from burns and surgical wards of this hospital during 1 year period (January to December 2008), after approval from the Institutional Ethics Committee. Out of 145 isolates, 142 were from pus/wound swabs and 3 were from endotracheal secretions.
They were further tested for Metallo-β-lactamase (MBL) production by modified-EDTA disc synergy test (MEDST)[12] and double disc synergy test (DDST).[13] For MEDST, an overnight broth culture of the test strain, (opacity adjusted to 0.5 McFarland opacity standards) was used to inoculate a plate of Mueller-Hinton agar. After drying, a 10 μg imipenem disc and a blank filter paper disk (6 mm in diameter, Whatman filter paper no. 2) were placed 10 mm apart from edge to edge. Then, 10 μl of 0.5 M EDTA solution was applied to the blank disc, which results in approximately 1.5 mg/disc. After overnight incubation, the presence of an enlarged zone of inhibition is interpreted as EDTA disc synergy test positive.[12]
For DDST, test organisms were inoculated on to Mueller Hinton agar plates. A 0.5 M EDTA solution was prepared by dissolving 18.61 g of disodium EDTA.2H2O in 100 ml of distilled water and adjusted to pH 8.0 by using NaOH. The mixture was sterilized by autoclaving. Two 10 mg imipenem discs were placed on the surface of an agar plate. EDTA solution was added to one of them to obtain a desired concentration of 750 μg. After 18-24 hours of incubation at 37°C, an increase of ≥7 mm in the zone diameter of imipenem-EDTA disc as compared to imipenem disc was considered to be a positive test for the presence of MBL.[13]
Antibiotic susceptibility of all MBL-positive isolates was performed on Mueller Hinton agar by Kirby Bauer Disc Diffusion Method (KBDDM) according to CLSI guidelines.[14] Antibiotics tested were gentamycin (30 μg), amikacin (30 μg), netilmycin (30 μg), amoxycillin-clavulanic acid (30 μg), cefotaxime (30 μg), ceftriaxone (30 μg), ceftazidime (30 μg), cefepime (30 μg), ciprofloxacin (5 μg), ofloxacin (5 μg), piperacillin (100 μg), and piperacillin-tazobactam (100/10 μg).
A proforma was prepared and filled up for each patient from whom MBL-producers were isolated.
RESULTS
Out of 145 carbapenem-resistant isolates, 110 were from burns ward and 35 were from surgical wards. From pus/wound swabs, all were Pseudomonas aeruginosa (103) and amongst Acinetobacter species, 39 were from pus/wound swabs, and 3 were from endotracheal secretions.
Overall prevalence of MBLs in the carbapenem-resistant isolates was 26.9% (39/145) in this study, by both MEDST and DDST, of which 32.04% (33/103) were Pseudomonas aeruginosa and 14.29% (06/42) were Acinetobacter species. All MBL-positive isolates were from adults and males predominated (58.97%). Out of 110 carbapenem-resistant isolates from burns ward, 18 (16.36%) were MBLs and out of 35 from surgical wards, 21 (60%) were MBLs.
Out of 39 MBL-positive isolates, only 6.06% (2/33) P.aeruginosa and 16.67% (1/06) Acinetobacter species were susceptible to piperacillin-tazobactam and netilmycin, respectively. All MBL-positive isolates were resistant to all other antibiotics tested.
Table 1 shows the risk factors in patients with carbapenem-resistant MBL-positive and negative isolates. Both MBL-positive and MBL-negative isolates had multiple risk factors like >8 days hospital stay, catheterization, IV lines, previous antibiotic use, mechanical ventilation, endotracheal intubation, etc., but graft application and surgical intervention were significant risk factors for MBL-positives (‘P’ < 0.05) as compared to MBL-negatives, by Chi-square analysis.
Table 1.
Risk factors in patients with carbapenem-resistant MBL-positive and negative isolates
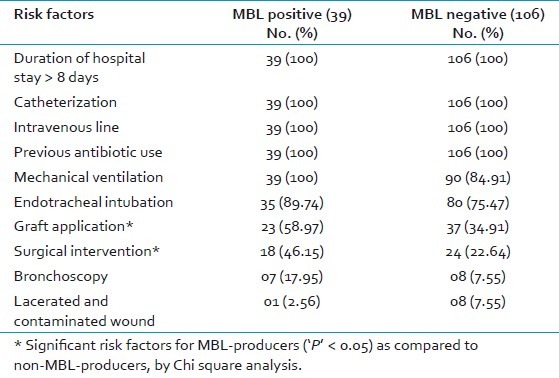
Overall 12 patients (30.77%) had a combination of first 6 risk factors [Table 1], and 11 patients (28.21%) had a combination of first 8 risk factors. In 18 burn patients from whom MBLs were isolated, combination of first 8 risk factors and combination of first 9 risk factors were seen in 6 patients; first 7 in 5 patients and all 10 in 1 patient [Table 1]. In 21 MBL-positive isolates from surgical wards, combinations of first 6 factors were seen in 12 patients; first 8 in 5 patients and first 5 in 4 patients.
Overall mortality in patients with MBL-positive isolates was 33.33% (13/39) and in patients with MBL-negative isolates was 25.47% (27/106). Of deaths due to MBLs, P. aeruginosa accounted for 36.36% (12/33) and Acinetobacter species accounted for 16.67% (1/6).
DISCUSSION
As MBLs will hydrolyze virtually all classes of β-lactams, there continued spread will be a clinical catastrophe.[1] With the global increase in the types of MBLs, an early detection is crucial.[7] Over the last decade, studies were on different methods of MBL detection in Pseudomonas and Acinetobacter species.[3–5] Though MIC detection is the gold standard phenotypic test, DDST and MEDST are comparable with the former and at the same time, are simple, reliable, less cumbersome and cheap, as per previous reports.[3–5] Lee et al, have reported 100% sensitivity and specifity of MEDST.[12] Therefore, these tests can be used in a small laboratory set up also. By both these tests, the prevalence of MBLs in burns and surgical wards was 26.9% in this study, and both the tests were comparable in the present study. In a previous study, in the same institute in intensive care areas, the prevalence of MBLs was 33.33%.[11]
A recent study in burn patients reported overall prevalence of 55% MBLs,[8] whereas in this study, overall prevalence is 26.9%. In burn patients, prevalence of MBL-producing Pseudomonas aeruginosa has been reported to be 16-19.5%.[7,9] A recent study from Tehran, Iran has reported a very high prevalence of 94.2% MBL-producing P. aeruginosa in burn patients.[15] In this study, though the prevalence of MBLs in Pseudomonas aeruginosa was much less than the former study, it was higher (32.04%) than in Acinetobacter species (14.29%). Our prevalence of MBLs in Pseudomonas correlates well with other studies (28.6%-36%).[3–5,11] Another recent study from South India has reported 62.5% MBLs in imipenem-resistant P. aeruginosa.[16]
Lee, et al,[4] have reported 14.2% MBLs in Acinetobacter species, which is almost similar to this study. A study from South India has reported 70.9% prevalence of MBLs in Acinetobacter species by DDST.[10] Our prevalence in Acinetobacter species was, however, only 14.29%. In another recent study from South India in burn patients, 15.7% strains were MBL-producers, with Acinetobacter baumannii being the predominant MBL producer.[16]
Apart from being carbapenem-resistant, all MBLs were resistant to important groups of antibiotics tested, including third generation cephalosporins, aminoglycosides and quinolones – a characteristic feature of MBL-producers.[1,3,15–17] Though one study showed 30% susceptibility to ceftazidime and 37.5% to gentamicin,[9] in this study, only 6.06% of Pseudomonas aeruginosa and 16.67% of Acinetobacter species were susceptible to piperacillin-tazobactam and netilmycin, respectively. For MBLs, limited treatment options are available and the only therapeutic option may be polymyxins, but it should never be used as monotherapy.[1] It can be combined with an appropriate aminoglycoside. Combination therapy is often employed in treatment of MBL-producing Acinetobacter baumanii with imipenem/meropenem combined with ampicillin-sulbactam.[18]
Multiple risk factors (5 or more) were present in all MBL-positive patients. All had risk factors of hospital stay >8 days, catheterization, IV line, previous antibiotic use, and mechanical ventilation [Table 1]. All the above were major risk factors for carbapenem resistance also. Infection Control Fact Sheet of 2007 of a hospital mentions risk factors for acquisition of MBLs as prolonged hospitalization; prior antimicrobial therapy; treatment in ICU, hematology, and burns patients where antibiotic usage is high.[19] In this study also, we isolated MBLs from burns ward (16.36%), and all had hospital stay >8 days and previous antibiotic use. A major finding in this study was that graft application and surgical intervention were significant risk factors for MBL-producers (‘P’ < 0.05) as compared to non MBL-producers [Table 1].
Mortality of MBL-positive patients was 33.33% in this study, but in one report, it is very high (85.7%).[4] In a previous study from the same institute from intensive care areas, overall mortality was 46.15%.[11] Patients with MBL-producing Pseudomonas had a higher mortality (36.36%) than Acinetobacter species (16.67%), in accordance with other studies.[3,4]
Emergence of MBL-producing Pseudomonas aeruginosa and Acinetobacter species in this hospital is alarming and reflect excessive use of carbapenems and selective antibiotic pressure. Therefore, a strict antibiotic policy should be followed in every hospital to prevent further spread of MBLs. Clinicians should be made aware of the problem of MBLs, so that they can prescribe antibiotics judiciously. As most MBL-producing organisms are multidrug-resistant,[15] this might pose a therapeutic challenge to clinicians as well as to microbiologists. Timely implementation of proper infection control practices reduce, eliminate, and prevent establishment of antibiotic-resistant organisms as the predominant nosocomial flora of burn unit and prevent cross-contamination.[20]
Patients with serious thermal injuries require immediate specialized care in order to minimize morbidity and mortality.[16] Detection of MBLs by either DDST or MEDST should become mandatory in all microbiology laboratories for all carbapenem-resistant isolates, thereby reducing morbidity and mortality in these patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-β-lactamases: The quiet before the storm? Clin Microbiol Rev. 2005;18:306–25. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt T, Usman M, Ahmed RN, Saif I. Emergence of Metallo- β-lactamase producing Pseudomonas aeruginosa in Pakistan. J Pak Med Assoc. 2005;55:302–4. [PubMed] [Google Scholar]
- 3.Pitout JD, Gregson DB, Poirel L, McClure JA, Le P, Church DL. Detection of Pseudomonas aeruginosa producing Metallo-β-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43:3129–35. doi: 10.1128/JCM.43.7.3129-3135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K, Lee WG, Uh Y, Ha GY, Cho J, Chong Y. VIM- and IMP-type Metallo-β-lactamase producing Pseudomonas spp. and Acinetobacter spp. in Korean hospitals. Emerg Infect Dis. 2003;9:868–71. doi: 10.3201/eid0907.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behera B, Mathur P, Das A, Kapil A, Sharma V. An evaluation of four different phenotypic techniques for detection of Metallo-β -lactamase producing Pseudomonas aeruginosa. Indian J Med Microbiol. 2008;26:233–7. doi: 10.4103/0255-0857.39587. [DOI] [PubMed] [Google Scholar]
- 6.Jayakumar S, Appalaraju B. Prevalence of multi and pan drug resistant Pseudomonas aeruginosa with respect to ESBL and MBL in a tertiary care hospital. Indian J Pathol Microbiol. 2007;50:922–5. [PubMed] [Google Scholar]
- 7.Khosravi AD, Mihahi F. Detection of Metallo-β-lactamase producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis. 2008;60:125–8. doi: 10.1016/j.diagmicrobio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Altoparlak U, Aktas F, Celebi D, Ozkurt Z, Akcay MN. Prevalence of Metallo-β-lactamase among Pseudomonas aeruginosa and Acinetobacter baumanii isolated from burn wounds and in vitro activities of antibiotic combinations against these isolates. Burns. 2005;31:707–10. doi: 10.1016/j.burns.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Rajput A, Saxena R, Singh KP, Kumar V, Singh S, Gupta A, et al. Prevalence of antibiotic resistance pattern of Metallo-β-lactamase producing Pseudomonas aeruginosa from burn patients – Experience of an Indian tertiary care hospital. J Burn Care Res. 2010;31:264–8. doi: 10.1097/BCR.0b013e3181d0f4bf. [DOI] [PubMed] [Google Scholar]
- 10.Karthika UR, Rao SR, Sahoo S, Shashikala P, Kanungo R, Jayachandran S, et al. Phenotypic and genotypic assays for detecting the prevalence of metallo-beta-lactamases in clinical isolates of Acinetobacter baumanii from a South Indian tertiary care hospital. J Med Microbiol. 2009;54:430–5. doi: 10.1099/jmm.0.002105-0. [DOI] [PubMed] [Google Scholar]
- 11.De AS, Kumar SH, Baveja SM. Prevalence of Metallo-β-lactamase producing Pseudomonas aeruginosa and Acinetobacter species in intensive care areas in a tertiary care hospital. Indian J Crit Care Med. 2010;14:217–9. doi: 10.4103/0972-5229.76089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTA-disk synergy tests to screen Metallo-β-lactamase strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect Dis. 2001;7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge Test and the imipenem-EDTA Double Disk Synergy Test for differentiating Metallo-β-lactamase producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41:4623–9. doi: 10.1128/JCM.41.10.4623-4629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Performance Standards for Antimicrobial Susceptibility Testing; Twenty First Informational Supplement. Clinical Laboratory Standards Institute. 2011;31:62–5. M100-S21. [Google Scholar]
- 15.Saderi H, Lotfalipour H, Owlia P, Salimi H. Detection of Metallo-β-lactamase producing Pseudomonas aeruginosa isolated from burn patients in Teheran, Iran. Lab Med. 2010;41:609–12. [Google Scholar]
- 16.Bandekar N, Vinodkumar CS, Basavarajappa KG, Prabhakar PJ, Nagaraj P. Beta lactamases mediated resistance amongst gram negative bacilli in Burn infection. International J Biol Med Res. 2011;2:766–70. [Google Scholar]
- 17.Peshattiwar PD, Peerapur BV. ESBL and MBL mediated resistance in Pseudomonas aeruginosa: An emerging threat to clinical therapeutics. J Clin Diagn Res. 2011;5:1552–4. [Google Scholar]
- 18.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumanii. Antimicrob Agents Chemother. 2007;51:3471–84. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organisms with Metallo-beta-lactamases (MBLs). Infection Control Fact Sheet 2007. Infection Control and Hospital Epidemiology Unit, Alfred Hospital. 2007. [Last accessed on 2008 Sept 18]. Available from: http://www.Alfred.org.au/departments/index.html .
- 20.Weber J, McManus A. Infection control in burn patients. Burns. 2004;30:A16–24. doi: 10.1016/j.burns.2004.08.003. [DOI] [PubMed] [Google Scholar]


