Abstract
Free radicals are highly reactive and unstable compounds. These highly reactive molecules cause oxidative damage to cellular components such as DNA, proteins and lipids. They play central role in the mechanism of cell injury and cell death. Free radical scavengers either prevent these reactive species from being formed, or remove them before they can damage vital components of the cell. Oxidative stress defines an imbalance in production of oxidizing chemical species and their effective removal by protective antioxidants and scavenger enzymes. Evidence of massive oxidative stress is well established in critical illnesses characterized by tissue ischaemia-reperfusion injury and by an intense systemic inflammatory response such as during sepsis and acute respiratory distress syndrome, acute lung injury. Several clinical trials have been performed in order to reduce oxidative stress by supplementation of antioxidants alone or in combination with standard therapies. Antioxidant supplementation at an early stage of illness may lead to improved therapies in the treatment of critically ill patients. Several intravenous anaesthetic drugs act as reactive oxygen species scavengers. Anaesthetic preconditioning is of particular interest to anaesthesiologist, in which lasting protection of myocardium is elicited by brief exposure to a inhalational anaesthetic agent. These anasthetics may also mediate protective effects in other organs, such as the brain and kidney It is important for the anaesthesiologist to understand the mechanism of damage caused by free radicals and how free radical scavengers work so that this knowledge can be applied to varied pathological conditions. The topic was hand searched in text books and electronically searched from PubMed and Google scholar using text words.
Keywords: Anaesthesiology, antioxidants, critical care, free radicals, oxidative stress
INTRODUCTION
Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. Inturn, these radicals can start chain reactions that damage cells. Most of the potentially harmful effects of oxygen are due to reactive oxygen species (ROS), which have a tendency to donate oxygen to other substances. Many such reactive species are free radicals and have a surplus of one or more free-floating electrons rather than having matched pairs and are, therefore, unstable and highly reactive.[1] Types of free radicals include superoxide radicals (O2–), hydrogen peroxide (H2O2), hydroxyl ions (OH) and lipid peroxyl radical (LOO).[2]
Several protective systems operate in mammalian cells to prevent formation of free radicals or to scavenge excessive amounts already formed. These are known as preventive and chain breaking antioxidants or radical scavengers like catalases, glutathione peroxidase, superoxide dismutase (SOD), α-tocopherol (Vit. E), ascorbic acid (Vit. C), β carotene (Vit. A), selenium. Although about 4000 antioxidants have been identified, the best known are Vit. E, Vit. C and the carotenoids. Many other non-nutrient food substances, generally phenolic or polyphenolic compounds, display antioxidant properties and thus may be important for health.
RELEVANCE OF FREE RADICALS AND FREE RADICAL SCAVENGERS TO ANAESTHESIOLOGY AND CRITICAL CARE
Excessive generation of ROS is one of the mechanism incriminated in the pathogenesis of generalized (i.e. sepsis, transplantation, ischaemia/reperfusion injury, burns) or local (i.e. asthma, chronic obstructive pulmonary disease) inflammatory reactions. Ischaemia/reperfusion injury (IRI) occurs in a number of pathological conditions, including myocardial infarction, stroke, aortic surgery, cardiopulmonary bypass, organ transplantation, resuscitation and critical care. Massive and abrupt release of oxygen radicals after reperfusion triggers oxidative damage. Before critical surgeries or after resuscitation, it would be wise to find a suitable prophylactic treatment to avoid ischaemia/reperfusion damage.[3] Commonly used anaesthetic agents protect against renal IRI. Erguin Y et al. reported protective effect of propofol and ketamine in IRI in skeletal muscle in rats.[4] Pharmacological interventions like methylprednisolone, multivitamin antioxidant infusion, Vit. E infusion, amrinone, prostaglandin E 1, pentoxifylline, mannitol, trimetazidine, dextrose, allopurinol, and OKY046 have shown some promise in decreasing liver damage caused by occluded blood supply in liver resection surgery performed under vascular control.[5] The oxidative stress is more evident in patients with sepsis, systemic inflammatory response syndrome (SIRS), who develop multiple organ failure (MOF) and die.[6] ROS are central to cardiac IRI. They contribute to myocardial stunning, infarction and apoptosis and possibly to genesis of arrhythmias. Anaesthetic preconditioning (APC) is of particular interest to anaesthesiologists. In this phenomenon, lasting protection of myocardium is elicited by brief exposure to inhalational anaesthetic agent. Free radicals are known to act as second messengers in the preconditioning cell-signalling pathway.[7] Inhalational anaesthetics have been shown to enhance generation of free radicals in cardiac cells, probably by causing mild uncoupling of the mitochondrial electron transport chain. Hepatotoxicity attributable to halothane may result partly from conversion of halothane to free radicals by cytochrome P450 enzymes, but there is no evidence of similar reactions involving other inhalational anaesthetics in other organs. Stephan G. De Hert et al. concluded from experimental data that clinical concentrations of inhalational anaesthetics protect the myocardium from IRI, as shown by decreased infarct size and a more rapid recovery of contractile function on reperfusion.[8] Local anaesthetics lidocaine and procaine dose dependently preserve endothelium-dependent vasorelaxation against ROS attack potentially via H2O2 scavenging.[9]
The aim of this article is to highlight relevance of free radicals and free radical scavengers to anaesthesia practice, as scant literature is available relating this relevance. There is no dearth of literature relating this relevance to critical care, which is summarized in this article. Ischaemic and hypoxic insults to the brain during surgery and anaesthesia result in life threatening complications including stroke. These complications occur at the rate of 0.08-0.7% in general surgery and 1.4-3.8% in cardiac surgery.[10] Pharmacologic interventions, including calcium channel blockers, free radical scavengers and glutamate antagonists, have been introduced to prevent and/or ameliorate stroke.[11] Antioxidant supplementation at an early stage of illness may lead to improved therapies in the treatment of critically ill patients. Inhalational anaesthetics have been shown to protect against cardiac IRI by mechanism that are apparently independent of their effects to cause coronary vasodilatation or to depress cardiac contractility. Propofol has been shown to be protective in experimental models of injury in several organs, including brain, liver and heart.[7] This article stresses requirement of further studies to determine if the cellular protective effects of propofol translate into meaningful improvements in perioperative outcome. An alternative pathway for nitric oxide generation was discovered, wherein the inorganic anions nitrate (NO3) and nitrite (NO2), most often considered inert end products from nitric oxide generation can be reduced back to nitric oxide and other bioactive nitrogen oxide species. This nitrate-nitrite-nitric oxide pathway is regulated differently than the classic l-arginine-nitric oxide synthase nitric oxide pathway, and it is greatly enhanced during hypoxia and acidosis. Several researches now indicate that the nitrate-nitrite-nitric oxide pathway is involved in regulation of blood flow, cell metabolism, and signalling, as well as in tissue protection during hypoxia.[12] The fact that nitrate is abundant in our diet gives rise to interesting nutritional aspects in health and disease.
The topic was hand searched in text books. Electronic search of literature was made from PubMed and Google scholar using text words.
HISTORY
The term antioxidant originally was used to refer specifically to a chemical that prevented the consumption of oxygen. Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of unsaturated fats, which is the cause of rancidity.[13] However, it was the identification of vitamins A, C, E as antioxidants that revolutionised the field and led to the realization of the importance of antioxidants in the biochemistry of living organisms.[14,15] Research into how vitamin E prevents the process of lipid peroxidation led to the identification of antioxidants as reducing agents that prevent oxidative reactions, often by scavenging reactive oxygen species before they can damage cells.[16]
FREE RADICALS AND REACTIVE OXYGEN SPECIES
Harmful effects of oxygen are due to formation of oxygen derived free radicals (ODFR). A free radical is a molecule or fragment thereof that possesses an unpaired electron. They are very reactive and have an extremely short half-life (microseconds). The major toxic effects of ODFR are the destruction of cell and organelle membranes and breakage of DNA strands with resultant enzyme inactivation.[17] The membrane damage leads to increased intracellular Ca++ ion concentration with activation of membrane bound enzyme phospholipase A2 and initiation of arachidonic acid cascades (PG, TX, LT).[18] ROS produced in cells include hydrogen peroxide (H2 O2), hypochlorus acid (HOCl) and free radical such as hydroxyl radical (-OH), suproxide anion (O2-). Superoxide is the product of mitochondrial autooxidation or enzymatic generation by cytoplasmic enzymes such as xanthine oxidase, Cytochrome P450 and others. H2O2 is generated in peroxisomes or the dismutation of superoxides. Nitric oxide (NO) is another species that can act as a free radical. These radicals are both unstable and reactive. Free radicals play a central role in the mechanism of cell injury and cell death. These mechanisms include lipid peroxidation of plasma and organellar membranes, oxidative alteration of proteins and alteration in DNA integrity. Highly reactive species are usually compartmentalized in small vesicles such as peroxisomes or confined in mitochondria. Trauma can disrupt peroxisomes and increase generation of free radicals.
Some important internally generated sources of free radicals are-mitochondria, phagocytes, xanthine oxidase, arachidonate pathway, peroxisomes, inflammation, and ischaemia/reperfusion.
Some externally generated sources of free radicals are-cigarette smoke, environmental pollutants, radiation, UV light and certain drugs and pesticides.
Free radicals, however, are not always harmful. They also serve useful purposes in human body. Several observations indicate that the oxygen radicals in the living systems are probably necessary compounds in the maturation processes of cellular structures. The concentration of these radicals is stringently maintained, however at less than 10-9 mol/L.[19] WBCs release free radicals to destroy invading pathogenic microbes as a part of body's defence mechanism against disease. Hence the complete elimination of these radicals would not be possible, but also harmful. The balance between the production of free radicals and the antioxidant defences in the body is important. If there are too many free radicals produced and too few antioxidants, a condition of ‘oxidative stress’ develops which may cause chronic damage
OXIDATIVE STRESS IN DISEASE (FREE RADICAL DISEASES)
Oxidative stress is thought to contribute to the development of wide range of diseases.
CNS disorders
Calcium is ubiquitous second messenger in cells [Figure 1]. The rapid, uncontrolled increase in cystolic calcium levels initiates activation of numerous enzymes that contribute to cell injury. 1) Protease activation causes breakdown of cytoskeleton of the neuron; 2) lipases damage plasma membrane lipids and release arachidonic acid, which is metabolized by cyclooxygenases and lipoxygenases to yield free radicals and other mediators of cell injury; 3) activation of nitric oxide synthetase (NOS) leads to release of NO and, in turn, the generation of peroxynitrite, a highly reactive free radical, and activated endonucleases damage DNA, thereby rendering neurons susceptible to apoptosis. Injury to mitochondria leads to energy failure, free radical generation and release of cytochrome C (Cyt c) from mitochondria; the latter is one of the means by which neuronal apoptosis is initiated. NO is infact, a weak free radical that in turn leads to the generation of peroxynitrite which is a ‘killer substance’ used by macrophages. In cerebral ischaemia NO is probably both friend and foe. It is likely that during a period of focal ischaemia, the vasodilating effect of NO serves to augment collateral CBF. However in the postischaemic phase, NO (probably inducible NO of neuronal origin) contributes to neuronal injury.[20]
Figure 1.
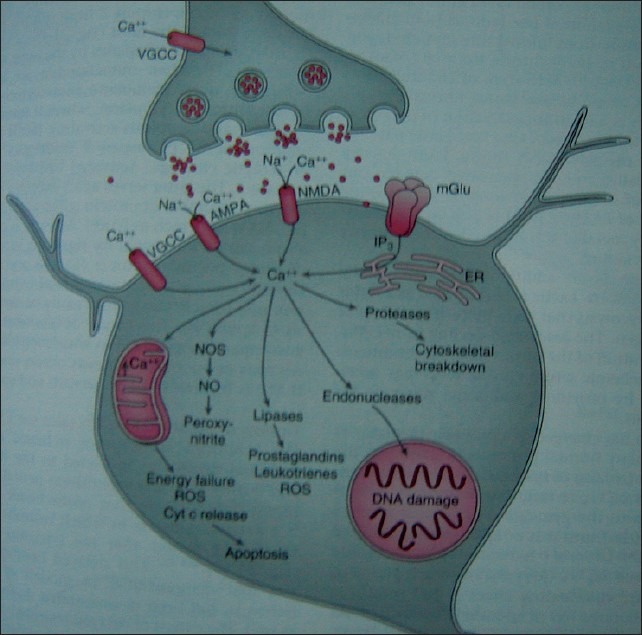
A.CNS disorders VGCC-voltage-gated calcium channels, AMPA-a-amino-3-hydroxy-5-methyl-4-isoxazopropionic acid, NMDAN- methyl-D-aspartate, mGlu-metabotropic glutamate receptors, IP3- inositol triphosphate, ER-endoplasmic reticulum, NOS-nitric oxide synthase, NO-nitric oxide, ROS-reactive oxygen species, Cyt c-cytochrome c Reproduced with permission from Miller's Anaesthesia, 6th edition, vol.no.1, page no.835, Copyright Elsevier Churchill Livingstone, 2005
Cardiovascular diseases
In cardiovascular disease, low density lipoprotein (LDL) oxidation appears to trigger the process of atherogenesis. Oxidized LDL is thought to reduce NO production by the endothelium, which leads to vasoconstriction. Enhanced platelet aggregation is also induced by oxidized LDL.[21,22] Low β-carotene concentration in adipose tissues were associated with a high risk of myocardial infarction in current smokers. Vitamin E intakes are associated with lowered risk of angina and mortality from ischaemic heart disease.
Biochemistry of ischaemia
When cellular hypoxia persists, the purine metabolite hypoxanthine and the xanthine oxidase accumulate intracellularly [Figure 2]. The hypoxanthine is derived from the catabolism of residual ATP whilst xanthine oxidase is produced from xanthine dehydrogenase under the hypoxic conditions. Xanthine dehydrogenase is a constituent of most cells and highly concentrated in intestinal mucosal epithelial cells and vascular endothelium. Consequently the intestines and lower limbs are locations where very high tissue concentration of XO accumulate during ischaemia.
Figure 2.
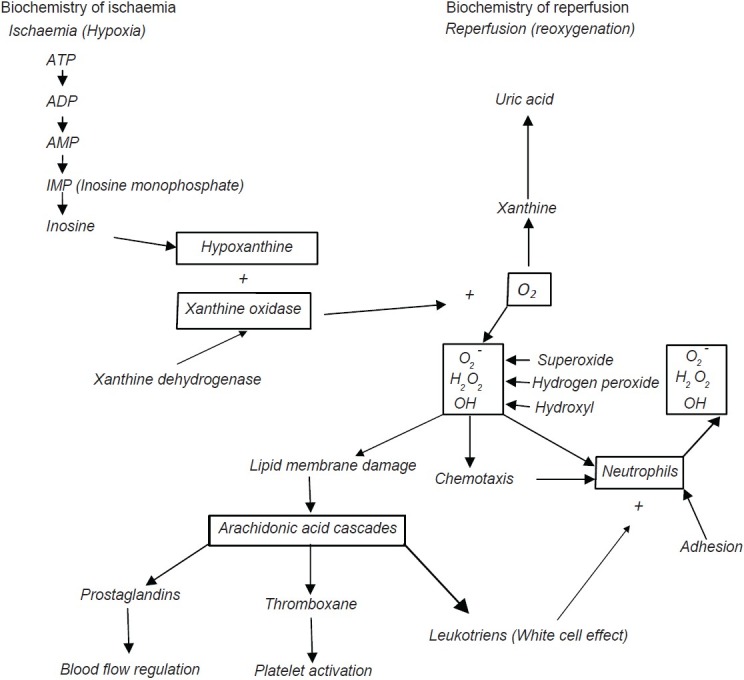
Pathophysiology of ischaemia - reperfusion injury
Biochemistry of reperfusion
When blood flow is restored to a postischaemic tissue, the accumulated XO and hypoxanthine react with freshly supplied oxygen to form purine metabolites (xanthine and uric acid) plus free radicals.
Critical care
Exaggerated production of ROS plays a major role in several aspects of septic shock, inflammation and ischaemia and reperfusion. ROS trigger DNA strand breakage with subsequent activation of the nuclear enzyme poly-adenosine 5’-diphosphate ribosyl synthetase and eventual severe energy depletion of the cells. Pharmacological evidence suggests that the peroxynitrite-poly-adenosine 5’-diphosphate ribosyl polymerase pathway contributes to the cellular injury in shock and endothelial injury.[23,24] Patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) showed a significant decrease in plasma levels of α-tocopherol, ascorbate, β-carotene and selenium and elevated levels of lipid peroxidation products.[6]
Diabetes mellitus
Oxidative stress is crucial to the aetiology of diabetes mellitus and ensuing damage to different tissues and organs.[25] Hyperglycaemia causes the generation of ROS leading to increased oxidative stress in a variety of tissues. In the absence of an appropriate compensatory response to endogenous antioxidants, such as Vit. C and E, catalase, glutathione and SOD, oxidative stress dominates, resulting in the activation of stress-sensitive intracellular signalling pathways. This plays a key role in the development of late complications of DM as well as mediating insulin resistance.[26]
Hydrogen peroxide is involved in the formation of many oxygen free radicals (OFRs). Thought to be involved in normal phagocyte function and host defence;released into phagosomes to destroy bacteria etc. May also be produced by radiation and certain chemicals and increased production has been implicated in many disease processes including pulmonary oxygen toxicity, halothane hepatitis, ARDS, paracetamol poisoning, burns, carcinogenesis, ischaemic colitis.
FREE RADICAL SCAVENGERS
Several protective systems operate in mammalian cells to prevent formation of free radicals or to scavenge excessive amounts already formed. Free radical scavengers either prevent reactive oxygen species from being formed, or remove them before they can damage vital components of the cell. They are known as preventive and chain breaking antioxidants. The first group includes catalases, glutathione peroxidases and superoxide dismutase (SOD) i.e. enzymatic mechanism of inactivation of ODFR (oxygen derived free radicals). The second group, chain breaking antioxidants or ‘radical scavengers’ are compounds capable of transferring hydrogen to free radicals. This group includes physiological antioxidants like ascorbic acid, α tocopherol and β carotene. The preventive antioxidants eliminate the species involved in the initiation of free radical chain reaction, whereas the chain breaking antioxidants repair oxidizing radical directly. Antioxidants are classified into two broad divisions, depending on whether they are soluble in water (hydrophilic) e.g. Vit. C or in lipids (hydrophobic) e.g. β carotene and Vit. E which are membrane bound. Water soluble antioxidants react with oxidants in the cell cytosol and the blood plasma, while lipid soluble antioxidants protect cell membranes from lipid peroxidation. These compounds may be synthesized in the body or obtained from diet. About 4000 antioxidants have been identified. Some of the antioxidants like glutathione, ubiquinol and uric acid are produced during normal metabolism in the body. Several essential minerals including selenium, copper, manganese and zinc are necessary for the formation or activity of peroxidases, SOD and catalase. Hence, if the nutritional supply of these minerals is inadequate, enzymatic defences against free radicals may be impaired.
APPLICATION OF FREE RADICAL SCAVENGERS IN
Neurological disorders
Antioxidants are commonly used as medication to treat various forms of brain injury. Superoxide dismutase mimetics, Sodium Pentothal and Propofol are used to treat reperfusion injury and traumatic brain injury. General anaesthetics penetrate into brain parenchyma and prevent oxidative injury to neurons by several mechanisms 1) General anaesthetics slow cerebral utilization of oxygen and glucose, inhibit oxidative metabolism in neutrophils and prevent redox changes in haemoglobin, thereby may inhibit free radical generation 2) Thiopental and propofol directly scavenge ROS and inhibit lipid peroxidation 3) Anaesthetics may prevent the elevation of extracellular glutamate concentration and inhibit the activation of excitatory glutamatergic receptors that augment oxidative stress after ischaemia.[27] Propofol is a peroxynitrite scavenger. Melatonin is a powerful antioxidant that can easily cross cell membrane and blood brain barrier. It facilitates sleep by acting as a natural sedative. FRS appear to prevent oxidative stress in neurons and prevent apoptosis and neurological damage. FRSs are effective in all cell types of neurovascular unit and should be considered as a potential therapeutic approach for stroke.[28] Amifostine 500mg intraaortic infusion during spinal cord ischaemic phase significantly attenuated the spinal cord oxidative injury in rabbits.[29] NASCIS ll (National Acute Spinal Cord Injury Studies) clinical trials have shown that an antioxidant dosing regimen with methylprednisolone begun within 8 hours after spinal cord injury enhances chronic neurological recovery. Phase lll trials with more selective and effective antioxidant U74006F (tirilazad mesylate) are being conducted to indicate the importance of inhibition of posttraumatic free radical reactions in the injured CNS.[30] Edaravone mainly showed a prophylactic effect on neurons against glutamate neurotoxicity, possibly through the inhibition of neurons via the suppression of ROS production.[31]
Cardiovascular diseases
Lidocaine and procaine dose dependently preserve endothelium-dependent vasorelaxation against ROS attack potentially via H2O2 scavenging.[9] Lidocaine is more effective than bupivacaine and ropivacaine in protecting human erythrocytes submitted to an oxidative stress.[32]
Respiratory diseases
Oxidative stress is important in the pathogenesis of ALI/ARDS. Patients with ARDS showed a significant decrease in plasma levels of α-tocopherol, ascorbate, β-carotene and selenium and elevated levels of lipid peroxidation products. Free radical scavenging has protective effect in pulmonary oxygen toxicity and ARDS. FRS Edaravone has protective effect in acute pancreatitis associated lung injury in rat lungs.[33]
Critical care
Oxygen free radical scavengers (SOD, allopurinol) are being assessed in experimental haemorrhagic and septic shock models.[34] Treatment with SODmimetics has been shown to prevent the cellular energetic failure associated with shock and ischaemia-reperfusion and to prevent tissue damage associated with conditions. Salvemini D et al. presented a study to support the potential development of SODmimetics as novel and effective agents in the area of critical care medicine.[23] Treatment with melatonin has been shown to prevent in vivo the delayed vascular decompensation and the cellular energetic failure associated with shock, inflammation and ischaemia/reperfusion injury. Recently it has been demonstrated that melatonin inhibits the activation of poly (ADP-ribose) synthetase and prevents the organ injury associated with shock, inflammation and ischaemia/reperfusion.[24]
CONCLUSION
As oxidative stress might be an important part of many human diseases, the use of free radical scavengers in pharmacology is being intensively studied, particularly as treatment for stroke and neurodegenerative diseases. Commonly used anaesthetic agents protect against ischaemia/reperfusion injury. An intense study of oxygen radical-mediated mechanism may lead to improved therapies in the treatment of critically ill patients. Antioxidants are also widely used as ingredients in dietary supplements in the hope of maintaining health and preventing diseases such as cancer and coronary heart disease. Further studies will be required to determine if the cellular protective effects of anaesthetic agents translate into meaningful improvements in perioperative outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bagchi K, Puri S. Free radicals and antioxidants in health and diseases. East Mediterr Health J. 1998;4:350–60. [Google Scholar]
- 2.Crider BA, Mortimer AJ. Anaesthesia for vascular surgery. In: Healy TEJ, Knight PR, editors. Wylie and Churchill- Davidson's A Practice of Anaesthesia. 7th ed. London: Arnold a member of the Hodder Headline Group; 2003. p. 768. [Google Scholar]
- 3.Dogan Z, Yuzbasioglu MF, Kurutas EB, Yildiz H, Coskuner I, Sinoglu N, et al. Thiopental improves renal ischaemia-reperfusion injury. Ren Fail. 2010;323:391–5. doi: 10.3109/08860221003611752. [DOI] [PubMed] [Google Scholar]
- 4.Erguin Y, Darendeli S, Imrek S, Kilinc M, Oksuz H. The comparison of the effects of anaesthetic doses of ketamine, propofol and etomidate on ischaemia/reperfusion injury in skeletal muscles. Fundam Clin Pharmacol. 2010;24:215–22. doi: 10.1111/j.1472-8206.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu Amara M, Guruswamy KS, Hori S, Glantzounis G, Fuller B, Davidson BR. Pharmacological interventions versus no pharmacological intervention for ischaemia reperfusion injury in liver resection surgery performed under vascular control. Cochrane Database Syst Rev. 2009;4:CD007472. doi: 10.1002/14651858.CD007472.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, et al. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 7.Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection-The relevance to anaesthesia practice. Anesth Analg. 2005;101:1275–87. doi: 10.1213/01.ANE.0000180999.81013.D0. [DOI] [PubMed] [Google Scholar]
- 8.Stephen G, De Hert, Turani F, Mathur S, Stowe DF. Cardioprotection with volatileanaesthetics-mechanisms and clinical implications. Anesth Analg. 2005;100:1584–93. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- 9.Lee JM, Suh JK, Jeong KS, Cho SY, Kim DW. Antioxidant effect of lidocaine and procaine on reactive oxygen species induced endothelial dysfunction in the rabbit abdominal aorta. Korean J Anesthesiol. 2010;59:104–10. doi: 10.4097/kjae.2010.59.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Kai S, Koyama T, Daigo H, Adachi T, Fukuda K, et al. General anaesthetics inhibit erythropoietin induction under hypoxic conditions in the mouse brain. PLoS One. 2011;6:e29378. doi: 10.1371/journal.pone.0029378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg MD. Current status of neuroprotection for cerebral ischaemia: Synoptic overview. Stroke. 2009;40:S111–4. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weitzberg E, Hezel M, Lundberg JO. Nitrate-nitrite-nitric oxide pathway: Implications for anaesthesiology and intensive care. Anesthesiology. 2010;113:1460–75. doi: 10.1097/ALN.0b013e3181fcf3cc. [DOI] [PubMed] [Google Scholar]
- 13.German J. Food processing and lipid oxidation. Advances in Experimental Medicine and Biology. 1999;459:23–50. doi: 10.1007/978-1-4615-4853-9_3. [DOI] [PubMed] [Google Scholar]
- 14.Jacob R. Three eras of Vit. C discovery. Subcell Biochem. 1996;25:1–16. [PubMed] [Google Scholar]
- 15.Knight J. Free radicals: Their history and current status in aging and disease. Ann Clin Lab Sci. 1998;28:331–46. [PubMed] [Google Scholar]
- 16.Wolf G. The discovery of the antioxidant function of Vit. E: The contribution of Henry A. Matill. J Nutr. 2005;135:363–6. doi: 10.1093/jn/135.3.363. [DOI] [PubMed] [Google Scholar]
- 17.Ernster L. Biochemistry of reoxygenation injury. Crit Care Med. 1988;16:947–53. doi: 10.1097/00003246-198810000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Morel DR. Role of arachidonic acid metabolism in ARDS. In: Vincent JL, editor. Update in intensive care And emergency medicine. Vol. 8. New York: Springer; 1989. pp. 115–24. [Google Scholar]
- 19.Prayor WA. Free radical reactions and their importance in biochemical systems. Fed Proc. 1973;32:1862–9. [PubMed] [Google Scholar]
- 20.Patel PM, Drummond JC. Cerebral physiology and the effects of anaesthetics andtechniques. In: Miller RD, editor. Miller's Anaesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 835–6. [Google Scholar]
- 21.Faggiotto A, Poli A, Catapano AL. Antioxidants and coronary artery disease. Curr Opin Lipidol. 1998;9:541–9. doi: 10.1097/00041433-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Adams AK, Wermuth EO, McBride PE. Antioxidant vitamins and the prevention of coronary heart disease. Am Fam Physician. 1999;60:895–904. [PubMed] [Google Scholar]
- 23.Salvemini D, Cuzzocrea S. Therapeutic potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med. 2003;31(suppl 1):S29–38. doi: 10.1097/00003246-200301001-00005. [DOI] [PubMed] [Google Scholar]
- 24.Cuzzocrea S, Reiter RJ. Pharmacological action of melatonin in shock, inflammation andischaemia/reperfusion injury. Eur J Pharmacol. 2001;24:1–10. doi: 10.1016/s0014-2999(01)01175-x. [DOI] [PubMed] [Google Scholar]
- 25.Kodiha M, Stochaj U. Nuclear Transport: A switch for the oxidative stress-signallingcircuit. J Signal Transduct. 2012;2012:208650. doi: 10.1155/2012/208650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fardoun RZ. The use of Vit. E in Type 2 diabetes mellitus. Clin Exp Hypertens. 2007;29:135–48. doi: 10.1080/10641960701361601. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JX, Gelb AW. Free radicals, antioxidants and neurologic injury: Possible relationship to cerebral protection by anaesthetics. J Neurosurg Anesthesiol. 2002;14:66–79. doi: 10.1097/00008506-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Lee BJ, Egi Y. Free radical scavengers in stroke. Brain Res. 2010;11:1307. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chronidoca F, Apostolakis E. Amifostine in spinal cord ischaemic phase. J Cardiothorac Surg. 2009;4:50. doi: 10.1186/1749-8090-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall ED, Braughler JM. Free radicals in CNS injury. Res Publ Assoc Res Nerv Ment Dis. 1993;347:781–6. [PubMed] [Google Scholar]
- 31.Hisano K, Watanabe M, Morimoto Y. Protective effects of the free radical scavenger edaravone against glutamate neurotoxicity in nearly pure neuronal culture. J Anesth. 2009;23:363–9. doi: 10.1007/s00540-009-0766-z. [DOI] [PubMed] [Google Scholar]
- 32.Lenfant F, Lahet JJ, Courderot-Masuyer C, Freysz M, Rochette L. Lidocaine has better antioxidant potential than ropivacaine and bupivacaine: In vitro comparison in a model of human erythrocytes submitted to an oxidative stress. Biomed Pharmacother. 2004;58:248–54. doi: 10.1016/j.biopha.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Yang T, Mao YF, Liu SQ. Edaravone-protective effect of free radical scavenger on acute pancreatitis associated lung injury. Eur J Pharmacol. 2010;25:152–7. doi: 10.1016/j.ejphar.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Karkema JM, Singh G, Wang P. Pharmacologic agents in the treatment of ischaemia, heamorrh- agic shock and sepsis. J Crit Care. 1992;7:189. [Google Scholar]


