Abstract
Background:
The maintenance of oxygenation is a commonly encountered problem in obese patients undergoing laparoscopic cholecystectomy. There is no specific guideline on the ventilation modes for this group of patients. Although several studies have been performed to determine the optimal ventilatory settings in these patients, the answer is yet to be found. The aim of this study was to evaluate the efficacy of pressure-controlled ventilation (PCV) in comparison with volume-controlled ventilation (VCV) for maintaining oxygenation during laparoscopic cholecystectomy in obese patients.
Methods:
One hundred and two adult patients of ASA physical status I and II, Body Mass Index of 30–40 kg/m2, scheduled for laparoscopic cholecystectomy were included in this prospective randomized open-label parallel group study. To start with, all patients received VCV. Fifteen minutes after creation of pneumoperitoneum, they were randomized to receive either VCV (Group V) or PCV (Group P). The ventilatory parameters were adjusted accordingly to maintain the end-tidal CO2 between 35 and 40 mmHg. Respiratory rate, tidal volume, minute ventilation and peak airway pressure were noted. Arterial blood gas analyses were done 15 min after creation of pneumoperitoneum and at 20-min intervals thereafter till the end of the surgery. All data were analysed statistically.
Results:
Patients in Group P showed a statistically significant (P < 0.05) higher level of PaO2 and lower value of PAO2–PaO2 than those in Group V.
Conclusion:
PCV is a more effective mode of ventilation in comparison with VCV regarding oxygenation in obese patients undergoing laparoscopic cholecystectomy.
Keywords: Laparoscopic cholecystectomy, obesity, pressure-controlled ventilation, volume-controlled ventilation
INTRODUCTION
Obesity is a well-established risk factor for cholelithiasis for which laparoscopic cholecystectomy is the routinely performed surgery.[1] Maintenance of oxygenation is one of the many problems in the anaesthetic management of obese patients.[2] Respiratory mechanics during laparoscopy are affected by obesity and pneumoperitoneum, but vary little with body position.[3] There is no guideline for selection of ventilatory modes in morbidly obese patients.[4] Developments in our understanding of pressure–volume curves and the recent demonstration of microscopic shear stress lung injury have changed the whole concept of safe ranges of pressure and volume in mechanical ventilation.[5]
The traditional approach of using large tidal volumes in volume-controlled ventilation (VCV) during laparoscopic surgery in obese patients causes cardiovascular embarrassment, rise in peak inspiratory pressure and plateau pressure without significant improvement in arterial oxygenation.[6] Moreover, high tidal volume causes excessive stretch of non-dependent lung regions and promotes alveolar rupture, leading to volutrauma.[7]
On the contrary, the decelerating inspiratory flow used during pressure-controlled ventilation (PCV) generates high initial flow rate, causing more rapid alveolar inflation. This mechanical effect of PCV allows a homogeneous distribution of ventilation leading to better ventilation–perfusion matching. At the same time, pressure limits and uniform distribution of forces within the lung reduce the risk of volu- and barotraumas.[5]
A previous cross-over study in non-obese patients undergoing laparoscopic urological procedures did not find any significant short-term benefit of PCV over VCV with respect to pulmonary mechanics, gas exchange and cardiac functions.[8] Another study comparing PCV with VCV during laparoscopic gastric banding surgery in obese patients has revealed that PCV improves oxygenation without any side-effects.[9]
Considering the results of the previous studies, we designed this study to evaluate and compare the efficacy of VCV and PCV in maintaining adequate oxygenation in obese patients undergoing laparoscopic cholecystectomy, although the null hypothesis states that PCV is equally efficacious compared with VCV in maintaining oxygenation in obese patients undergoing laparoscopic cholecystectomy.
METHODS
After obtaining approval of the Institutional Ethics Committee and written informed consent from the patients, this prospective, randomized, open-label, parallel group study was undertaken on 102 patients in the surgical OT of IPGME and R, Kolkata. The inclusion criteria were patients of either sex, aged between 18 and 39 years, of ASA physical status I and II, with Body Mass Index between 30 and40 kg/m2, no major obstructive or restrictive pulmonary disease (defined as <70% of predicted value for pulmonary function test variables of volume and flow), PaCO2 between 35 and 45 mmHg and scheduled for laparoscopic cholecystectomy. Exclusion criteria were patient refusal, anticipated difficult intubation, inability to maintain stable ventilator settings for 30 min, patients with a history of obstructive or restrictive type of lung diseases, known hypersensitivity to any drugs used in this study, ASA physical status III and IV and conversion to laparotomy.
Sample size calculation
A pilot study involving 20 patients (10 patients in each group) was performed to determine the difference of mean PaO2 between groups and the within-group standard deviation. The difference of means was found to be 30 and within-group standard deviation as 60. From this data and assuming a probability of Type I error as 5%, it was calculated that a minimum of 73 patients would be required for the study to be able to reject the null hypothesis with a power of 85%. A total of 1252 patients were needed initially to get 102 patients (51 patients in each group) incorporated into the study.
Following complete pre-anaesthetic evaluation, a peripheral venous access and an arterial access were secured and a baseline arterial blood sample was drawn for arterial blood gas (ABG) analysis. Routine continuous monitoring including pulse rate, blood pressure (BP), oxygen saturation (SpO2), respiratory rate, capnometry and electrocardiogram were done throughout the procedure. The pre-operative readings as well as the readings in intra-operative period were recorded at an interval of 15 min and considered for statistical analysis. All members of the team provided care to all the patients. A standard protocol for general endotracheal anaesthesia with controlled ventilation was conducted in all patients.
All patients received oral alprazolam 0.5 mg the night before the surgery; intravenous inj ranitidine 50 mg and inj metoclopramide 10 mg 2 h pre-operatively, inj glycopyrrolate 0.2 mg intravenously 30 min pre-operatively and inj fentanyl 2 mcg/kg 5 min before induction. After proper pre-oxygenation, patients were induced with inj propofol 2 mg/kg. The trachea was intubated with a PVC-cuffed endotracheal tube of appropriate size after achieving adequate relaxation with inj suxamethonium 1 mg/kg. The lungs were ventilated with 50% nitrous oxide, 50% oxygen and isoflurane 0.9–1%. Muscle relaxation was maintained with inj vecuronium bromide 0.08 mg/kg followed by additional top-up doses of 0.02 mg/kg, maintaining muscle relaxation at <2 twitches (using a train-of-four sequence) of adductor pollicis muscle measured every 15 min. Bispectral index was used to monitor the level of anaesthesia (BIS maintained between 40 and 60). Inj tramadol 2 mg/kg was administered for additional perioperative analgesia.
To start with, all patient's lungs were ventilated (Penlon Prima SP, AV 800 ventilator. Penlon Limited. Abingdon Science Park.Barton Lane. Abingdon OX14 3PH. United Kingdom.) using VCV with a tidal volume of 8 mL/kg and inspiratory/expiratory ratio of 1:2. Respiratory rate was adjusted to obtain an end-tidal CO2 (EtCO2) of 35–40 mmHg. After pneumoperitoneum (intra-abdominal pressure 10–12 mmHg), patients were placed in a 25° head-up position. Fifteen minutes after pneumoperitoneum, patients were randomized with the help of a computer-generated random number list to receive either VCV (Group V; n=51) or PCV (Group P; n=51).
In Group V, ventilation was continued with a tidal volume of 8 mL/kg. The initial tidal volume was increased by 1 mL/kg every 5 min until 12 mL/kg, and the respiratory rate was increased by 2/min every 5 min till 20/min to maintain EtCO2 between 35 and 40 mmHg. Patients in Group V were shifted to laparotomy when EtCO2 could not be maintained with tidal volume of 12 mL/kg and respiratory rate of 20/min. Following fall in EtCO2 <35 mmHg, the respiratory rate was decreased by 2/min every 5 min till 8/min, with a decrease in tidal volume of 1 mL/kg until 6 mL/kg.
In Group P, the airway pressure not exceeding 35 cmH2O was set to provide a tidal volume of 8 mL/kg. Respiratory rate was adjusted to keep an EtCO2 of 35–40 mmHg. Following an increase in EtCO2, the respiratory rate was increased by 2/min every 5 min till 20/min, achieving the target EtCO2. Following a fall in EtCO2 <35 mmHg, the respiratory rate was decreased by 2/min every 5 min till 8/min, with a decrease in airway pressure by 2 mmHg every 5 min until 10 cmH2O. Patients in PCV requiring airway pressure >35 cmH2O and respiratory rate >20/min to maintain normocarbia were shifted to VCV and were dropped from the study.
Arterial blood samples were drawn 15 min after establishment of pneumoperitoneum and at 20-min intervals thereafter till the end of the surgery. Patients requiring conversion from laparoscopy to laparotomy were excluded from the study. Laparotomy was advised when the patient was unable to maintain SpO2 >95% and EtCO2 <45 mmHg.
Neuromuscular blockade was reversed if the train-of-four ratio was ≥2 twitches with inj neostigmine 0.05 mg/kg and inj glycopyrrolate 0.01 mg/kg. The patient was extubated after fulfilling the criteria of adequate reversal.
Statistical analysis
All data were entered into an excel spread sheet and analysed using standard statistical software like SPSS and Statistica. All normally distributed numerical data were analysed using the Unpaired t test and all categorical data were analysed using the Pearson's Chi-square test. A P<0.05 was considered statistically significant. For intra-group analysis, a repeated measure ANOVA was performed to estimate the changes between any two time points within a group.
RESULTS
The study included 102 patients randomized into two groups of 51 patients according to the ventilation mode (Group V=VCV, Group P=PCV) [Figure 1].
Figure 1.
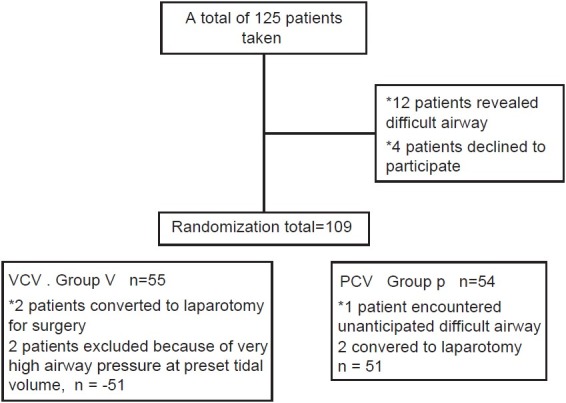
Randomization and sample selection process
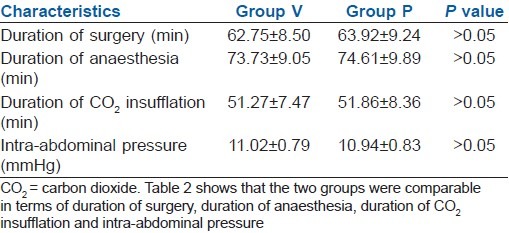
The two groups were comparable in terms of demographic profile [Table 1], duration of surgery, duration of anaesthesia, duration of CO2 insufflation and intra-abdominal pressure [Table 2].
Table 1.
Demographic profile (mean±SD)
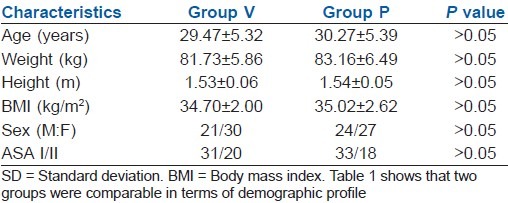
Table 2.
Duration of surgery, anaesthesia, CO2 insufflation time and peak airway pressure (mean±SD)
Haemodynamic variables were similar in both groups [Table 3].
Table 3.
Haemodynamic variables (mean±SD)
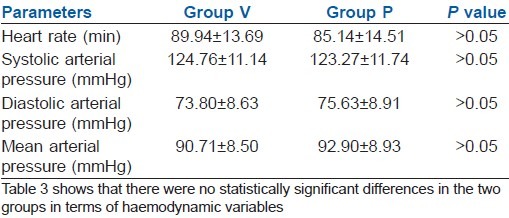
Table 4 showed that 35 min after establishment of pneumoperitoneum, statistically significant (P>0.05) higher respiratory rate and tidal volumes were required in Group V compared with Group P. Fifteen minutes and 35 min after establishment of pneumoperitoneum, statistically significant (P>0.05) higher minute ventilation was required and statistically significant (P>0.05) higher peak airway pressures were generated in Group V than in Group P.
Table 4.
Ventilatory parameters (mean±SD)
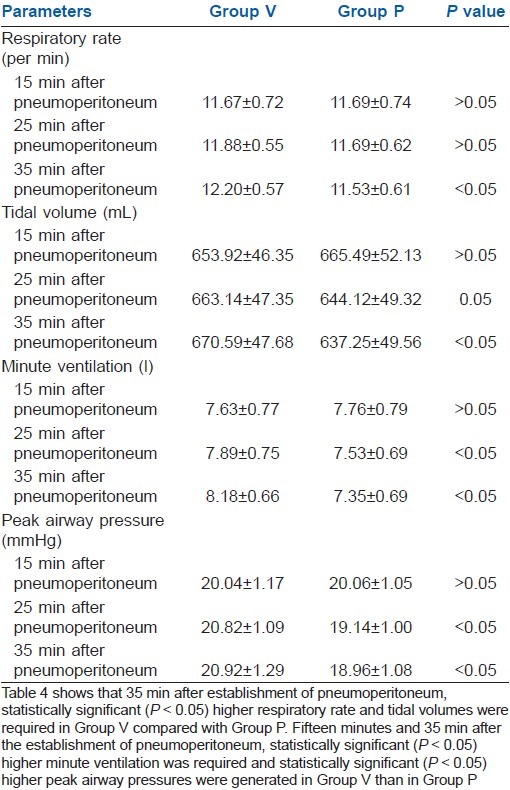
Table 5 showed that SpO2 and EtCO2 were comparable in both groups.
Table 5.
SpO2 and EtCO2 (mean±SD)
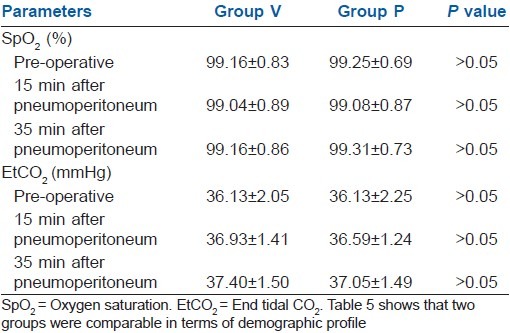
Table 6 showed no statistically significant difference in pH, PaCO2 and PaCO2–EtCO2 values in the two groups. Fifteen minutes after pneumoperitoneum, PaO2 and PAO2–PaO2 were comparable in both the groups. But, 35 min after pneumoperitoneum, the patients in Group P showed a statistically significant (P>0.05) higher value of PaO2 and lower value of PAO2–PaO2 in comparison with the patients in Group V.
Table 6.
Arterial blood gas analyses (mean±SD)
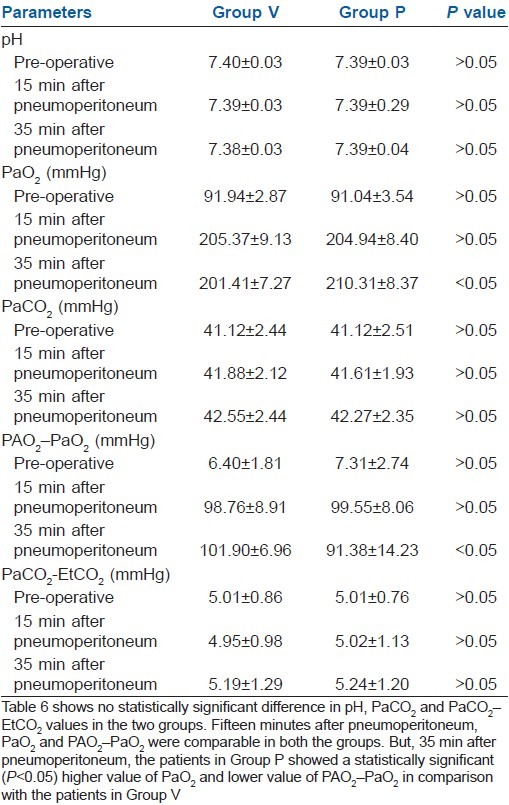
Figure 2 showed that 35 min after establishment of pneumoperitoneum, there was a significant rise in PaO2 in Group P than that in Group V.
Figure 2.
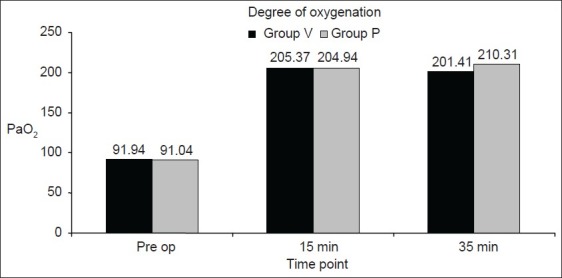
Degree of oxygenation in the two groups of patients pre-operatively and 15 and 35 min after the establishment of pneumoperitoneum. It showed that 35 min after the establishment ofpneumoperitoneum, there was a significant rise in PaO2 in Group P than that in Group V
No patient required conversion to laparotomy. No patient was shifted to VCV from PCV.
DISCUSSION
Reduced pulmonary compliance in obese individuals leads to decreased functional residual capacity (FRC), vital capacity and total lung capacity. Reduced FRC can result in lung volumes below closing capacity in the course of normal tidal ventilation, leading to small airway closure, ventilation–perfusion mismatch, right-to-left shunting and, ultimately, arterial hypoxaemia. Anaesthesia worsens this situation such that up to 50% reduction in FRC occurs in obese anaesthetized patients compared with 20% in the non-obese.[10] Moreover, pneumoperitoneum causes 30% lower static compliance and 68% higher inspiratory resistance in supine anaesthetized obese patients compared with those of normal weight patients.[3] During laparoscopy, diminished FRC, ventilation–perfusion mismatch and pulmonary shunting contribute to a decrease in arterial oxygenation, which is further exaggerated in the obese patients.[11]
Ventilation–perfusion inequalities in obese patients during laparoscopy may require about 15–25% increase in minute ventilation to maintain normocarbia.[12] But, the increase in tidal volume or respiratory rate does not improve arterial oxygenation.[2]
Although several studies have been performed to determine the optimal ventilatory settings in these patients, the answer is yet to be found.[13] The primary goal of mechanical ventilation is the maintenance of adequate gas exchange, which must be achieved with minimum lung injury and the lowest possible degree of haemodynamic impairment.[14]
The large tidal volumes used in VCV to maintain normocarbia during laparoscopic surgery in obese patients may lead to certain deleterious effects. High inflation volumes that intend to aggressively recruit atelectatic lung units overdistend the normal alveoli, leading to alveolar rupture and volutrauma. It can also exert an inflammatory lung injury similar to adult respiratory distress syndrome. The inflammatory mediators in the lung can also be released into systemic circulation, resulting in inflammatory injury to the distant organs.[7]
Monitoring EtCO2 is an adequate guide for determining the minute ventilation required to maintain normocarbia,[12] and it provides a reasonable approximation of PaCO2 in healthy patients undergoing laparoscopic cholecystectomy.[15] Therefore, in our study, we maintained EtCO2 between 35 and 40 mmHg in both the groups for comparison of oxygenation between the two groups. After pneumoperitoneum, it takes about 15 min for PaCO2 to reach a plateau.[12] Thus, in this study, arterial blood samples were taken for analysis 15 min after the establishment of pneumoperitoneum.
In our study, 35 min after the creation of pneumoperitoneum, significantly lower respiratory rate was required to maintain normocarbia in Group P compared with Group V. Although the difference in respiratory rate was statistically significant, it was not clinically relevant (12.20±0.57 in Group V vs. 11.53±0.61 in Group P).
Statistically significant higher tidal volume and minute ventilation were required for maintenance of normocarbia during VCV compared with PCV. It can be explained by the fact that large tidal volume in VCV mainly ventilates the non-dependent portion of the lung, leading to excessive stretching of those regions without improving the overall ventilation.[7] On the contrary, in PCV, recruitment of collapsed alveoli due to high flow rate in the early inspiratory phase leads to improved lung ventilation.[5] Although the delivery of tidal volume and minute ventilation were lower in PCV, adequate CO2 elimination was achieved due to overall improvement in lung ventilation. Thus, the adverse consequences of large tidal volume delivery like rise in peak pressure, plateau pressure, volutrauma and inflammatory lung injury could be avoided in PCV.
Balick-Weber et al.[8] noted no significant difference in arterial oxygenation in patients receiving PCV compared with the patients receiving VCV. But, in our study, 35 min after establishment of pneumoperitoneum, a significantly higher PaO2 value was found in Group P than that in Group V. Cadi et al.[9] observed a similar finding in their study. The improved oxygenation in PCV may be explained by the delivery of “square wave”[5] of pressure to the patient's airway. Pressure develops rapidly due to the very high flow at the initiation of inspiration followed by rapid flow deceleration. By delivering a larger proportion of tidal volume early in the inspiratory phase, the lung is maintained at a higher volume recruiting more alveoli to participate in gas exchange.[5] PCV is also associated with increased mean airway pressure, a ventilatory parameter found to correlate with oxygenation status.[5]
In our study, the patients receiving PCV (Group P) showed a significantly lower value of alveolar–arterial oxygen gradient (PAO2–PaO2) compared with the patients receiving VCV (Group V). This observation is consistent with the result of the study conducted by Cadi et al.[9] This can be explained by the decelerating inspiratory flow profile in PCV that enhances the distribution of ventilation among alveolar lung units improving gas exchange.[5] Moreover, in VCV, higher intra-thoracic pressure decreases the venous inflow into the thorax, leading to a fall in preload and the compression of pulmonary blood vessels raises pulmonary vascular resistance, which can impede right ventricular stroke output.[7] Fall in right ventricular stroke output causes ventilation–perfusion mismatch in the lung, resulting in hypoxaemia. It can be supported by the previous study[16] that found lower pulmonary vascular resistance and higher cardiac index in patients receiving PCV in comparison with the patients receiving VCV.
Balick-Weber et al.[8] found lower peak airway pressures during PCV than during VCV in patients undergoing laparoscopic urological procedures. In this study also the peak airway pressures were significantly lower in Group P than that in Group V. It can be explained by the decelerating flow pattern and the earlier dissipation of flow resistance in PCV.[8] Thus, in PCV, the peak pressure is limited, reducing the chance of barotraumas. Conversely, the higher tidal volumes and increased peak airway pressures in VCV cause alveolar over distension, leading to lung injury.[14]
The use of positive end-expiratory pressure (PEEP) to increase FRC and to improve oxygenation in obese patients is questionable. Although the use of PEEP is of proven value for improving oxygenation in situations involving respiratory failure, its role in anaesthetized patients is controversial.[13] It has been suggested that PEEP may fail to improve oxygenation because increased alveolar pressure increases the shunt fraction.[2]
CONCLUSION
In conclusion, it can be stated that PCV is a better choice than VCV in obese patients undergoing laparoscopic cholecystectomy because of its dual advantages. On one hand, it improves arterial oxygenation and on the other hand, it can limit the chance of lung injury.
The limitations of this study were lack of direct measure of FRC, lung compliance and plateau pressure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Neugebauer E, Troidl H, Kum CK, Eypasch E, Miserez M, Paul A. The E.A.E.S. Consensus development conferences on laparoscopic cholecystectomy, appendicectomy and hernia repair. Consensus statements. September 1994. Surg Endosc. 1995;9:550–63. doi: 10.1007/BF00206852. [DOI] [PubMed] [Google Scholar]
- 2.Sprung J, Whalley DG, Falcone T, Wilks W, Navratil JE, Bourke DL. The effects of tidal volume and respiratory rate on oxygenation and respiratory mechanics during laparoscopy in morbidly obese patients. Anesth Analg. 2003;97:268–74. doi: 10.1213/01.ane.0000067409.33495.1f. [DOI] [PubMed] [Google Scholar]
- 3.Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, Hammel J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg. 2002;94:1345–50. doi: 10.1097/00000539-200205000-00056. [DOI] [PubMed] [Google Scholar]
- 4.Baerdemaeker LD, Herten CV, Gillardin JM, Pattyn P, Mortier EP, Szegedi LL. Comparison of volume-controlled and pressure-controlled ventilation during laparoscopic gastric banding in morbidly obese patients. Obes Surg. 2008;18:680–5. doi: 10.1007/s11695-007-9376-8. [DOI] [PubMed] [Google Scholar]
- 5.Nichols D, Haranath S. Pressure control ventilation. Crit Care Clin. 2007;23:183–99. doi: 10.1016/j.ccc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Bardoczky GI, Yernault JC, Houben JJ, Hollander AA. Large tidal volume ventilation does not improve oxygenation in morbidly obese patients during anesthesia. Anesth Analg. 1995;81:385. doi: 10.1097/00000539-199508000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Marino PL, Sutin KM, editors. Princiles of Mechanical Ventilation, The ICU Book. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2007. pp. 457–71. [Google Scholar]
- 8.Balick-Weber CC, Nicolas P, Hedreville-Montout M, Blanchet P, Stephan F. Respiratory and haemodynamic effects of volume-controlled vs pressure-controlled ventilation during laparoscopy: A cross-over study with echocardiographic assessment. Br J Anaesth. 2007;99:429–35. doi: 10.1093/bja/aem166. [DOI] [PubMed] [Google Scholar]
- 9.Cadi P, Guenoun T, Journois D, Chevallier JM, Diehl JL, Safran D. Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. Br J Anaesth. 2008;100:709–16. doi: 10.1093/bja/aen067. [DOI] [PubMed] [Google Scholar]
- 10.Ogunnaike BO, Whitten CW. In: Anesthesia and obesity, Clinical Anesthesia. 5th ed. Barash PG, Cullen BF, Stoelting RK, editors. Philadelphia: Lippincott Williams and Wilkins; 2006. pp. 1040–52. [Google Scholar]
- 11.Morgan GE, Mikhail MS, Murray MJ, editors. Anesthesia for Patients with Respiratory Disease, Clinical Anesthesiology. 4th ed. New York: Lange Medical books, McGraw-Hill Companies; 2006. pp. 571–84. [Google Scholar]
- 12.Joris JL. In: Anesthesia for Laparoscopic Surgery, Miller's Anesthesia. 6th ed. Miller RD, editor. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 2285–306. [Google Scholar]
- 13.Perilli V, Sollazzi L, Bozza P, Modesti C, Chierichini A, Tacchino RM, et al. The effects of the reverse Trendelenberg position on respiratory mechanics and blood gases in morbidly obese patients during bariatric surgery. Anesth Analg. 2000;91:1520–5. doi: 10.1097/00000539-200012000-00041. [DOI] [PubMed] [Google Scholar]
- 14.Koh SO. Mode of mechanical ventilation: Volume controlled mode. Crit Care Clin. 2007;23:161–7. doi: 10.1016/j.ccc.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham AJ, Nolan C. In: Anesthesia for Minimally Invasive Procedures, Clinical Anesthesia. 5th ed. Barash PG, Cullen BF, Stoelting RK, editors. Philadelphia: Lippincott Williams and Wilkins; 2006. pp. 1061–71. [Google Scholar]
- 16.Auler JO, Junior, Carmona MJ, Silva MH, Silva AM, do Amaral RV. Haemodynamic effects of pressure-controlled ventilation versus volume-controlled ventilation in patients submitted to cardiac surgery. Clin Intensive Care. 1995;6:100–6. doi: 10.3109/tcic.6.3.100.106. [DOI] [PubMed] [Google Scholar]


