Abstract
Objective:
The epigenetic basis for human asthma is not well studied, particularly among older adults. This study investigated the methylation profiles in sputum DNA among older adults with asthma, using a population of smokers.
Methods:
This was a cross-sectional study using the Lovelace Smokers Cohort, a population of former and current smokers aged ≥ 40 years in New Mexico. One hundred eighty-four smokers with asthma were compared with 511 smoker control subjects with a similar smoking history, after carefully excluding those with COPD. Environmental exposures were assessed by a standard questionnaire. Postbronchodilator spirometry was performed. Induced sputum was analyzed for the methylation prevalence of 12 selected asthma-related genes using nested methylation-specific polymerase chain reaction assay.
Results:
Asthma was associated with a greater number of methylated genes and, specifically, with methylated protocadherin-20 gene in sputum DNA compared with control subjects with a similar smoking history. These associations remained significant after adjustment for covariates as well as Bonferroni correction. A synergistic interaction was noted between two methylated genes (protocadherin-20 and paired box protein transcription factor-5α) in sputum DNA on the odds for asthma (P = .009). Interestingly, the epigenetic-asthma associations were not explained by the environmental factors studied. Further, methylated genes in sputum DNA, including the protocadherin-20 gene, identified a symptomatically more severe asthma phenotype in a subgroup analysis.
Conclusions:
Asthma is associated with methylation of selected genes, such as protocadherin-20 gene, in sputum DNA. If future studies establish causality, novel demethylating interventions to prevent and treat asthma among older smokers may be possible.
Asthma, an obstructive inflammatory airway disease affecting 39.9 million people in the United States,1 may have an epigenetic basis.2 Epigenetics is the study of changes in gene function through heritable changes in chromatin structure without a change in DNA sequence. Epigenetic changes (involving promoter methylation or demethylation of cytosines at cytosine-phosphate-guanine [CpG] sites in genes) regulate diverse cellular functions, including inflammation.3 Epigenetic changes are also potentially modifiable (unlike genetic mutations) and provide an exciting possibility of novel interventions to prevent and treat asthma. Epigenetic patterns in sputum cells that include leukocytes and epithelial cells from the aerodigestive tract, as opposed to those in whole blood cells, may be more relevant to studies of airway diseases. We have developed a sensitive and specific approach, the nested methylation-specific polymerase chain reaction (PCR) assay, that allows interrogation for the presence of methylated alleles of genes selectively silenced in sputum cells.4,5
Most asthma studies involve children and young adults and exclude older adults. Adult-onset asthma currently forms the majority of asthma cases seen at and after middle age, particularly among women.6 Unlike pediatric-onset asthma, adult-onset asthma is more strongly associated with female sex, obesity, nonatopic state, and smoking.6 Although cigarette smoking is a known risk factor for adult-onset asthma, most asthma studies carefully exclude smokers for fear of overlap with COPD, another obstructive lung disease.6‐11 Thus, asthma in older adults, particularly smokers, remains a common but woefully understudied disease. Our objective was to identify profiles of methylated genes in sputum DNA of subjects with asthma, using a population of older adults with past or current history of smoking but without COPD. If future studies establish causality, novel epigenetic interventions to prevent and treat asthma in older adults may be possible.
Materials and Methods
Study Population
In this cross-sectional study, subjects were drawn from 1,861 eligible participants enrolled between 2001 and 2007 in the Lovelace Smokers Cohort, a well-characterized cohort in New Mexico.12,13 This large cohort disproportionately enrolled women who had ever smoked to study the susceptibility to the development of obstructive lung disease, since women are underrepresented in most studies of airflow obstruction in the United States.14 The catchment area was Albuquerque, New Mexico, and its surrounding communities, comprising a diverse population of approximately 700,000 persons living at altitudes of approximately 1,500 m above sea level. Participants were recruited from the community through newspaper or television advertisements and were paid a small stipend for their participation. This study was approved by the Western Institutional Review Board (Olympia, Washington; WIRB Protocol #20031684).
Inclusion Criteria
Subjects were included if they were 40 to 75 years of age, smokers (former or current) with ≥ 10 pack-years of smoking history, and able to understand English. In addition, subjects were required to undergo spirometric testing and sputum induction in the same time frame as obtaining the medical history. Of the 1,861 subjects enrolled in the original cohort, we included 184 smokers with asthma and 511 smoker control subjects in this study.
Asthma was defined by a self-reported provider diagnosis of ever having asthma and/or spirometric reversibility. Subjects with asthma who also met the definition of COPD (see later in this section) were excluded. Reversibility was defined by an increase in absolute value by ≥ 12% and 200 mL compared with baseline in FEV1 and/or FVC during a single testing session with bronchodilator administration.15 COPD was defined by a self-reported provider diagnosis of having COPD and/or presence of irreversible spirometric airflow obstruction (ie, lack of reversibility in a setting of postbronchodilator FEV1/FVC value of ≤ 70%). The control subjects were smokers, both current and former, as defined by the absence of all of the following criteria: (1) any self-reported lung disease, (2) spirometric airflow obstruction (defined by a prebronchodilator FEV1/FVC ratio above both the Hankinson-defined lower limit of normal15 and GOLD [Global Initiative for Chronic Obstructive Lung Disease]-defined value of 70%16), and (3) bronchodilator reversibility (as defined here).
Exclusion Criteria
We carefully excluded those with COPD (168), asthma-COPD overlap state (161), chronic bronchitis and other lung diseases (119), missing data (35), minimal smoking history (108), and test samples of inadequate quality (572). For this purpose, asthma-COPD overlap state was defined by those subjects who met the definitions described here of both asthma and COPD. Chronic bronchitis was defined by a self-report of a persistent cough and phlegm production for at least 3 months per year in 2 consecutive years. “Other lung diseases” were defined by self-report of provider-diagnosed lung disease that did not meet the discrete definitions described here of asthma, COPD, chronic bronchitis, and asthma-COPD overlap state.
Study Measurements
Outcome measures and assessment of exposure variables were obtained at the same visit for all participants. All tests were conducted at Lovelace Scientific Resources, Albuquerque, New Mexico. Information related to demographics, respiratory diseases, quality of life, and smoking was obtained by self-report from all study participants via a questionnaire, based on the validated American Thoracic Society (ATS) 1978 Adult Questionnaire and the St. George Respiratory Questionnaire (SGRQ).17,18 BMI was measured using standardized methods.19
Prebronchodilator and postbronchodilator spirometry were obtained on all subjects by registered respiratory therapists, strictly adhering to the 1994 ATS guidelines.20 After completion of prebronchodilator spirometry, all subjects were given two puffs of albuterol (90 μg/spray metered dose inhaler) with a LiteAire dual valve spacer (Thayer Medical Corporation), and spirometry was repeated after 15 min.20 Participants were requested not to take any inhalers for 4 h prior to their appointment. Vmax Encore 22 (Viasys Respiratory Care) and KoKo (Ferraris Respiratory) spirometers were used. Both machines met the 1994 ATS recommendations and were calibrated daily and checked at three different injection speeds, as per the ATS guidelines.20 Additionally, respiratory therapists were monitored and periodically recredentialed as part of a standardized laboratory proficiency testing plan. Only spirometric tests that met the ATS criteria were included in the analyses.
Induced sputum was collected and stored in Saccomanno fixative. Three slides were made for each sputum sample to check for adequacy, as defined by the presence of lung macrophages or Curschmann spirals.21 Adequate sputum samples from each study subject were taken for DNA isolation by protease digestion followed by phenol chloroform extraction and ethanol precipitation. Nested methylation-specific PCR assays were used to detect methylated alleles in DNA recovered from the sputum samples, as previously described.5 We studied the promoter methylation of a panel of 12 genes selected due to their known involvement with oxidative damage, DNA repair pathways, and cell fate determination of epithelial or hematopoietic cells that are closely associated with chronic inflammatory conditions, as found in patients with asthma.22‐24 These genes included PCDH20 (protocadherin 20 gene); SULF2 (extracellular sulfatase 2 gene); GATA4 and GATA5 (GATA binding protein-4 and -5 transcription factor genes); PAX5α and PAX5β (paired box protein 5 transcription factor genes); p16; MGMT (O6-methylguanine-DNA methyltransferase gene); RASSF1α (Ras association domain family member 1α gene), DAPK (death-associated protein kinase gene); DAL1 (differentially expressed in adenocarcinoma of the lung 1 gene); and JPH3 (junctophilin 3 gene). The nested methylation-specific PCR assay, as published by Belinsky et al,4,25 designs stage II methylation-specific primer pairs for each gene to include three to five CpGs in each primer, thus, six to 10 CpGs for each gene. Stringent annealing temperatures are used in the PCR reaction such that amplification of the allele only occurs if the majority of the CpGs under the primer are methylated. The specificity of this approach has been confirmed by the bisulfite sequencing of the PCR products, and those results show that the CpG sites between the primers that comprise the amplified sequence are indeed methylated.4,25 The methylation-specific PCR assays were thus optimized to be highly specific for the region amplified. As an additional quality control measure, a subset of sputum samples that gave positive methylation products were reanalyzed with 100% validation by a second method using restriction enzyme digestion that can discriminate methylation status of CpGs within the resulting PCR product.4,25
Statistical Analysis
Summary statistics, including means, SDs, medians, and interquartile ranges for continuous variables and proportions for categorical variables, were obtained. The χ2 test was used for analysis of categorical variables and the t test for continuous variables. Multivariable binary logistic regression models were used for binary outcomes. Covariates considered in the adjusted models included sex, age, heavy smoking, current smoking, obesity, educational attainment (high school or better), wood smoke exposure, and Hispanic ethnicity. Heavy smoking was defined as ≥ 40 pack-years (based on the observed mean cut point of 39.4 pack-years in the cohort). All analyses were conducted in SAS 9.2. A two-sided P value of < .05 was generally considered statistically significant. However, to adjust for multiple comparisons involving individual genes, a Bonferroni correction for the 12 genes was applied using a P value of < .0042 to determine statistical significance.
Results
Smokers with asthma were more likely to have higher SGRQ scores (ie, greater respiratory symptoms, activity difficulties, and impact on daily life); greater prevalence of postbronchodilator spirometric obstruction26; and lower absolute postbronchodilator FEV1/FVC ratio, as compared with smoker control subjects (Table 1). In addition, smokers with asthma were more likely to be obese than smoker control subjects, in both unadjusted and adjusted analyses (Tables 1, 2, respectively). Of particular note, the asthma and control groups were not different with respect to their smoking history.
Table 1.
—Unadjusted Characteristics of Smokers With Asthma Compared With Smoker Control Subjects
| Characteristic | Asthma (n = 184) | Smoker Control Subjects (n = 511) | P Value |
| Male sex | 26.6 | 23.7 | .43 |
| Age, y | 53.3 ± 9.2 | 54.5 ± 9.1 | .15 |
| Hispanic ethnicity | 23.4 | 22.3 | .77 |
| ≥ High school education | 69.6 | 69.1 | .90 |
| Obese | 41.3 | 29.4 | .003 |
| BMI, kg/m2 | 29.6 ± 6.4 | 28.3 ± 5.9 | .01 |
| Pack-y of cigarettes | 33.6 ± 16.6 | 35.0 ± 17.2 | .30 |
| Current cigarette smoke exposure | 60.3 | 59.9 | .92 |
| Wood smoke exposure | 27.2 | 22.3 | .18 |
| SGRQ total score | 21.2 ± 16.5 | 16.8 ± 14.3 | .001 |
| SGRQ symptom score | 31.1 ± 21.5 | 25.7 ± 20.6 | .002 |
| SGRQ impact score | 11.6 ± 13.6 | 7.9 ± 10.6 | < .001 |
| SGRQ activity score | 30.0 ± 23.8 | 26.1 ± 22.3 | .046 |
| FEV1/FVC < LLN per NHANES III | 1.1 | 0.0 | .02 |
| Absolute FEV1/FVC ratio | 75.3 ± 5.5 | 77.0 ± 5.1 | < .001 |
| % predicted FEV1, NHANES III | 93.9 ± 13.6 | 94.3 ± 13.2 | .74 |
| Rapid % FEV1 decliners | 32.4 | 28.1 | .33 |
Data are presented as % or mean ± SD. Spirometric tests were obtained postbronchodilator; obese status was defined by BMI ≥ 30 kg/m2; LLN uses the NHANES III reference standards.26 Rapid decliners were defined by the highest tertile of percent annual decline in FEV1 obtained from the difference between the baseline and final follow-up visits among 142 and 413 subjects with asthma and control subjects, respectively. LLN = lower limit of normal; NHANES = Third National Health and Nutrition Examination Survey; SGRQ = St. George Respiratory Questionnaire.
Table 2.
—Multivariable Analysis of the Association Between Selected Environmental Characteristics and Asthma Compared With Smoker Control Subjects
| Asthma (n = 184) |
||
| Exposure Variable | OR (95% CI) | P Value |
| Male sex | 1.18 (0.80-1.76) | .41 |
| Age, y | 0.99 (0.97-1.01) | .27 |
| Hispanic ethnicity | 0.98 (0.64-1.52) | .94 |
| ≥ High school education | 1.04 (0.70-1.53) | .86 |
| Obese (BMI ≥ 30 kg/m2) | 1.71 (1.20-2.44) | .003a |
| Current cigarette smoke exposure | 0.97 (0.67-1.42) | .89 |
| Heavy cigarette smoke exposure | 0.86 (0.55-1.33) | .49 |
| Wood smoke exposure | 1.22 (0.82-1.82) | .32 |
Heavy cigarette smoke exposure is defined by pack-y of cigarettes ≥ 40; for age, the OR represents a 7% increase in the odds of COPD per 1 year of age. Of note, the obesity association remained significant even after additional simultaneous adjustment for the methylation status of the four genes PCDH20 (protocadherin 20 gene), SULF2 (extracellular sulfatase 2 gene), GATA4 (GATA binding protein-4 transcription factor gene), and PAX5α (paired box 5 protein transcription factor gene).
Represents significant associations.
As compared with smoker control subjects, sputum DNA from smokers with asthma showed a greater mean number of methylated genes as well as a greater prevalence for at least four (of 12) genes methylated (Table 3). Examination of individual genes showed that the prevalence for methylation of PCDH20 was associated with asthma (Table 3, unadjusted analyses). The former association remained significant even after Bonferroni correction for multiple comparisons for 12 genes. In multivariable analyses, the association of asthma with either methylation of ≥ 4 genes or of individual PCDH20 remained significant and unchanged even after adjustment for environmental factors as covariates (Table 4).
Table 3.
—Unadjusted Prevalence for Gene Methylation in Sputum DNA Among Smokers With Asthma Compared With Smoker Control Subjects
| Gene Methylation | Asthma (n = 184) | Smoker Control Subjects (n = 511) | P Value |
| Total number of methylated genes | 2.6 ± 2.2 | 2.2 ± 2.0 | .02 |
| Methylation index ≥ 4 genes | 31.5 | 23.1 | .02 |
| PCDH20 methylation | 46.2 | 34.1 | .0035 |
| SULF2 methylation | 38.0 | 29.9 | .04 |
| GATA4 methylation | 41.8 | 34.6 | .08 |
| PAX5α methylation | 13.6 | 12.1 | .61 |
| p16 methylation | 14.7 | 16.4 | .58 |
| MGMT methylation | 27.2 | 24.1 | .40 |
| RASSF1α methylation | 1.6 | 0.2 | .03 |
| DAPK methylation | 19.0 | 15.1 | .21 |
| GATA5 methylation | 19.0 | 17.2 | .58 |
| PAX5β methylation | 8.7 | 7.4 | .58 |
| DAL1 methylation | 9.2 | 7.8 | .56 |
| JPH3 methylation | 25.0 | 24.5 | .88 |
Data are presented as prevalence (%) or mean ± SD. Bonferroni correction for multiple comparisons involving 12 genes requires a P value < .0042 for analysis of individual genes. DAL1 = differentially expressed in adenocarcinoma of the lung 1 gene; DAPK = death-associated protein kinase gene; GATA5 = GATA binding protein-5 transcription factor gene; JPH3 = junctophilin 3 gene; MGMT = O6-methylguanine-DNA methyltransferase gene; PAX5β = paired box 5 protein transcription factor gene; RASSF1α = Ras association domain family member 1α gene. See Table 2 legend for expansion of other abbreviations.
Table 4.
—Multivariable Analysis of the Association Between Gene Methylation in Sputum DNA and Asthma Compared With Smoker Control Subjects
| Asthma (n = 184) |
||||
| Unadjusted |
Adjusted |
|||
| Exposure Variable | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Methylation index ≥ 4 genes | 1.53 (1.06-2.23) | .03 | 1.56 (1.06-2.28) | .02 |
| PCDH20 methylation | 1.66 (1.18-2.34) | .0035 | 1.71 (1.20-2.44) | .003 |
| SULF2 methylation | 1.44 (1.01-2.04) | .04 | 1.46 (1.01-2.09) | .04 |
| GATA4 methylation | 1.36 (0.96-1.92) | .08 | 1.34 (0.94-1.91) | .10 |
| PAX5α methylation | 1.14 (0.69-1.87) | .61 | 1.14 (0.68-1.90) | .62 |
The variables adjusted included sex, age, heavy smoking, current smoking, obesity, educational attainment (high school or better), wood smoke exposure, and Hispanic ethnicity. Four of a panel of 12 genes are depicted in this table. Bonferroni correction for multiple comparisons involving 12 genes requires a P value < .0042 for analysis of individual genes. See Table 2 legend for expansion of abbreviations.
Smokers with asthma with methylated PCDH20 were indistinguishable from those without methylation in terms of their lung function but showed significantly greater concomitant methylation of most other genes in their sputum, particularly SULF2 (e-Table 1 (379.3KB, pdf) ). Further, subjects with “symptomatically most severe” asthma when compared with subjects with “least severe” asthma (ie, those in the highest vs lowest tertiles of SGRQ symptom subscale scores) were more likely to show gene methylation in their sputum DNA, particularly of PCDH20 and SULF2 (Table 5); the latter association remained significant even after the Bonferroni correction for multiple comparisons for 12 genes.
Table 5.
—Unadjusted Comparison of Characteristics of Patients With Asthma in the Highest Tertile of SGRQ Symptom Subscale Scores, Compared With Those in the Lowest Tertile Group
| Characteristic | High Tertile Symptom Subscale Score (n = 61) | Low Tertile Symptom Subscale Score (n = 60) | P Value |
| Male sex | 27.9 | 18.3 | .21 |
| Age, y | 49.5 ± 7.5 | 56.0 ± 8.7 | < .001 |
| Hispanic ethnicity | 29.5 | 16.7 | .09 |
| ≥ High school education | 54.1 | 73.3 | .03 |
| Obese (BMI ≥ 30 kg/m2) | 42.6 | 36.7 | .50 |
| BMI, kg/m2 | 30.1 ± 7.1 | 28.1 ± 5.6 | .09 |
| Current cigarette smoke exposure | 85.3 | 30.0 | < .001 |
| Pack-years of cigarettes | 32.6 ± 15.8 | 32.7 ± 13.3 | .98 |
| Wood smoke exposure | 39.3 | 16.7 | .006 |
| SGRQ symptom score | 56.2 ± 14.3 | 9.3 ± 6.8 | < .001 |
| Absolute FEV1/FVC ratio | 75.9 ± 5.7 | 74.9 ± 5.6 | .36 |
| FEV1 % predicted (NHANES III) | 95.5 ± 14.1 | 95.3 ± 13.0 | .94 |
| Total number of methylated genes | 3.0 ± 2.1 | 2.0 ± 2.1 | .003 |
| Methylation index ≥ 4 genes | 49.2 | 21.7 | .002 |
| PCDH20 methylation | 57.4 | 38.3 | .04 |
| SULF2 methylation | 55.7 | 28.3 | .002 |
| GATA4 methylation | 45.9 | 38.3 | .40 |
| PAX5α methylation | 14.8 | 15.0 | .970 |
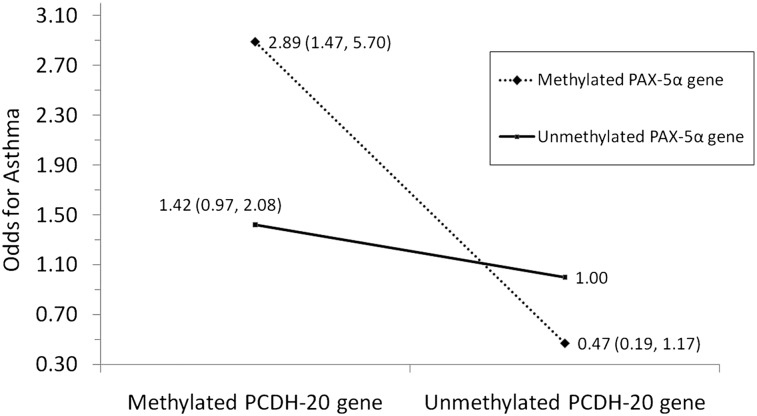
Additionally, no significant gene-environment interactions for PCDH20 were noted among smokers with asthma. On the contrary, a synergistic (multiplicative) interaction between the methylation of PCDH20 and PAX5α on the adjusted odds for asthma was observed (P = .009) (Fig 1).
Figure 1.
A significant synergistic (multiplicative) interaction between methylated PCDH-20 gene and PAX-5α gene in sputum was seen on the adjusted odds for asthma among smokers (P = .009). PAX-5α = paired box 5 protein transcription factor; PCDH-20 = protocadherin 20.
Additional analyses were performed to address whether environment-asthma associations were explained by epigenetics and, conversely, whether epigenetic-asthma associations were explained by the environment. The association between obesity and asthma did not change substantially after adjusting for the epigenetic findings in sputum (Table 2 footnote). Similarly, the epigenetic-asthma associations also did not change after adjusting for obesity and the remaining covariates (Table 4).
Alternative Analytic Strategy
Subgroup analyses among women smokers alone revealed similar epigenetic-asthma associations as for the entire group (no significant sex-epigenetic interactions were noted on asthma). Use of more stringent clinical definitions of asthma generally showed similar results, but the associated decrease in sample size among cases resulted in loss of power (data not shown). On the other hand, our use of a less stringent definition of asthma (expanded to include all obstructive lung diseases—asthma, COPD, asthma-COPD overlap state, and chronic bronchitis) resulted in similar methylation associations as described here with asthma alone (e-Table 3 (379.3KB, pdf) ). Similar methylation associations were also seen when subjects with asthma or asthma-COPD overlap state were together compared with smoker control subjects (data not shown).
Discussion
Older adult smokers with asthma demonstrate epigenetic changes in sputum DNA, specifically PCDH20 methylation, when compared with control subjects with a similar smoking history. We further demonstrate a synergistic interaction between two methylated genes (PCDH20 and PAX5α) in sputum DNA on the odds for asthma in this population. Interestingly, the epigenetic-asthma associations are not explained by the environmental factors studied, nor are the obesity-asthma associations explained by the methylated genes in sputum. Further, the methylation of genes in sputum DNA identifies a symptomatically more severe disease phenotype among subjects with asthma.
Cadherins, including protocadherins, have functions in cell adhesion and signal transduction pathways,27 and protocadherin-1 gene (a related member of the cadherin family) has been previously associated with asthma in both mice and humans.28,29 Although PCDH20 is expressed by human lung tissue,30 its potential role in regulating asthma pathophysiology needs further evaluation. Interestingly, our finding that PCDH20 methylation may be associated with a symptomatically more severe asthma phenotype supports the clinical relevance of studying gene methylation status in sputum (Table 5). Nevertheless, the cross-sectional nature of our study does not allow us to establish the direction of the association (ie, whether gene methylation precedes airway disease or is a consequence of airway disease).
Our study showed obesity to be associated with asthma in older adults, thus confirming findings previously described among children and young adults. Although obesity is associated with epigenetic effects,31,32 we did not find that it explained away the epigenetic associations with asthma. Since epigenetic modifications may constitute a memory of prior environmental exposures including those occurring in utero,33 we may not have had sufficiently detailed information about prior exposures to explain our epigenetic associations in this study. Alternatively, epigenetic events may be triggered by the diseased phenotype itself, thus bypassing environmental triggers.32
Interestingly, subjects with asthma with methylation of PCDH20 also showed concomitant methylation of most other genes in their sputum (e-Table 1 (379.3KB, pdf) ). Further, we demonstrate that multiple methylated genes jointly affect the odds for asthma (Fig 1). This phenomenon of multiple methylated genes in respiratory samples has not been previously reported in asthma but is similar to the field effect described with cancer.34
The strengths of our study include the use of postbronchodilator spirometry to assess reversibility, strict adherence to the 1994 ATS guidelines in the performance of spirometry, state-of-the-art validated PCR assays to measure methylation, use of sputum cells to study methylation instead of blood or buccal cells (whose epigenetic patterns may be irrelevant to the airway), exclusion of those with COPD or asthma-COPD overlap state, and conservative Bonferroni correction for multiple comparisons. To our knowledge, this is the first study of sputum methylation profiles in subjects with asthma.
We also recognize several limitations to our study. Our study cohort may not be representative of all smokers in New Mexico. However, the smoking behavior in this study is consistent with that observed in representative surveys of New Mexico.35 Our definitions of asthma are not confirmed by methacholine bronchoprovocation and are thus subject to misclassification bias. However, this misclassification is likely to be nondifferential between the methylated and unmethylated groups and unlikely to produce a spurious effect. Although we have differentiated smokers into smoker control subjects, asthma, COPD, chronic bronchitis and asthma-COPD overlap state, we recognize that such discrete differentiation may not always be possible in clinical practice. Our results may be generalizable primarily to the asthma phenotype that occurs in the older, overweight/obese smoker, particularly women. However, this phenotype is clinically relevant since it often represents difficult-to-manage disease.36 Although we arbitrarily selected 12 biologically relevant genes based on their associations with inflammation and DNA repair pathways, it is likely that there are many other genes associated with inflammation and oxidative stress in asthma. However, ours is one of the few sentinel studies that argue for studying the potential role for methylated genes in sputum of subjects with asthma. Thus, longitudinal epidemiologic and functional genetic studies are needed in the future to establish a possible causal association between environmental exposures, airway epigenetic changes, and incident asthma.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Sood: contributed to conception and design of the study, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Mr Petersen: contributed to acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Dr Blanchette: contributed to acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Dr Meek: contributed to conception and design of the study, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Ms Picchi: contributed to acquisition of data or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Dr Belinsky: contributed to conception and design of the study, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Dr Tesfaigzi: contributed to conception and design of the study, drafting the article or revising it critically for important intellectual content, and approving the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Mr Petersen has received payment as an employee of Lovelace Respiratory Research Institute for analysis work that was funded by research grants and contracts from GlaxoSmithKline, AstraZeneca, Novartis AG, Amgen Inc, and Merck & Co, Inc. Drs Sood, Blanchette, Meek, Belinsky, and Tesfaigzi and Ms Picchi have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors played no role in the design, conduct, and analysis of the study or the writing of the manuscript.
Other contributions: This work was performed at the Lovelace Respiratory Research Institute, Albuquerque, NM.
Additional information: The e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- ATS
American Thoracic Society
- CpG
cytosine-phosphate-guanine
- DAL1
differentially expressed in adenocarcinoma of the lung 1 gene
- DAPK
death-associated protein kinase gene
- GATA4
GATA binding protein-4 transcription factor gene
- GATA5
GATA binding protein-5 transcription factor gene
- JPH3
junctophilin 3 gene
- MGMT
O6-methylguanine-DNA methyltransferase gene
- PAX5α and PAX5β
paired box 5 protein transcription factor genes
- PCDH20
protocadherin 20 gene
- PCR
polymerase chain reaction
- RASSF1α
Ras association domain family member 1α gene
- SGRQ
St. George Respiratory Questionnaire
- SULF2
extracellular sulfatase 2 gene
Footnotes
Funding/Support: This study was supported by the State of New Mexico (appropriation from the Tobacco Settlement Fund) and from the National Institutes of Health [Grants K23 HL 094531-01 and CTSA 1ULRR031977-01 (A. S.), RO1 ES015482 (Y. T.), and R01 CA 097356 (S. B.)].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.American Lung Association Trends in asthma morbidity and mortality. July 2011. American Lung Association website. http://www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf. Accessed October 1, 2011
- 2.Schwartz DA. Epigenetics and environmental lung disease. Proc Am Thorac Soc. 2010;7(2):123-125 [DOI] [PubMed] [Google Scholar]
- 3.Adcock IM, Tsaprouni L, Bhavsar P, Ito K. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007;19(6):694-700 [DOI] [PubMed] [Google Scholar]
- 4.Belinsky SA, Liechty KC, Gentry FD, et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66(6):3338-3344 [DOI] [PubMed] [Google Scholar]
- 5.Belinsky SA, Palmisano WA, Gilliland FD, et al. Aberrant promoter methylation in bronchial epithelium and sputum from current and former smokers. Cancer Res. 2002;62(8):2370-2377 [PubMed] [Google Scholar]
- 6.Sood A, Qualls B, Thyagarajan M, et al. Adult-onset asthma is the dominant phenotype in women of age 40 years [abstract]. Am J Respir Crit Care Med. 2011;183(Meeting Abstracts):A3746 [Google Scholar]
- 7.Vesterinen E, Kaprio J, Koskenvuo M. Prospective study of asthma in relation to smoking habits among 14,729 adults. Thorax. 1988;43(7):534-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamrozik E, Knuiman MW, James A, Divitini M, Musk AW. Risk factors for adult-onset asthma: a 14-year longitudinal study. Respirology. 2009;14(6):814-821 [DOI] [PubMed] [Google Scholar]
- 9.King ME, Mannino DM, Holguin F. Risk factors for asthma incidence. A review of recent prospective evidence. Panminerva Med. 2004;46(2):97-110 [PubMed] [Google Scholar]
- 10.Troisi RJ, Speizer FE, Rosner B, Trichopoulos D, Willett WC. Cigarette smoking and incidence of chronic bronchitis and asthma in women. Chest. 1995;108(6):1557-1561 [DOI] [PubMed] [Google Scholar]
- 11.Rönmark E, Lundbäck B, Jönsson E, Jonsson AC, Lindström M, Sandström T. Incidence of asthma in adults—report from the Obstructive Lung Disease in Northern Sweden Study. Allergy. 1997;52(11):1071-1078 [DOI] [PubMed] [Google Scholar]
- 12.Sood A, Stidley CA, Picchi MA, et al. Difference in airflow obstruction between Hispanic and non-Hispanic White female smokers. COPD. 2008;5(5):274-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sood A, Petersen H, Blanchette CM, et al. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010;182(9):1098-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152-2158 [DOI] [PubMed] [Google Scholar]
- 15.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968 [DOI] [PubMed] [Google Scholar]
- 16.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2008. Global Initiative for Chronic Obstructive Lung Disease website. www.goldcopd.com. Accessed October 1, 2011
- 17.Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6 pt 2):1-120 [PubMed] [Google Scholar]
- 18.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31 [DOI] [PubMed] [Google Scholar]
- 19.Gordon CC, Chumlea WC, Roche AF. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Publishers, Inc; 1988 [Google Scholar]
- 20.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107-1136 [DOI] [PubMed] [Google Scholar]
- 21.Saccomanno G, Archer VE, Auerbach O, Saunders RP, Brennan LM. Development of carcinoma of the lung as reflected in exfoliated cells. Cancer. 1974;33(1):256-270 [DOI] [PubMed] [Google Scholar]
- 22.Chau BN, Diaz RL, Saunders MA, et al. Identification of SULF2 as a novel transcriptional target of p53 by use of integrated genomic analyses. Cancer Res. 2009;69(4):1368-1374 [DOI] [PubMed] [Google Scholar]
- 23.Hagman J, Lukin K. “Hands-on” regulation of B cell development by the transcription factor Pax5. Immunity. 2007;27(1):8-10 [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18(5):2901-2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60(21):5954-5958 [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187 [DOI] [PubMed] [Google Scholar]
- 27.Aaron SD, Vandemheen KL, Boulet LP, et al. ; Canadian Respiratory Clinical Research Consortium Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179(11):1121-1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koppelman GH, Meyers DA, Howard TD, et al. Identification of PCDH1 as a novel susceptibility gene for bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(10):929-935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koning H, Brouwer U, Hylkema MN, et al. Regulation of protocadherin-1 expression in mouse lung and experimental asthma [abstract]. Am J Respir Crit Care Med. 2011;183(Meeting Abstracts):A1347 [Google Scholar]
- 30.Imoto I, Izumi H, Yokoi S, et al. Frequent silencing of the candidate tumor suppressor PCDH20 by epigenetic mechanism in non-small-cell lung cancers. Cancer Res. 2006;66(9):4617-4626 [DOI] [PubMed] [Google Scholar]
- 31.Campión J, Milagro F, Martínez JA. Epigenetics and obesity. Prog Mol Biol Transl Sci. 2010;94291-347 [DOI] [PubMed] [Google Scholar]
- 32.Franks PW, Ling C. Epigenetics and obesity: the devil is in the details. BMC Med. 2010;888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabesch M, Michel S, Tost J. Epigenetic mechanisms and the relationship to childhood asthma. Eur Respir J. 2010;36(4):950-961 [DOI] [PubMed] [Google Scholar]
- 34.Tessema M, Yu YY, Stidley CA, et al. Concomitant promoter methylation of multiple genes in lung adenocarcinomas from current, former and never smokers. Carcinogenesis. 2009;30(7):1132-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System User’s Guide. Atlanta, GA: US. Department of Health and Human Services, Centers for Disease Control and Prevention; 1998:585 [Google Scholar]
- 36.Moore WC, Meyers DA, Wenzel SE, et al. ; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement