Abstract
Background:
Bronchiectasis is a potentially serious condition characterized by permanent and abnormal widening of the airways, the prevalence of which is not well described. We sought to describe the trends, associated conditions, and risk factors for bronchiectasis among adults aged ≥ 65 years.
Methods:
A 5% sample of the Medicare outpatient claims database was analyzed for bronchiectasis trends among beneficiaries aged ≥ 65 years from 2000 to 2007. Bronchiectasis was identified using International Classification of Diseases, Ninth Revision, Clinical Modification claim diagnosis codes for acquired bronchiectasis. Period prevalence was used to describe sex- and race/ethnicity-specific rates, and annual prevalence was used to describe trends and age-specific rates. We estimated trends using Poisson regression and odds of bronchiectasis using multivariate logistic regression.
Results:
From 2000 to 2007, 22,296 people had at least one claim for bronchiectasis. The 8-year period prevalence of bronchiectasis was 1,106 cases per 100,000 people. Bronchiectasis increased by 8.7% per year. We identified an interaction between the number of thoracic CT scans and race/ethnicity; period prevalence varied by a greater degree by number of thoracic CT scans among Asians compared with whites or blacks. Among people with one CT scan, Asians had a 2.5- and 3.9-fold higher period prevalence compared with whites and blacks.
Conclusions:
Bronchiectasis prevalence increased significantly from 2000 to 2007 in the Medicare outpatient setting and varied by age, sex, and race/ethnicity. This increase could be due to a true increase in the condition or an increased recognition of previously undiagnosed cases.
Bronchiectasis is an uncommon, but potentially serious condition related to abnormal widening of the airway passages. Recurrent lung infections; foreign objects in the airways; and defective lung clearance mechanisms, such as the inability to properly clear mucus, can lead to bronchiectasis.1,2 Symptoms of bronchiectasis include, but are not limited to, hemoptysis, chronic cough, sputum production, and shortness of breath.2‐4 Bronchiectasis treatment is aimed at minimizing further damage to the airways through inflammation reduction, infection prevention, and bronchopulmonary hygiene.3 Treatment is usually long term, and some patients may benefit from surgical resection of the lung or lung transplantation.2
The current prevalence of bronchiectasis is not well described.3,5,6 To our knowledge, only one prior study estimated bronchiectasis prevalence for the United States. This retrospective cohort study estimated a prevalence of 52.3 cases of bronchiectasis per 100,000 adults, with higher rates among women and older individuals.6 The hospital admission rate of bronchiectasis in Hong Kong was found to be 16.4 per 100,000 people in 1990,7 suggesting a high prevalence in Asian countries.8 Other studies have also shown high rates for women and older adults,6,8‐12 as well as associations with diseases such as rheumatoid arthritis and humoral immunodeficiencies.3,13
We previously analyzed hospital discharge data from the Agency for Healthcare Quality and Research and found an increasing trend in bronchiectasis-associated hospitalizations from 1993 to 2006.14 Bronchiectasis-associated hospitalizations were highest among women and people aged > 60 years. However, that study was limited to an inpatient population in select states, leaving open questions of generalizability and confounders regarding access to care. Additionally, the use of CT scans has been increasing,15,16 and the observed increase in bronchiectasis could reflect an artifact of increased CT scan use with improved diagnostic capacity for detecting previously unrecognized bronchiectasis. For example, if more thoracic CT scans are being ordered for a variety of conditions, this increased scanning would result in an increased opportunity for detecting incidental findings, such as bronchiectasis.17,18
Because prior studies reported a high prevalence of bronchiectasis among older people6,8,14 and the Medicare Part B population aged ≥ 65 years represents about 91% of the total US population aged ≥ 65 years,19 the Medicare Part B outpatient databases from the Centers for Medicare & Medicaid Services (CMS) provide an optimal resource for studying bronchiectasis in the United States. We undertook the current study to estimate the prevalence and trends for bronchiectasis in an outpatient population that is representative of the US population most affected by this condition.
Materials and Methods
Databases and Populations
We analyzed a 5% sample of the carrier and the denominator standard analytic files (SAFs) obtained from CMS. The 5% sample was randomly selected from all carrier claims by CMS. The carrier SAFs contain claims-level information from noninstitutional outpatient health-care providers. The denominator SAFs include annual demographic and enrollment information for each Medicare beneficiary (e-Appendix 1 (338.7KB, pdf) ).
Data Analysis and Ethical Review
We analyzed claims from 2000 to 2007 to estimate prevalence and trends of bronchiectasis and to describe comorbid conditions and geographic patterns over the 8-year period. Analysis was completed using SAS, version 9.2 (SAS Institute Inc) statistical software. We used the Medicare Denominator database to define the denominator population. An individual’s annual record was included in the analysis if all of the following criteria in that year were met: (1) residency within the 50 United States or the District of Columbia, (2) aged ≥ 65 years, and (3) no enrollment in a health maintenance organization. We compared the age, sex, and racial distribution in the study population to the general US census population aged ≥ 65 years in 2000 to determine how representative the 5% sample was relative to the US population.
Bronchiectasis claims were identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 494 (bronchiectasis), 494.1 (bronchiectasis with acute exacerbation), and 494.0 (bronchiectasis without acute exacerbation). A single summary record was created for each individual and each year. This record included count variables to indicate the number of claims with bronchiectasis, sex, age, race, number of thoracic CT scans, and comorbid conditions of interest. Analysis by racial or ethnic group used the racial/ethnic categories defined by CMS. Because bronchiectasis is a chronic condition, individual cases were considered if the patient had at least one primary or secondary claim of bronchiectasis in the time period of interest. We assessed potential bias resulting from this case definition by comparing overall demographics and specific outcomes to those that would result from a case definition of at least two bronchiectasis claims (e-Appendix 1 (338.7KB, pdf) ).
We calculated 8-year prevalence estimates from 2000 to 2007 and constructed a multivariate logistic regression model to assess race, sex, and CT scan use (e-Appendix 1 (338.7KB, pdf) ). Age-specific period prevalence estimates were not assessed because age is a time-dependent variable. We used ArcInfo, version 9.3.1 (Environmental Systems Research Institute, Inc) to map the period prevalence by state. We calculated age- and sex-specific annual prevalence of bronchiectasis and used Poisson regression to determine significance of annual trends from 2000 to 2007 (e-Appendix 1 (338.7KB, pdf) ).
To describe the most common conditions associated with bronchiectasis in this population, we assessed diagnosis claims among people with bronchiectasis (e-Appendix 1 (338.7KB, pdf) ), identifying the most common primary diagnosis claims and describing the frequency of conditions with known or suspected associations with bronchiectasis, such as rheumatoid arthritis and nontuberculous mycobacterial infection.20 We calculated the prevalence ratio for these conditions as the percentage with both bronchiectasis and the condition of interest divided by the percentage with the condition of interest among the general population. This study was not considered human subject research by the National Institutes of Health Office of Human Subjects Protection because the limited-use data set is not linkable to identifying information.
Results
The study population included > 2 million unique individuals enrolled in Medicare Part B for at least 1 month from 2000 through 2007. Medicare beneficiaries from all 50 states and the District of Columbia were represented. The demographic distribution was representative of the entire US population aged ≥ 65 years.
We identified 117,112 claims of bronchiectasis from 2000 to 2007 from 22,296 people for an average of approximately five claims per person during this period. These individuals included 13,971 women (63%). The demographics differed significantly between people with one claim of bronchiectasis and people with two or more claims of bronchiectasis, but the overall pattern of period and annual prevalence by sex remained the same (e-Appendix 1 (338.7KB, pdf) ). To reduce the possibility of bias due to differences in health-care utilization behaviors,21‐23 we used the case definition of at least one bronchiectasis claim in the period of interest.
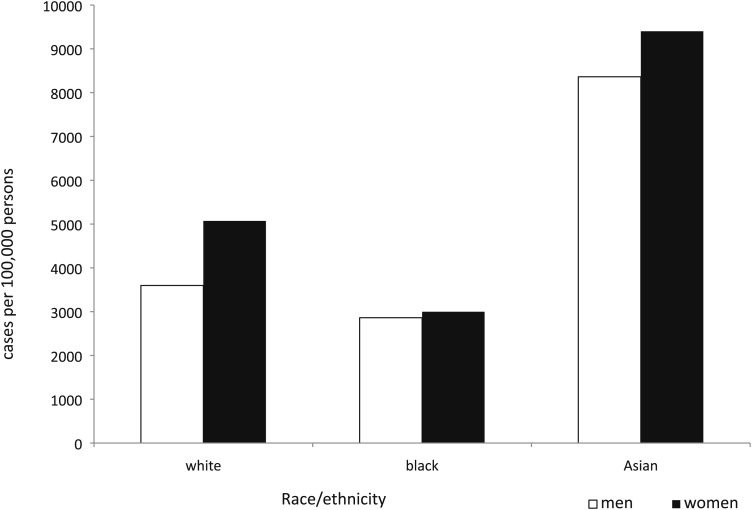
The overall 8-year period prevalence was 1,106 (95% CI, 1,092-1,121) cases of bronchiectasis per 100,000 people. Period prevalence for Asians was higher than for blacks or whites within each stratum of thoracic CT scans. We identified a statistically significant interaction between the number of thoracic CT scans and race/ethnicity, with period prevalence among Asians varying to a greater degree than that for whites and blacks. Among people with one CT scan, Asians had a 2.5- and 3.9-fold higher period prevalence compared with whites and blacks. Within people who had two to three scans, the relative prevalence estimates for Asians compared with whites and blacks were 2.0 and 3.0, respectively (Fig 1). Using a logistic regression model containing variables for sex, race, thoracic CT scans, and an interaction term for number of thoracic CT scans and race, we identified a 36% increase in the odds of bronchiectasis among women compared with men (OR, 1.36; 95% CI, 1.32-1.40).
Figure 1.
Bronchiectasis period prevalence by sex and race/ethnicity among people with two or three thoracic CT scans, 2000 to 2007.
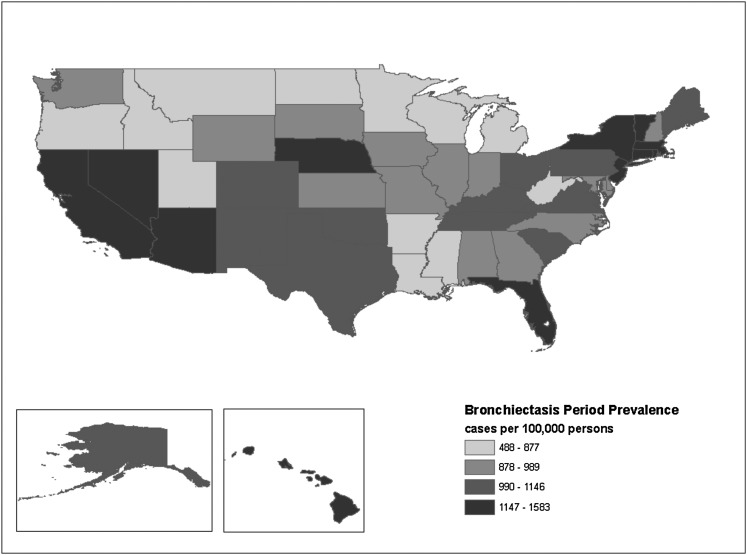
A threefold increased prevalence was observed between the state with the highest period prevalence (Massachusetts, 1,583 [95% CI, 1,467-1,700] cases per 100,000 population) and the state with the lowest prevalence (Montana, 488 [95% CI, 339-637] cases per 100,000 population). No clear pattern of clustering by geographic region at the state level was evident (Fig 2).
Figure 2.
Map of bronchiectasis period prevalence, 2000 to 2007.
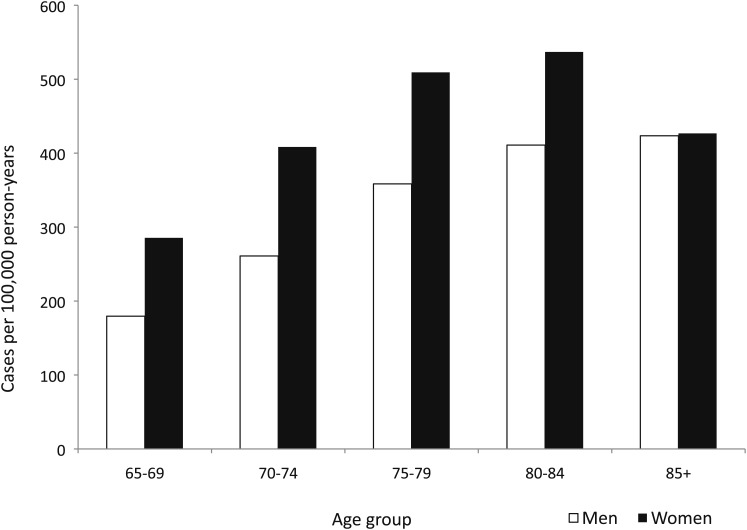
The overall average annual prevalence of bronchiectasis in the physician outpatient setting was 370 (95% CI, 367-374) cases of bronchiectasis per 100,000 person-years. Prevalence increased with age up to 85 years, and women aged 80 to 84 years had the highest average annual prevalence (537 [95% CI, 523-551] cases per 100,000 person-years). Within each age group up to age 85 years, women had a 1.3- to 1.6-fold increased prevalence relative to men (Fig 3).
Figure 3.
Average annual prevalence of bronchiectasis by age and sex, 2000 to 2007.
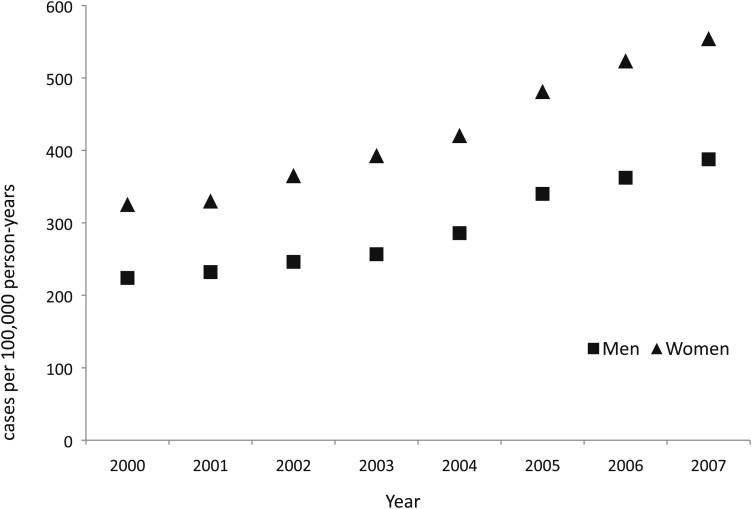
Overall, the annual percentage change (APC) of bronchiectasis was 8.74% (95% CI, 7.70%-9.79%), representing a significant increase in bronchiectasis from 2000 through 2007. The APC was similar for men (9.10%; 95% CI, 7.64%-10.58%) and women (8.70%; 95% CI, 7.80%-9.60%) (Fig 4). The APC for thoracic CT scans increased by 10.3% (95% CI, 8.2%-12.3%) per year during the same period.
Figure 4.
Trend in annual prevalence of bronchiectasis by sex.
Conditions identified as higher among people with bronchiectasis compared with those without bronchiectasis from the initial screening of all claims were acute bronchitis, unspecified pneumonia, shortness of breath, cough, and other respiratory distress. In a focused comparison with conditions suspected to be associated with bronchiectasis, we further identified pulmonary nontuberculous mycobacterial disease (PNTM), rheumatoid arthritis, and lung malignancies as each having an increased prevalence among people with bronchiectasis in both men and women. The prevalence ratio of PNTM among individuals with bronchiectasis compared with those without bronchiectasis was twofold greater among women than men. The prevalence ratio of postinflammatory pulmonary fibrosis, lung malignancies, and rheumatoid arthritis among people with and without bronchiectasis was similar for men and women (Table 1).
Table 1.
—Comorbid Conditions by Men and Women for People With and Without Bronchiectasis, 2000 to 2007
| Men |
Women |
|||||
| Condition | Individuals With Bronchiectasis (n = 8,325) | Individuals Without Bronchiectasis (n = 842,757) | RP | Individuals With Bronchiectasis (n = 13,971) | Individuals Without Bronchiectasis (n = 1,156,878) | RP |
| PNTM | 217 (2.61) | 480 (0.06) | 43.5 | 752 (5.38) | 638 (0.06) | 89.67 |
| Acute bronchitis | 5,046 (60.61) | 207,621 (24.64) | 2.46 | 9,110 (65.21) | 329,981 (28.52) | 2.29 |
| Rheumatoid arthritis | 636 (7.64) | 29,260 (3.47) | 2.20 | 1,749 (12.52) | 75,494 (6.53) | 1.92 |
| Postinflammatory pulmonary fibrosisa | 2,975 (35.74) | 49,447 (5.87) | 6.09 | 4,778 (34.20) | 62,892 (5.44) | 6.29 |
| Lung malignancies | 1,080 (12.97) | 38,564 (4.58) | 2.83 | 1,112 (7.96) | 36,196 (3.13) | 2.54 |
| Inflammatory bowel disease | 186 (2.23) | 7,976 (0.95) | 2.35 | 327 (2.34) | 13,410 (1.16) | 2.02 |
| Other genetic disordersb | 177 (2.13) | 1,211 (0.14) | 15.21 | 252 (1.80) | 1,764 (0.15) | 12.0 |
Data are presented as No. (%), unless otherwise indicated. PNTM = pulmonary nontuberculous mycobacterial disease; RP = relative prevalence.
Postinflammatory pulmonary fibrosis: International Classification of Diseases, Ninth Revision, Clinical Modification code 515.
Congenital cartilage deficiency, situs inversus, common variable immunodeficiency, IgG deficiency, allergic bronchopulmonary aspergillosis, α1-antitrypsin deficiency.
Because we saw little difference in the prevalence ratios by sex, we then grouped these comorbid conditions across sex groups and looked at racial/ethnic differences. Interestingly, the absolute prevalence and prevalence ratio for most of the conditions for people with and without bronchiectasis was similar for whites, blacks, and Asians (Table 2). People with bronchiectasis were 50 to 75 times more likely to have PNTM than those without bronchiectasis, with the highest prevalence ratio among whites. Among people with bronchiectasis, about 20% (4,507) had none of the comorbidities listed in Tables 1 and 2.
Table 2.
—Comorbidities by Race/Ethnicity for People With and Without Bronchiectasis, 2000 to 2007
| White |
Black |
Asian |
|||||||
| Condition | With Bronchiectasis (n = 19,839) | Without Bronchiectasis (n = 1,724,735) | RP | With Bronchiectasis (n = 1,075) | Without Bronchiectasis (n = 166,354) | RP | With Bronchiectasis (n = 587) | Without Bronchiectasis (n = 33,626) | RP |
| PNTM | 902 (4.55) | 986 (0.06) | 75.83 | 18 (1.67) | 56 (0.03) | 55.67 | 32 (5.45) | 37 (0.11) | 49.55 |
| Acute bronchitis | 12,736 (64.20) | 476,345 (27.62) | 2.32 | 558 (51.91) | 33,005 (19.84) | 2.62 | 380 (64.74) | 10,638 (31.64) | 2.05 |
| Rheumatoid arthritis | 2,057 (10.37) | 86,949 (5.04) | 2.06 | 122 (11.35) | 9,700 (5.83) | 1.95 | 92 (15.67) | 2,569 (7.64) | 2.05 |
| Postinflammatory pulmonary fibrosisa | 6,922 (34.89) | 100,409 (5.82) | 5.99 | 368 (34.23) | 7,225 (4.34) | 7.89 | 194 (33.05) | 1,376 (4.09) | 8.08 |
| Lung malignancies | 1,963 (9.89) | 66,295 (3.84) | 2.58 | 129 (12.00) | 5,813 (3.49) | 3.44 | 40 (6.81) | 885 (2.63) | 2.59 |
| Inflammatory bowel disease | 467 (2.35) | 19,384 (1.12) | 2.10 | 24 (2.23) | 1,156 (0.69) | 3.23 | 9 (1.53) | 265 (0.79) | 1.94 |
| Other genetic disordersb | 406 (2.05) | 2,666 (0.15) | 13.67 | 10 (0.93) | 176 (0.11) | 8.45 | 1 (0.17) | 28 (0.08) | 2.13 |
Data are presented as No. (%), unless otherwise indicated. See Table 1 legend for expansion of abbreviations.
Postinflammatory pulmonary fibrosis: International Classification of Diseases, Ninth Revision, Clinical Modification code 515.
Congenital cartilage deficiency, situs inversus, common variable immunodeficiency, IgG deficiency, allergic bronchopulmonary aspergillosis, α1-antitrypsin deficiency.
Discussion
We analyzed CMS databases to describe nationally representative patterns of prevalence and trends of bronchiectasis in the older adult US outpatient population. Based on the number of cases identified in this analysis and extrapolating to the 2007 US population aged ≥ 65 years, we estimate that > 190,000 unique cases of bronchiectasis were assessed by a physician in the older adult US population in that year. Bronchiectasis prevalence increased significantly from 2000 to 2007 among both men and women. Although some of this increase is likely due to increasing use of CT scans since 2000, the burden of recognized disease nonetheless clearly increased from 2000 to 2007. One study identified a high proportion of asymptomatic individuals with bronchiectasis among individuals with a chest CT scan in a health screening program, suggesting that a large proportion of undiagnosed cases may exist.8 This large number of undiagnosed cases indicates a need for greater awareness of this condition.
Overall, the burden of disease was greater among women than among men, and prevalence increased with age for both sexes. These findings are consistent with previous studies.6,10‐12 People identified as Asian had a 1.4- to 2.5-fold increased prevalence relative to whites, with the greatest relative prevalence among people with a higher number of CT scans. This increased prevalence among the Asian subgroup was not explained by differences in the prevalence of comorbidities, which tended to be similar across sex and racial/ethnic groups. The increased prevalence among Asians has not been previously described for the US population; however, some studies suggested a high prevalence in Asian countries.7,8 Of note is our finding that the relative prevalence for Asians compared with whites or blacks varied by CT scan use; that is, with increasing CT scan use, the prevalence was greater for Asians compared with whites and blacks. The reasons for the increased likelihood of a bronchiectasis diagnosis among Asians with increased CT scan use are not clear but may warrant further research.
We used comorbid conditions to identify patterns of possible underlying pathogenic processes, such as those due to infectious or inflammatory conditions. Prevalence estimates were higher for inflammatory conditions (rheumatoid arthritis or inflammatory bowel disease) and infectious conditions (PNTM) among people with bronchiectasis than for those without bronchiectasis. Although PNTM was a relatively rare condition even among people with bronchiectasis (2%-5%), the prevalence among those with bronchiectasis compared with the general population was strikingly higher, suggesting an important interaction between bronchiectasis and PNTM.
The racial and ethnic categories of white, black, and Asian encompass a wide variety of ethnicities and may serve as surrogates for unidentified behavioral risk factors or genetic susceptibility because associations between specific human leukocyte antigen alleles and bronchiectasis have been identified.24,25 Although we identified Asians as having the highest prevalence of bronchiectasis, we are unsure how broadly this applies to the diverse groups included in the category. For example, Native Hawaiian and Pacific Islanders have a variety of different risk factors compared with Asian Americans.26‐28 Although Medicare data are not subdivided by the ethnic groups that comprise the Asian population, future studies could explore analyses by Asian subgroup.
The lack of geographic clustering could indicate that bronchiectasis has little or no association with geographic variables that exist on a statewide scale. However, we did not look at geographic patterns on smaller geographic subunits, such as by county or by urban or rural region; therefore, we do not know whether there may be geographic patterns that depend on local coding practices, CT scan use, ethnic variation, or other unknown factors.
There are limitations inherent in the use of Medicare claims data for epidemiologic analysis. One example of a limitation that should be considered while interpreting these results is the potential bias from excluding individuals with health maintenance organization coverage. Differences in these populations, especially with regard to socioeconomic or health status, are unknown. The use of ICD-9-CM codes for identifying cases of bronchiectasis and comorbid conditions is also a limitation in our study. ICD-9-CM codes are primarily used for billing purposes and only secondarily for epidemiologic research.29 Additionally, the use of ICD-9-CM codes for identifying cases of bronchiectasis has not been validated. Differences in regional billing practices or changes in Medicare reimbursement may affect coding30 and, consequently, the current study results. Because we were limited to ICD-9-CM codes, we may be overestimating or underestimating the true prevalence of bronchiectasis. However, given the relative rarity of this condition and only recent awareness of its impact, we are more likely to be underestimating the true prevalence. Limitations using ICD-9-CM codes also apply to the comorbidity analysis because we were unable to determine the detailed clinical pathology associated with the ICD-9-CM comorbidity code.
Conclusions
We observed an increasing prevalence of bronchiectasis in the outpatient Medicare population and an overall higher prevalence for women and Asians. The increased prevalence among Asians was observed at all levels of thoracic CT scan use and was greatest with four to six scans across the 8-year period. This increasing prevalence may be due to increased recognition of previously undiagnosed cases or a true increase in incidence, highlighting the need for increased awareness of this condition. Overall, this representative study of > 2 million adults aged ≥ 65 years provides further evidence for the increased burden among Asians and women and identifies an increasing trend in the elderly outpatient population in the United States.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Ms Seitz and Dr Prevots had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ms Seitz: contributed to the study concept and design; data acquisition and management; statistical and epidemiologic analyses; interpretation of results; drafting of the manuscript; and manuscript preparation, revision, final edit, and approval of the final version.
Dr Olivier: contributed to the study concept and design; interpretation of results; and manuscript preparation, revision, final edit, and approval of the final version.
Dr Adjemian: contributed to the interpretation of results and manuscript preparation, revision, final edit, and approval of the final version.
Dr Holland: contributed to the interpretation of results and manuscript preparation, revision, final edit, and approval of the final version.
Dr Prevots: contributed to the study concept and design; data acquisition and management; statistical and epidemiologic analyses; interpretation of results; drafting of the manuscript; and manuscript preparation, revision, final edit, and approval of the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: This research was completed as part of the NIH intramural research program. The authors had full control in the design of the study, analysis of the data, and preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect those of the US Department of Health and Human Services.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- APC
annual percentage change
- CMS
Centers for Medicare & Medicaid Services
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PNTM
pulmonary nontuberculous mycobacterial disease
- SAF
standard analytic file
Footnotes
Funding/Support: This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Barker AF, Bardana EJ., Jr Bronchiectasis: update of an orphan disease. Am Rev Respir Dis. 1988;137(4):969-978 [DOI] [PubMed] [Google Scholar]
- 2.Rosen MJ. Chronic cough due to bronchiectasis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1):122S-131S [DOI] [PubMed] [Google Scholar]
- 3.Barker AF. Bronchiectasis. N Engl J Med. 2002;346(18):1383-1393 [DOI] [PubMed] [Google Scholar]
- 4.Wynn-Williams N. Bronchiectasis: a study centred on Bedford and its environs. BMJ. 1953;1(4821):1194-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fall A, Spencer D. Paediatric bronchiectasis in Europe: what now and where next?. Paediatr Respir Rev. 2006;7(4):268-274 [DOI] [PubMed] [Google Scholar]
- 6.Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med.. 2005;12(4):205-209 [Google Scholar]
- 7.Tsang KW, Tipoe GL. Bronchiectasis: not an orphan disease in the East. Int J Tuberc Lung Dis. 2004;8(6):691-702 [PubMed] [Google Scholar]
- 8.Kwak HJ, Moon JY, Choi YW, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku J Exp Med. 2010;222(4):237-242 [DOI] [PubMed] [Google Scholar]
- 9.Ellis DA, Thornley PE, Wightman AJ, Walker M, Chalmers J, Crofton JW. Present outlook in bronchiectasis: clinical and social study and review of factors influencing prognosis. Thorax. 1981;36(9):659-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108(4):955-961 [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest. 1998;113(5):1329-1334 [DOI] [PubMed] [Google Scholar]
- 12.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162(4 pt 1):1277-1284 [DOI] [PubMed] [Google Scholar]
- 13.Crofton J, Douglas A. Bronchiectasis. Crofton and Douglas’s Respiratory Diseases. St Louis, MO: Blackwell Scientific Publications; 1981:417-430 [Google Scholar]
- 14.Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest. 2010;138(4):944-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284 [DOI] [PubMed] [Google Scholar]
- 16.Rao VM, Levin DC, Parker L, Frangos AJ, Sunshine JH. Trends in utilization rates of the various imaging modalities in emergency departments: nationwide Medicare data from 2000 to 2008. J Am Coll Radiol. 2011;8(10):706-709 [DOI] [PubMed] [Google Scholar]
- 17.Schragin JG, Weissfeld JL, Edmundowicz D, Strollo DC, Fuhrman CR. Non-cardiac findings on coronary electron beam computed tomography scanning. J Thorac Imaging. 2004;19(2):82-86 [DOI] [PubMed] [Google Scholar]
- 18.Elgin EE, O’Malley PG, Feuerstein I, Taylor AJ. Frequency and severity of “incidentalomas” encountered during electron beam computed tomography for coronary calcium in middle-aged army personnel. Am J Cardiol. 2002;90(5):543-545 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services Overview Medicare Enrollment Reports, Medicare Enrollment, Aged Beneficiaries: As of July 2006. Baltimore, MD: Centers for Medicare & Medicaid Services; 2006 [Google Scholar]
- 20.Boyton RJ. Regulation of immunity in bronchiectasis. Med Mycol. 2009;47(suppl 1):S175-S182 [DOI] [PubMed] [Google Scholar]
- 21.Wenneker MB, Epstein AM. Racial inequalities in the use of procedures for patients with ischemic heart disease in Massachusetts. JAMA. 1989;261(2):253-257 [PubMed] [Google Scholar]
- 22.Escarce JJ, Epstein KR, Colby DC, Schwartz JS. Racial differences in the elderly’s use of medical procedures and diagnostic tests. Am J Public Health. 1993;83(7):948-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg KC, Hartz AJ, Jacobsen SJ, Krakauer H, Rimm AA. Racial and community factors influencing coronary artery bypass graft surgery rates for all 1986 Medicare patients. JAMA. 1992;267(11):1473-1477 [PubMed] [Google Scholar]
- 24.Boyton RJ, Smith J, Jones M, et al. Human leucocyte antigen class II association in idiopathic bronchiectasis, a disease of chronic lung infection, implicates a role for adaptive immunity. Clin Exp Immunol. 2008;152(1):95-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyton RJ, Smith J, Ward R, et al. HLA-C and killer cell immunoglobulin-like receptor genes in idiopathic bronchiectasis. Am J Respir Crit Care Med. 2006;173(3):327-333 [DOI] [PubMed] [Google Scholar]
- 26.Bitton A, Zaslavsky AM, Ayanian JZ. Health risks, chronic diseases, and access to care among US Pacific Islanders. J Gen Intern Med. 2010;25(5):435-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Maternal, pregnancy, and birth characteristics of Asians and Native Hawaiians/Pacific Islanders—King County, Washington, 2003-2008. MMWR Morb Mortal Wkly Rep. 2011;60(7):211-213 [PubMed] [Google Scholar]
- 28.Moy KL, Sallis JF, David KJ. Health indicators of Native Hawaiian and Pacific Islanders in the United States. J Community Health. 2010;35(1):81-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40(5 pt 2):1620-1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia DC, Krushat WM, Fagan AB, Tebbutt JA, Kusserow RP. Accuracy of diagnostic coding for Medicare patients under the prospective-payment system. N Engl J Med. 1988;318(6):352-355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement