Abstract
Background
There are few data available regarding the specificity and modifiability of major cardiovascular (CV) risk factors in patients with premature versus (vs) late-onset coronary artery disease (CAD). This study was designed to analyze and compare these risk factors.
Patients and methods
Data from 15,381 consecutive patients (mean age, 62.3 ± 11.7 years; female, 33.8%) hospitalized with CAD were collected from a large-scale registry (Transparency Registry to Objectify Guideline-Oriented Risk Factor Management) and analyzed. The patients were divided into two groups, depending on age at inclusion: group 1 patients (n = 5725; mean age, 50.5 ± 7.2 years) were males aged < 55 years and females aged < 65 years; group 2 patients (n = 9656; mean age, 69.4 ± 7.4 years) were males aged > 55 years and females aged > 65 years and had a low-density lipoprotein cholesterol level of >100 mg/dL on admission to cardiac rehabilitation. Besides the conventional risk factors, lipoprotein(a) concentrations and glucose tolerance were measured facultatively. Univariate (chi-square test) and multivariate logistic regression models were used.
Results
Cigarette smoking (group 1 at 31.5% vs group 2 at 9.4%; P < 0.001), family history of CAD (group 1 at 43.6% vs group 2 at 26.5%; P < 0.001), and dyslipidemia (group 1 at 92.7% vs group 2 at 91.8%; P < 0.001) were dominant risk factors in the younger group. Arterial hypertension (group 1 at 71.4% vs group 2 at 87.0%; P < 0.001) and diabetes (group 1 at 23.5% vs group 2 at 30.1%; P < 0.001) were dominant risk factors in the older group. Impaired glucose tolerance and diabetes were less frequent in the younger group (Ptrend = 0.038), and identical lipoprotein(a) concentration levels of >30 mg/dL were found in both groups (8.0%; P = 0.810). Modification of lipid profile and blood pressure was more effective in the younger group (low-density lipoprotein cholesterol < 100 mg/dL: group 1 at 66.3% vs group 2 at 61.1%; systolic blood pressure < 140 mmHg: group 1 at 91.7% vs group 2 at 83.0%; P < 0.001).
Conclusion
CV risk factors differ markedly between premature and non-premature CAD. Cardiac rehabilitation provides an opportunity to reinforce secondary prevention after acute coronary syndrome.
Keywords: acute coronary syndrome, premature manifestation, cardiovascular risk factors, diabetes, cholesterol
Introduction
While the majority of patients with coronary artery disease (CAD) are elderly, the proportion of younger individuals with a premature manifestation of CAD (P-CAD) is continually growing. This is owing to an increase in obesity and diabetes mellitus as well as adverse lifestyles (including smoking and physical inactivity) even in children and young adults.1,2 Premature CAD is defined as the first manifestation of CAD in male patients under 55 years of age and in female patients under 65 years of age.3 The cumulative burden of conventional modifiable cardiovascular (CV) risk factors including smoking, atherogenic dyslipidemia, diabetes, and being overweight play a key role in the progression of atherosclerotic vascular damage.4 Thus, the global risk of genetic, metabolic, and environmental risk factors determines the age of onset of CAD.
The present study was designed to analyze and compare major CV risk factors for patients with P-CAD and those with late-onset CAD. Furthermore, the authors assessed the target value attainment during an inpatient cardiac rehabilitation program and determined the measurement frequency of extended CV risk factors including lipoprotein(a) [Lp(a)] and impaired glucose tolerance (IGT) in the daily routine of German cardiac rehabilitation centers.
Methods
Patients
In total, 15,381 consecutive patients with CAD (mean age, 62.3 ± 11.7 years; 33.8% female) who were hospitalized for inpatient cardiac rehabilitation in 101 German rehabilitation centers were included in a nationwide multi-centric longitudinal registry (the Transparency Registry to Objectify Guideline-Oriented Risk Factor Management [TROL]). Patients were divided into two groups, depending on age at inclusion: group 1 had patients with P-CAD (men aged < 55 years, women aged < 65 years); group 2 had patients with late-onset CAD (men aged > 55 years, women aged > 65 years) and low-density lipoprotein cholesterol (LDL-C) levels > 100 mg/dL (>2.6 mmol/L) on admission to a cardiac rehabilitation center.
On admission, anthropometric parameters (age, gender, body mass index), type of coronary index event (ST elevation myocardial infarction [STEMI], non-STEMI, unstable angina, stable angina), and revascularization procedure (percutaneous coronary intervention, coronary artery bypass grafting [CABG]) or conservative strategy were assessed. Furthermore, comorbidities including peripheral arterial disease, previous stroke, carotid stenosis > 50%, and chronic obstructive pulmonary disease were documented.
Risk factor assessment and target values
To assess the CV risk profile, total cholesterol, LDL-C, and high-density lipoprotein cholesterol (HDL-C) levels, triglyceride (TG) level, and arterial blood pressure (BP) were determined in all patients. Lipid levels, BP, and medication were recorded on admission and again on discharge. In respect to medication, the observation comprised beta-blockers, ACE inhibitors, angiotensin II type 1 receptor blockers, statins, and cholesterol resorption antagonists.
Furthermore, family history of premature atherosclerosis, smoking behavior, and glucometabolic state (manifest diabetes mellitus) was assessed. Facultatively, the emerging CV risk factors Lp(a) and IGT were determined. IGT was defined as a fasting plasma glucose level of ≥100 mg/dL but <126 mg/dL or a 2-hour oral glucose tolerance test (OGTT) plasma level of ≥140 mg/dL but <200 mg/dL. Diabetes was characterized by a fasting plasma glucose level ≥126 mg/dL and/or a 2-hour OGTT plasma glucose level of ≥200 mg/dL.5
The target BP value for patients after acute coronary syndrome (ACS) was <140/90 mmHg. The target HDL-C levels were >40 mg/dL (>1 mmol/L) and the target LDL-C levels were <100 mg/dL (<2.6 mmol/L). All patients were to achieve a fasting glucose level of <126 mg/dL (<7.0 mmol/L) and a TG level of <150 mg/dL (<1.7 mmol/L).
The local laboratories at the respective rehabilitation centers, which are obliged to take part in quality control measures at regular intervals, performed the laboratory tests.
Cardiac rehabilitation program
In Germany, the vast majority of patients at high cardiac risk – in particular, after ACS or CABG – are entitled to undergo inpatient rehabilitation therapy. This is conducted in specialized institutions and usually lasts 3–4 weeks. According to recent guidelines, rehabilitation programs include individualized physical training, disease information, structured teaching programs for reduction of CV risk factors, and psychological support.6 The protocol of the present study was reviewed and approved by the ethics review board of the Bavarian Chamber of Physicians, Munich.
Statistical analysis
Discrete variables are given as counts and percentages, and continuous variables are given as mean plus or minus standard deviation. Univariate between-group comparisons for nominal variables were performed using chi-square test, Cochran-Armitage trend tests were used for ordinal variables, and estimation of variance (F-test) was used for continuous variables. Logistic regression analysis with backward selection was applied to determine the simultaneous influence of conventional CV risk factors on belonging to the premature or the late-onset CAD group. A nominal P-value of < 0.05 was considered statistically significant. No adjustment for multiplicity was performed. Analyses were performed using statistical software (SAS, v 9.2; SAS Institute Inc, Cary, NC).
Results
Group 1 patients had a mean age of 50.5 ± 7.2 years, while the mean age of group 2 patients was 69.4 ± 7.4 years (P < 0.001). Female gender, a higher body mass index, STEMI, non-STEMI, and interventional revascularization during the acute coronary event were found to be more frequent in group 1. Group 2 patients demonstrated a significantly higher proportion of all comorbidities, including peripheral arterial disease, stroke, carotid stenosis, and chronic obstructive pulmonary disease, and group 2 patients were treated to a greater extent with aortocoronary bypass grafting during the target coronary event. Because of a complex revascularization approach in some patients (primary percutaneous coronary intervention of target vessel and consecutive CABG), the summary of interventions exceeds 100% in both age groups (Table 1).
Table 1.
Baseline characteristics and functional parameters
| Group 1 (n = 5725) | Group 2 (n = 9656) | P-value | |
|---|---|---|---|
| Age (years)* | 50.5 ± 7.2 | 69.4 ± 7.4 | <0.001 |
| Female (%) | 37.6 | 31.6 | <0.001 |
| Height (cm)* | 171.9 ± 9.3 | 169.4 ± 9.2 | <0.001 |
| Weight (kg)* | 82.8 ± 15.6 | 78.5 ± 13.5 | <0.001 |
| Body mass index (kg/m2)* | 30.0 ± 4.7 | 27.4 ± 4.1 | <0.001 |
| Diagnosis | |||
| STEMI (%) | 55.3 | 39.9 | <0.001 |
| NSTEMI (%) | 15.0 | 11.3 | <0.001 |
| Unstable AP (%) | 18.8 | 24.0 | <0.001 |
| Stabile CAD | 18.9 | 24.8 | <0.001 |
| Therapy | |||
| Primary PCI (%) | 67.2 | 48.6 | <0.001 |
| CABG (%) | 38.1 | 56.7 | <0.001 |
| Conservative (%) | 22.3 | 28.9 | <0.001 |
| Comorbidity | |||
| PAD (%) | 6.9 | 13.9 | <0.001 |
| Stroke (%) | 7.2 | 13.1 | <0.001 |
| Carotid stenosis > 50% (%) | 3.1 | 8.2 | <0.001 |
| COPD (%) | 7.1 | 11.3 | <0.001 |
Note:
Data presented as mean plus or minus standard deviation.
Abbreviations: STEMI, ST elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; AP, angina pectoris; CAD, coronary artery disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; PAD, peripheral arterial disease.
In univariate analysis, current cigarette smoking and a positive family history of CAD were discovered in a higher proportion in the younger group, whereas arterial hypertension predominated in group 2 (Table 2). On admission, almost a quarter of patients in the younger group offered manifest diabetes mellitus, while every third late-onset patient was diabetic (group 1 at 23.5% versus [vs] group 2 at 30.1%; P < 0.001). The OGTT was performed in 20.0% of group 1 patients compared with 6.1% of group 2 patients without a documented history of glucometabolic disorder. Of these patients, IGT was identified in every tenth patient (11.2%) in group 1 and in every sixth patient (16.0%) in group 2. Manifest diabetes was identified by OGTT in 2.0% of group 1 patients vs 2.3% of group 2 patients (Ptrend = 0.038). Lp(a) was determined in 20.2% of group 1 patients and 4.8% of group 2 patients, with identical pathologic levels > 30 mg/dL in both groups (8.0%).
Table 2.
Conventional and extended cardiovascular risk factors
| Group 1 (n = 5725) | Group 2 (n = 9656) | P-value | |
|---|---|---|---|
| Conventional risk factors | |||
| Dyslipidemia (%) | 92.7 | 91.8 | 0.04 |
| Arterial hypertension (%) | 71.4 | 87.0 | <0.001 |
| Current smoker (%) | 31.5 | 9.4 | <0.001 |
| Former smoker (%) | 48.7 | 40.0 | <0.001 |
| Family history (%) | 43.6 | 26.5 | <0.001 |
| Diabetes mellitus (%) | 23.5 | 30.1 | <0.001 |
| OGTTa | |||
| Performed [n (%)] | 842 (20.0%) | 393 (6.1%) | <0.001 |
| IGT [n (%)] | 94 (11.2) | 63 (16.0) | 0.038 |
| Diabetes [n (%)] | 17 (2.1) | 9 (2.3) | 0.038 |
| Lp(a) | |||
| Determined [n (%)] | 1154 (20.2) | 446 (4.8) | <0.001 |
| >30 mg/dL [n (%)] | 90 (7.8) | 38 (8.2) | 0.810 |
| Determined Lp(a) (mg/dL)b | 10.8 ± 20.9 | 10.3 ± 22.3 | 0.022 |
Notes:
In patients without documented diabetes;
data presented as mean plus or minus standard deviation.
Abbreviations: OGTT, oral glucose tolerance test; IGT, impaired glucose tolerance; Lp(a), lipoprotein(a).
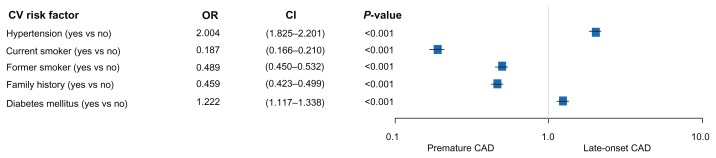
In multivariate analysis, arterial hypertension and diabetes favored late-onset CAD, whereas particularly current and former cigarette smoking as well as family history of CAD were significantly correlated with premature CAD (Figure 1).
Figure 1.
Cardiovascular (CV) risk factors associated with premature versus (vs) late-onset coronary artery disease (CAD).
Abbreviations: OR, odds ratio; CI, confidence interval.
Risk factor management
On admission to cardiac rehabilitation, the mean systolic BP of group 1 patients was lower than that of group 2 patients (group 1 at 129.7 ± 20.6 mmHg vs group 2 at 135.2 ± 21.0 mmHg; P < 0.001), whereas the mean diastolic BP was higher (group 1 at 79.3 ± 11.8 mmHg vs group 2 at 77.7 ± 11.3 mmHg; P < 0.001) in the younger patients. Further BP control was achieved in both age groups (systolic BP: −5.2 mmHg in group 1 and −4.8 mmHg in group 2, P < 0.001; diastolic BP: −10.3 mmHg in group 1 and −12.0 mmHg in group 2, P = 0.074). All lipid fractions could be optimized during cardiac rehabilitation. BP and lipid profile on admission and on discharge are demonstrated in Table 3.
Table 3.
Trend of arterial hypertension, lipid profile, and plasma glucose level during cardiac rehabilitation
| Admission | Discharge | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Group 1 | Group 2 | P-value | Group 1 | Group 2 | P-value | |
| RR syst (mmHg) | 129.7 ± 20.6 | 135.2 ± 21.0 | <0.001 | 119.5 ± 13.2 | 123.3 ± 14.0 | <0.001 |
| RR diast (mmHg) | 79.3 ± 11.8 | 77.7 ± 11.3 | <0.001 | 74.1 ± 8.6 | 73.0 ± 8.7 | <0.001 |
| Total-C (mg/dL) | 199.8 ± 47.6 | 215.3 ± 38.6 | <0.001 | 164.4 ± 33.4 | 168.5 ± 33.1 | <0.001 |
| LDL-C (mg/dL) | 121.9 ± 38.5 | 137.6 ± 29.0 | <0.001 | 91.8 ± 26.6 | 97.2 ± 26.9 | <0.001 |
| HDL-C (mg/dL) | 42.5 ± 13.3 | 44.7 ± 12.6 | <0.001 | 43.0 ± 12.0 | 44.8 ± 12.0 | <0.001 |
| TG (mg/dL) | 172.8 ± 78.5 | 157.5 ± 65.4 | <0.001 | 149.9 ± 66.7 | 134.3 ± 53.2 | <0.001 |
| FG (mg/dL) | 102.0 ± 27.9 | 108.6 ± 30.0 | <0.001 | 99.7 ± 23.1 | 104.5 ± 22.8 | <0.001 |
Note: Data other than P-values are presented as mean plus or minus standard deviation.
Abbreviations: RR syst, systolic blood pressure; RR diast, diastolic blood pressure; Total-C, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, highdensity lipoprotein cholesterol; TG, triglyceride; FG, fasting glucose plasma level.
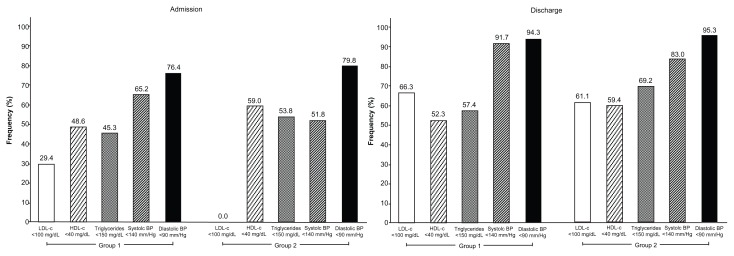
Target value attainment differed significantly between groups. LDL-C levels < 100 mg/dL on discharge were more frequent in group 1, whereas TG levels < 150 mg/dL and HDL-C levels > 40 mg/dL was found to be more frequent in group 2. On discharge from rehabilitation the proportion of systolic BPs below 140 mmHg and diastolic BPs below 90 mmHg was high (Figure 2).
Figure 2.
Target value attainment after cardiac rehabilitation: comparison of groups 1 and 2.
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BP, blood pressure.
ACE inhibitors (group 1 at 77.1% vs group 2 at 78.9%; P = 0.01) were given to a lesser extent in group 1 than in group 2; beta-blockers (group 1 at 92.2% vs group 2 at 88.4%; P < 0.001) were given to a greater extent in group 1 than in group 2. The prescription of angiotensin II type 1 receptor blockers was comparably low between both groups (group 1 at 13.4% vs group 2 at 12.5%; P = 0.133), 94.7% of group 1 patients vs 89.1% of group 2 patients (P < 0.001) received statins, and 39.0% of group 1 patients vs 45.5% of group 2 patients received cholesterol resorption inhibitors (P < 0.001).
Discussion
There were three important findings from this study. First, based on 15,381 consecutive ACS patients, the authors found considerable difference of CV risk factor pattern between patients with P-CAD and patients with late-onset CAD. Second, in both groups the proportion of patients with diabetes or IGT was notably high; nevertheless, the frequency of routine performance of OGTT in post-ACS patients without known glucometabolic disorder is limited. Finally, cardiac rehabilitation after an acute coronary event is a powerful tool to optimize attainment of target BP values in both age groups. The influence of cardiac rehabilitation is shown predominantly in the younger group, whereas lipid profile, particularly TG and HDL-C levels, is less modifiable during the 3 weeks of cardiac rehabilitation.
Risk factor pattern of patients with premature vs late-onset CAD
Corresponding to other data,7 the authors found a “malignant triad” of smoking, LDL-C-related dyslipidemia, and family history of CAD particularly in younger patients. The proportion of current or former cigarette smokers in group 1 was notable at about 80%, whereas only 49.4% of group 2 patients reported regular current or former tobacco consumption. This indicates an age-dependent divergent importance of the connection between smoking and microvascular damage. Smoking initiates and promotes atherosclerosis by altering cardiac hemodynamics, causing dyslipidemia and increased production of free oxygen radicals by oxidative stress of nicotine. 7 According to the INTERHEART study,4 cigarette smoking is associated with a higher CV risk than diabetes, arterial hypertension, abdominal obesity, and adverse psychosocial factors, and it was outperformed only by a raised apolipoprotein B/apolipoprotein A-I ratio. Predominantly young smoking women are at risk for premature manifestation of CAD followed by a significant reduction in life expectancy. 8,9 Despite this, the results of the three EUROASPIRE surveys demonstrate an increase in smoking rates, particularly in younger female patients, from 1996 to 2007.2 Panagiotakos et al10 reported that cigarette smoking was the most important risk factor for having a myocardial infarction in individuals under the age of 36 years, with a sixfold increased risk for reinfarction after the first acute coronary event. In comparison with nonsmokers, cigarette smoking is associated with an average lifetime loss of 10 years.11 Although an excess risk continues for more than 20 years after smoking cessation,12 the quitting after CABG gained approximately 3 years’ life expectancy and has shown to be the most important therapeutic approach in the follow-up of surgical revascularization.13 In 2007, Germany started a smoking restriction in the public and hospitality sectors. After just 1 year, this law implementation was associated with a 13.3% decline in angina pectoris and an 8.6% decline in myocardial infarction, saving approximately 7.7 million Euros in hospitalization costs.14 Thus, smoking cessation and interdisciplinary precautions to prevent smoking relapse should remain stringent goals, particularly in young patients with CAD.
Furthermore, young patients are characterized by a higher incidence of LDL-C-related dyslipidemia; the proportion (>90%) is comparable with those of other large-scale registries. 7 Although dyslipidemia is strongly associated with a genetic component, modifiable cofactors including physical inactivity and being overweight have been shown to reinforce lipid profile disorders.15
A comparable adverse causal complex is applicable for arterial hypertension, which was found in the majority of younger patients (although to a lesser extent than in older patients). In such a constellation, arterial hypertension, dyslipidemia, smoking, and being overweight can act as a trigger for the manifestation of CAD, particularly in younger patients.16
Besides remediable CV risk parameters, the authors found an impressive proportion of group 1 patients with a positive family history of P-CAD. Andresdottir et al17 reported that independently of obesity, cholesterol, and BP levels, up to 16.6% of coronary events were attributable to family history of CAD. The importance of family history is considerable, particularly in young women; nevertheless, young women with a genetic background of CAD generally demonstrate less CV risk awareness and worse lifestyle behaviors than those without family history of CAD.18 A large-scale study including more than 50,000 individuals reported that paternal as well as maternal history of myocardial infarction up to a manifestation age > 80 years is associated with increased CAD risk for the following generation.19 The highest risk was described for women aged < 50 years with premature maternal infarction, with an odds ratio of 2.57, which is comparable with the prognostic impact of smoking or manifest diabetes. In the presence of coexisting CV risk factors the importance of family history of CAD is further increased.20
Glucometabolic disorders
Type 2 diabetes constitutes one of the common final results of sedentary behavior, obesity, and adverse eating behavior, whereas each point separately offers an additional major CV risk factor.21,22 In the light of increased mortality of diabetics with ACS compared with nondiabetic patients, the fundamental goal should be the evaluation of a pathologic glucose metabolism by a routinely performed OGTT.23 Astonishingly, particularly in the older group the OGTT was performed quite rarely across the German rehabilitation centers.
In the observed patients, the percentage of diabetics was comparable with prior studies, with a proportion of 13%–30%.2,20,24 The OGTT identified IGT, a prognostic parameter,25 in a high percentage of patients and predominantly in the older group. In the Euro Heart Survey on diabetes and the heart,26 the rate of patients with unknown IGT who were referred to undergo elective or acute angiography was remarkable at 39%. This high prevalence of glucometabolic disorders is confirmed by other study groups.27 Lankisch et al28 reported that if all patients with a fasting glucose of ≥90 mg/dL would be referred to undertake the OGTT, the identification rate of diabetes would be increased from 28.1% (usually the OGTT is performed if the fasting glucose level is ≥126 mg/dL) to 93.8%. Notably, the OGTT performed immediately after a STEMI did not provide reliable information on the long-term glucometabolic state; thus, a delay after the acute coronary event may be desirable.29
Extended CV risk factors
In young adults with P-CAD, intravascular thrombogenesis as a complex interplay of a procoagulant state, fibrinolysis, endothelial dysfunction, and inflammation may be an additional parameter besides conventional CV risk factors.30 A near normal LDL-C level can be found in up to 40% of CAD patients; thus, discussion regarding emerging CV risk factors – including high-sensitivity C-reactive protein, Lp(a), and homocysteine – causing the inflammation theory of CAD is ongoing.31
Lp(a) is a genetically determined lipid fraction that has proven to be a relevant cofactor in the genesis and progression of atherosclerosis. The pharmacological approach in lowering Lp(a) levels is still limited, although reducing the oxidized LDL-C levels in patients with pathologic Lp(a) concentration levels may be of prognostic influence.32 Additionally, more recently a genetic background for the prognostic implication of Lp(a) independent of LDL-C has been described.33 The increased awareness of accompanying risk factors in younger patients might explain the higher rate of Lp(a) measurements in group 1 during cardiac rehabilitation; however, the Lp(a) concentration was only determined in every fifth group 1 patient and in <5% of group 2 patients. Whereas other authors describe a higher rate of pathologic Lp(a) levels in P-CAD patients,34 the present authors found comparable increased values across both age groups. However, while 92% of young patients revealed an elevated LDL-C level, a determination of Lp(a) levels and other risk factors should be used to estimate overall risk for CAD progression. In the case of elevated Lp(a) in patients with P-CAD, an aggressive lipid-lowering treatment with statins at low to normal values should be aimed for.35
Risk factor management
Cardiac rehabilitation provides a substantial benefit in management of major CV risk factors including lipid profile and BP in patients with CV diseases.36 The 3- to 4-week inpatient cardiac rehabilitation in Germany is designed according to the recommendations of national and European Society of Cardiology guidelines37 and offers the opportunity not only to monitor patients after ACS but also to reinforce secondary preventive approaches in the long-term treatment of CAD. Cardiac rehabilitation includes a gradual increase in activity levels, as well as continuous and individually focused information about effective lifestyle modification, smoking cessation, and dietary counseling.
Although recent guidelines recommend target BP values of <130/80 mmHg for patients with manifest CAD, the present authors focused on conservative values of <140/90 mmHg because of the limited treatment period during cardiac rehabilitation. In the TROL, after a 3-week inpatient cardiac rehabilitation program, BP values of <140/90 mmHg were attained in a high proportion of all patients, with a predominance shown in younger patients because of higher baseline BP levels in the late-onset CAD group. Only diastolic BP in group 2 patients could be further optimized, which can be explained by an even lower diastolic BP in older patients due to increased vascular stiffness. The comparable medication across both groups might be a crucial point of reduced efficacy in BP therapy, indicating the need for intensified management of hypertensive patients of a particularly advanced age.
The European Society of Cardiology’s guidelines on CVD prevention in clinical practice have been recently updated.37 Of note, these guidelines already suggest stronger lipid goals of LDL-C for all ACS patients, irrespective of a glucometabolic situation below 70 mg/dL (1.8 mmol/L). Regarding LDL-C targets, the present authors still focused on the 100 mg/dL (2.6 mmol/L) threshold according to National Cholesterol Education Program – Adult Treatment Panel III (NCEP ATP III) criteria.38 The NCEP ATP III target value was achieved by a moderate proportion of both patient groups during cardiac rehabilitation. An early and intensive statin therapy is superior to moderate statin doses, particularly in patients after ACS, and leads to a reduced mortality by −25% over a follow-up period of 2 years.39 Compared with other large registries (eg, the Global Registry of Acute Coronary Events or the Register of Information and Knowledge about Swedish Heart Intensive Care Admissions) with a prescription rate of <50%, in the TROL the statin therapy rates of 94.7% and 89.1% in groups 1 and 2, respectively, can hardly be increased.40,41 Because of a high prescription rate of statins, the LDL-C lipid fraction demonstrated a favorable trend during cardiac rehabilitation; however, the less influenceable HDL-C and fasting TG levels remained inadequate. Plasma TG levels in particular were above the NCEP ATP III limits in every second patient from group 1, implying a prolongation of comprehensive lifestyle change. Nevertheless, there is no strong evidence that fasting TG levels < 150 mg/dL are associated with a decreased coronary risk. It remains to be analyzed whether non-fasting TG levels are better indicators for further events.38 Similarly, the target values for HDL-C could only be attained in over half of the patients in both groups. Because of the independent prognostic impact of TG and reduced HDL-C on CAD progression these lipid values, as well as LDL-C, should be carefully observed.42
To identify patients with a constellation of high CV risks and offer them eligible and sustentative interventions is highly desirable. In patients with established CAD, participation in regular, individually designed public training programs has been proven to reduce plasma lipids, inflammation parameters, excess weight, depression, and psychosocial stress levels.43,44 Aerobic exercise training is associated with reduced mortality, in healthy individuals, those with a CV risk profile, and cardiac patients.45 Recent European guidelines recommend physical activity at a mild to moderate intensity for 30 minutes at least three times a week.37
In Germany, many health insurance companies support preventive medicine sports groups with regular training sessions within the framework of heart disease management programs.46
Although there is compelling evidence of beneficial outcomes, the divergence between guideline-based recommendations and the clinical reality in treatment of CAD patients is worrisome. The large-scale European REACH (Reduction of Atherothrombosis for Continued Health) registry demonstrated a 24% reduction in CV event rate and an 11% reduction in mortality if conventional risk factors are strictly adhered to; nevertheless, this treatment is deemed to be adequate in only 60% of patients with established atherosclerotic disease.47 The three EUROASPIRE surveys2,48 describe a continuing gap between theory and practice in lifestyle interventions and drug therapy. Thus, there is an urgent need for increased sensibility regarding CV risk factor control and enhanced individual responsibility in CAD treatment.
Study limitations
This study has several limitations. First, the study was conducted as a prospective observational study, performed in German rehabilitation clinics. The results reflect the conditions of selected patients with stringent observation of risk factors, lifestyle, and medication. Participation in the cardiac rehabilitation was voluntary and not without a selection bias. Second, in the given registry the authors exclusively focused on documentation of age-dependent CV risk factors and their modifiability during inpatient cardiac rehabilitation. Follow-up and outcome data after optimization of CV risk factors were missing.
Differences regarding the physical activity level were not registered and should be acknowledged. Furthermore, the authors do not have data regarding eating behavior in both groups. As only instantaneous data on the constellation of risk during cardiac rehabilitation were collected, the authors do not have information regarding continuous data – including exercise and dietary data prior to admission.
Because of the limited measurement rate, reliable information regarding Lp(a) levels was missed. Third, there was an inhomogeneity in characteristics of CAD within the groups, including patients with stable CAD, those after an acute coronary event, and those after interventional and/or surgical revascularization. Finally, newly described risk factors such as high-sensitivity C-reactive protein were excluded from the observation because of the high percentage of surgically revascularized patients with postoperatively increased C-reactive protein levels.
Conclusion
In patients with P-CAD, cigarette smoking and family history of CAD were found to be more frequent in younger patients than in their older counterparts. Even in young patients, the incidence of glucometabolic disorder, including IGT and manifest diabetes, is alarming high. Because of the synergistic effect of CV risk factors and positive family history, the reduction of modifiable risk parameters and smoking cessation is strongly required – particularly in younger patients with P-CAD. Cardiac rehabilitation after an acute coronary event is a powerful tool to optimize attainment of target lipid profile and BP values in both age groups. The influence of cardiac rehabilitation is shown predominantly in the younger group, and it may have a positive effect on patient-related outcome.
Acknowledgments
The TROL was run in cooperation with the German Society for Prevention and Rehabilitation of Cardiovascular Diseases. The registry was supported by an educational grant-in-aid from MSD Sharp and Dohme GmbH, Munich-Haar, Germany.
Footnotes
Disclosure
KB and BK are employees of MSD. No competing financial interests exist for any authors. HV received travel expenses from MSD Sharp and Dohme GmbH and Essex Pharma GmbH. Parts of this work have been presented at the annual congress of the European Society of Cardiology in 2005 in Stockholm, Sweden.
References
- 1.Franklin BA, Trivax JE, Vanhecke TE. New insights in preventive cardiology and cardiac rehabilitation. Curr Opin Cardiol. 2008;23(5):477–486. doi: 10.1097/HCO.0b013e32830b360b. [DOI] [PubMed] [Google Scholar]
- 2.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U for EUROASPIRE Study Group. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373(9667):929–940. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 3.Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyörälä K. Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Eur Heart J. 1998;19(10):1434–1503. doi: 10.1016/s0021-9150(98)90209-x. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, et al. for INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Department of Noncommunicable Disease Surveillance, World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Part 1. Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. Report no WHO/NCD/NCS/99.2. [Google Scholar]
- 6.Karoff M, Held K, Bjarnason-Wehrens B. Cardiac rehabilitation in Germany. Eur J Cardiovasc Prev Rehabil. 2007;14(1):18–27. doi: 10.1097/HJR.0b013e3280128bde. [DOI] [PubMed] [Google Scholar]
- 7.Schoenenberger AW, Radovanovic D, Stauffer JC, et al. for AMIS Plus Investigators. Acute coronary syndromes in young patients: presentation, treatment and outcome. Int J Cardiol. 2011;148(3):300–304. doi: 10.1016/j.ijcard.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Heitzer T, Meinertz T. Prevention of coronary heart disease: smoking. Z Kardiol. 2005;94(Suppl 3):III/30–42. doi: 10.1007/s00392-005-1306-y. German. [DOI] [PubMed] [Google Scholar]
- 9.Grundtvig M, Hagen TP, German M, Reikvam A. Sex-based differences in premature first myocardial infarction caused by smoking: twice as many years lost by women as by men. Eur J Cardiovasc Prev Rehabil. 2009;16(2):174–179. doi: 10.1097/HJR.0b013e328325d7f0. [DOI] [PubMed] [Google Scholar]
- 10.Panagiotakos DB, Rallidis LS, Pitsavos C, Stefanadis C, Kremastinos D. Cigarette smoking and myocardial infarction in young men and women: a case-control study. Int J Cardiol. 2007;116(3):371–375. doi: 10.1016/j.ijcard.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teo KK, Ounpuu S, Hawken S, et al. for INTERHEART Study Investigators. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(8536):647–658. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 13.Van Domburg RT, op Reimer WS, Hoeks SE, Kappetein AP, Bogers AJ. Three life-years gained from smoking cessation after coronary artery bypass surgery: a 30-year follow-up study. Am Heart J. 2008;156(3):473–476. doi: 10.1016/j.ahj.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Sargent JD, Demidenko E, Malenka DJ, Li Z, Gohlke H, Hanewinkel R. Smoking restrictions and hospitalization for acute coronary events in Germany. Clin Res Cardiol. 2012;101(3):227–235. doi: 10.1007/s00392-011-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hata Y, Nakajima K. Life-style and serum lipids and lipoproteins. J Atheroscler Thromb. 2000;7(4):177–197. doi: 10.5551/jat1994.7.177. [DOI] [PubMed] [Google Scholar]
- 16.Hurrell C, Wietlisbach V, Jotterand V, et al. High prevalence of major cardiovascular risk factors in first-degree relatives of individuals with familial premature coronary artery disease: the GENECARD project. Atherosclerosis. 2007;194(1):253–264. doi: 10.1016/j.atherosclerosis.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Andresdottir MB, Sigurdsson G, Sigvaldason H, Gudnason V for Reykjavik Cohort Study. Fifteen percent of myocardial infarctions and coronary revascularizations explained by family history unrelated to conventional risk factors: the Reykjavik Cohort Study. Eur Heart J. 2002;23(21):1655–1663. doi: 10.1053/euhj.2002.3235. [DOI] [PubMed] [Google Scholar]
- 18.Patel MJ, de Lemos JA, Philips B, et al. Implications of family history of myocardial infarction in young women. Am Heart J. 2007;154(3):454–460. doi: 10.1016/j.ahj.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104(4):393–398. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 20.Philips B, de Lemos JA, Patel MJ, McGuire DK, Khera A. Relation of family history of myocardial infarction and the presence of coronary arterial calcium in various age and risk factor groups. Am J Cardiol. 2007;99(6):825–829. doi: 10.1016/j.amjcard.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 21.Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. 2010;42(5):879–885. doi: 10.1249/MSS.0b013e3181c3aa7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 23.Bartnik M, Rydén L, Malmberg K, et al. for Euro Heart Survey Investigators. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart. 2007;93(1):72–77. doi: 10.1136/hrt.2005.086975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Völler H, Reibis R, Pittrow D, et al. Secondary prevention of diabetic patients with coronary artery disease in cardiac rehabilitation: risk factors, treatment and target level attainment. Curr Med Res Opin. 2009;25(4):879–890. doi: 10.1185/03007990902801360. [DOI] [PubMed] [Google Scholar]
- 25.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299–302. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drechsler K, Fikenzer S, Sechtem U, et al. The Euro Heart Survey – Germany: diabetes mellitus remains unrecognized in patients with coronary artery disease. Clin Res Cardiol. 2008;97(6):364–370. doi: 10.1007/s00392-008-0643-z. [DOI] [PubMed] [Google Scholar]
- 27.De la Hera JM, Delgado E, Hernández E, et al. Prevalence and outcome of newly detected diabetes in patients who undergo percutaneous coronary intervention. Eur Heart J. 2009;30(21):2614–2621. doi: 10.1093/eurheartj/ehp278. [DOI] [PubMed] [Google Scholar]
- 28.Lankisch M, Füth R, Schotes D, et al. High prevalence of undiagnosed impaired glucose regulation and diabetes mellitus in patients scheduled for an elective coronary angiography. Clin Res Cardiol. 2006;95(2):80–87. doi: 10.1007/s00392-006-0328-4. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen EC, Seljeflot I, Abdelnoor M, et al. Abnormal glucose regulation in patients with acute ST-elevation myocardial infarction: a cohort study on 224 patients. Cardiovasc Diabetol. 2009;8:6–14. doi: 10.1186/1475-2840-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pineda J, Marín F, Marco P, et al. The prognostic value of biomarkers after a premature myocardial infarction. Int J Cardiol. 2010;143(3):249–254. doi: 10.1016/j.ijcard.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 32.Erqou S, Kaptoge S, Perry PL, et al. for Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke R, Peden JF, Hopewell JC, et al. for PROCARDIS Consortium. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 34.Pineda J, Marín F, Marco P, et al. Premature coronary artery disease in young (age < 45) subjects: interactions of lipid profile, thrombophilic and haemostatic markers. Int J Cardiol. 2009;136(2):222–225. doi: 10.1016/j.ijcard.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Bassand JP, Hamm CW, Ardissino D, et al. for Task Force for Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of European Society of Cardiology. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28(13):1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 36.Sarrafzadegan N, Rabiei K, Kabir A, et al. Changes in lipid profile of patients referred to a cardiac rehabilitation program. Eur J Cardiovasc Prev Rehabil. 2008;15(4):467–472. doi: 10.1097/HJR.0b013e328300271f. [DOI] [PubMed] [Google Scholar]
- 37.Perk J, De Backer G, Gohlke H, et al. for Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice; European Association for Cardiovascular Prevention and Rehabilitation. European guidelines on cardiovascular disease prevention in clinical practice (version 2012. Eur Heart J. doi: 10.1093/eurheartj/ehs092. Epub May 3, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Grundy SM, Brewer HB, Jr, Leeman JI, Smith SC, Jr, Lenfant C for American Heart Association, National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–448. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 39.Baigent C, Keech A, Kearney PM, et al. for Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 40.Franklin K, Goldberg RJ, Spencer F, et al. for GRACE Investigators. Implications of diabetes in patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2004;164(13):1457–1463. doi: 10.1001/archinte.164.13.1457. [DOI] [PubMed] [Google Scholar]
- 41.Rydén L, Standl E, Bartnik M, et al. for Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 42.Kannel WB, Vasan RS. Triglycerides as vascular risk factors: new epidemiologic insights. Curr Opin Cardiol. 2009;24(4):345–350. doi: 10.1097/HCO.0b013e32832c1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavie CJ, Milani RV. Cardiac rehabilitation and exercise training in secondary coronary heart disease prevention. Prog Cardiovasc Dis. 2011;53(6):397–403. doi: 10.1016/j.pcad.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Vanhees L, Rauch B, Piepoli M, et al. for EACPR writing group. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular disease (Part III) Eur J Prev Cardiol. doi: 10.1177/2047487312437063. Epub May 25, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 46.Busse R. Disease management programs in Germany’s statutory health insurance system. Health Aff (Millwood) 2004;23(3):56–67. doi: 10.1377/hlthaff.23.3.56. [DOI] [PubMed] [Google Scholar]
- 47.Cacoub PP, Zeymer U, Limbourg T, et al. for REACH Registry Investigators. Effects of adherence to guidelines for the control of major cardiovascular risk factors on outcomes in the REduction of Atherothrombosis for Continued Health (REACH) Registry Europe. Heart. 2011;97(8):660–667. doi: 10.1136/hrt.2010.213710. [DOI] [PubMed] [Google Scholar]
- 48.EUROASPIRE I and II Group; European Action on Secondary Prevention by Intervention to Reduce Events. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. Lancet. 2001;357(9261):995–1001. doi: 10.1016/s0140-6736(00)04235-5. [DOI] [PubMed] [Google Scholar]