Abstract
Objectives
The aim of this study was to investigate the conformational change of arterial structure in the vasospastic lesion with optical coherence tomography.
Background
Coronary artery spasm plays an important role in the pathogenesis of ischemic heart diseases. The conformational change of each arterial layer during vasospasm has not been studied in detail.
Methods
We assessed 19 coronary arteries (10 spasm and 9 nonspasm lesions) with optical coherence tomography during the provocation test for coronary spasm. An intimal bump was defined as 1 or more intimal projections into the lumen that disappeared after the administration of nitroglycerine (NTG). Intimal gathering was defined as a folding/gathering of the intima, resulting in multiple kinks in the luminal contour that resolved after the administration of NTG.
Results
The spasm lesion more frequently showed an intimal bump at baseline and intimal gathering during spasm compared with the nonspasm lesion (spasm 80% vs. nonspasm 0%, p < 0.01, spasm 100% vs. nonspasm 0%, p < 0.01, respectively). The spasm lesion demonstrated a thicker maximum media thickness (spasm 0.24 ± 0.04 mm vs. nonspasm 0.12 ± 0.03 mm, p < 0.01) at baseline, whereas no differences were observed after the administration of NTG (spasm 0.13 ± 0.03 mm vs. nonspasm 0.13 ± 0.02 mm, p = 0.65).
Conclusions
Our results suggest that medial contraction occurs even in an asymptomatic state and facilitates the formation of an intimal bump in patients with vasospastic angina. Luminal narrowing during spasm is associated with intimal gathering without alteration of intimal area.
Keywords: coronary, imaging, optical coherence tomography, spasm
Coronary artery spasm plays an important role in the pathogenesis of ischemic heart disease, including variant angina, myocardial infarction with or without ST-segment elevation, and ventricular arrhythmia (1–5). Although the etiology of coronary artery spasm is believed to be due to transient abnormal or hypersensitive vascular smooth muscle in response to various stimuli (2,3), the lack of a tool for monitoring spasm in vivo has hampered our ability to study the pathophysiology of the vascular wall during spasm.
Optical coherence tomography (OCT) is a new high-resolution imaging (10 to 20 µm spatial resolution) modality (6–8). Optical coherence tomography has a unique ability to assess each intima and medial layer individually in vivo (9). We hypothesized that OCT could reveal detailed conformational changes that might occur in each arterial wall layer during vasospasm. The aim of this study was to use OCT to investigate the conformational change of the coronary artery wall microstructure in vasospastic lesions.
Methods
Subjects
We enrolled 19 patients who were admitted to Wakayama Medical University Hospital or Osaka Ekisaikai Hospital from October 2008 to July 2010 to evaluate spontaneous chest pain at rest by a provocation test with acetylcholine or ergonovine (Erg). Our exclusion criteria comprised severe stenosis in a coronary artery on coronary angiography, congestive heart failure, cardiomyopathy, history of fatal arrhythmia, history of prior myocardial infarction, history of cardiogenic shock, and chronic renal failure. This study complies with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Wakayama Medical University. We also obtained written informed consent from all participants before initial coronary angiography.
OCT imaging and provocation protocol
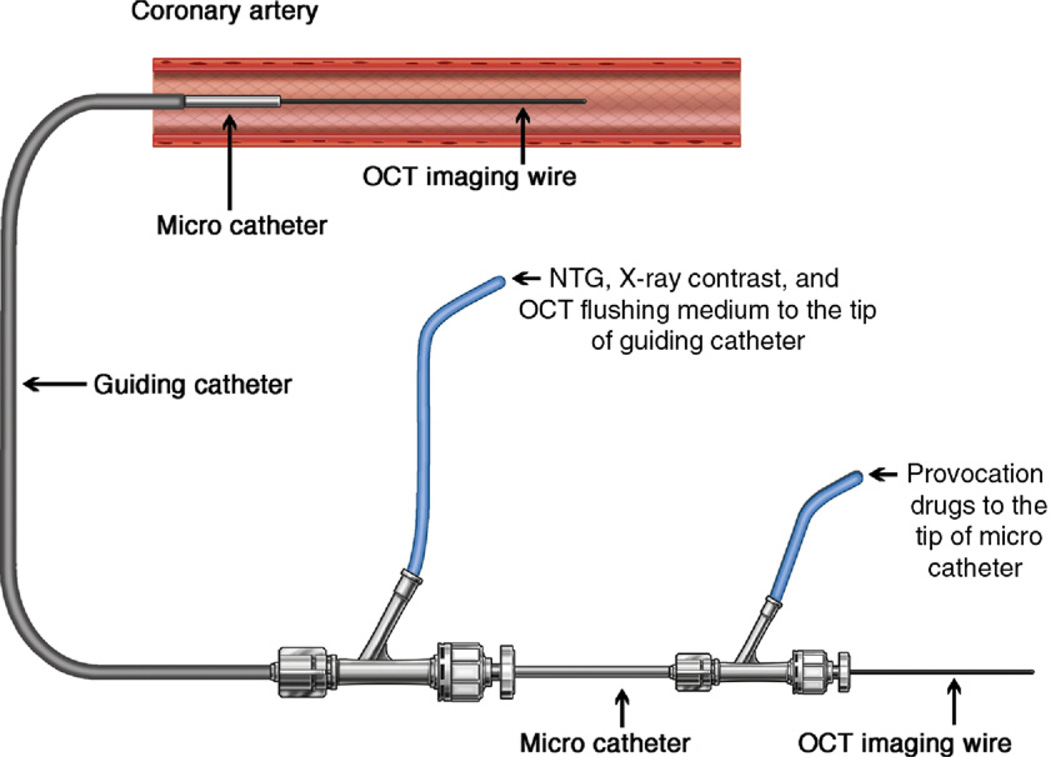
All calcium channel blockers and nitrates were discontinued 1 week before angiography. After completion of diagnostic coronary angiography, 1 coronary artery was randomly selected for the provocation test with OCT imaging (baseline-OCT) before the provocation test. We assembled special equipment for conducting the provocation test with OCT (Fig. 1). A 0.016-inch OCT image wire (ImageWire, LightLab Imaging, Westford, Massachusetts) with a continuous-flushing (nonocclusive) technique for OCT image acquisition was applied in this study (10). The coronary artery was imaged with an automatic pullback device traveling at 1 mm/s. After storing all raw data in the LightLab Imaging M2 console, the raw OCT data were transferred to open-source software, ImageJ (National Institutes of Health, Bethesda, Maryland) for image interpretation (11). After baseline-OCT imaging, incremental doses of acetylcholine (10, 20, 50 µg) or Erg (5, 10, 20, 40, 60 µg) were injected into the coronary artery from the tip of the microcatheter to provoke coronary spasm. During injection of acetylcholine or Erg, the coronary lumen diameter was simultaneously monitored under fluoroscopy with the intermittent injections of X-ray contrast from the tip of the guiding catheter. When spasm was provoked or the concentration of drugs reached at maximum dose, an infusion of provocation drug was immediately suspended and the administration route was locked. Then, OCT image acquisition was started (provocation-OCT). Immediately after the provocation-OCT imaging, a sufficient amount of nitroglycerine (NTG) was administered from the tip of the guiding catheter. After coronary spasm terminated, OCT imaging was repeated (NTG-OCT). The following conditions were considered to document spasm: 1) more than 0.2 mV ST-segment elevation was observed in at least 2 leads; 2) more than 0.1 mV horizontal or downsloping ST-segment depression; 3) more than 0.2 mV junctional type ST-segment depression; and 4) occurrence of new chest pain. Depending on the results of the provocation test, lesions were classified into a spasm lesion group or a nonspasm lesion group.
Figure 1. A System for the Provocation Test With OCT.
Two coupled Y-connectors make 3 channels; one is for optical coherence tomography (OCT) image wire, one is for provocation drugs, and the other is for nitroglycerine (NTG), X-ray contrast, and OCT flushing medium. Figure illustration by Craig Skaggs.
OCT image analysis
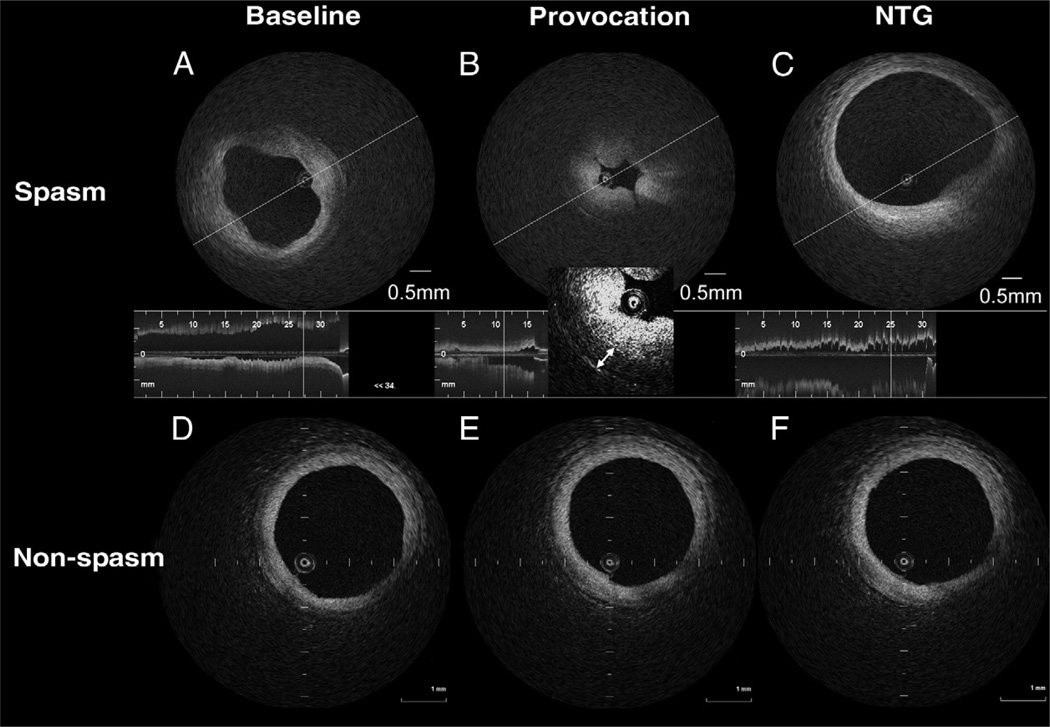
All OCT images were analyzed by 2 independent investigators (A.T. and M.M.), who were blinded to the clinical presentations. The site showing the most stenotic lumen in the provocation-OCT image was selected for analysis. We coregistered the baseline-OCT images, the provocation-OCT images, and the NTG-OCT images with information on the distance from anatomical landmarks, including side branches, the guiding catheter, or some morphological features, such as calcium deposits, vasa vasorum, cardiac veins, or pericardium. To evaluate the medial layer by OCT, we used the raw OCT images with adequately adjusted contrast and brightness as well as the logarithm of the OCT data for quantitative analysis of the media. Maximum intimal thickness, maximum medial thickness, lumen area, intimal area, and medial area at the site of spasm were measured by OCT according to previous methods (9). Reactivity to intracoronary NTG was calculated by the (lumen area in NTG-OCT) divided by (the lumen area in baseline-OCT), and reactivity to NTG of maximum medial thickness was calculated by (the maximum medial thickness with NTG-OCT) divided by (the maximum medial thickness at baseline-OCT). Intimal bump was defined as 1 or more smooth projections of the intima into the lumen that disappeared after the administration of NTG (Fig. 2A). Intimal gathering was defined as a gathering or intimal folding that resulted in multiple kinks in the luminal contour that resolved after the administration of NTG (Fig. 2B).
Figure 2. Representative OCT Images of a Spasm Lesion and a nonspasm Lesion.
Representative optical coherence tomography (OCT) images of a spasm lesion (A, B, C) and a nonspasm lesion (D, E, F). (A) The OCT showed intimal bumps that deformed the lumen. A thickened medial layer can be seen. (B) Medial thickness increases, causing luminal narrowing with intimal gathering. Medial thickness is 0.38 mm (double-headed arrow). (C) Both intimal bumps and gathering have disappeared. (D, E, F) No obvious morphological change can be seen in the nonspasm lesion throughout the provocation test. NTG = nitroglycerine.
Statistical analysis
Statistical analysis was performed with R version 2.11.1 (R Foundation for Statistical Computing) and StatView 5.0J (SAS Institute, Cary, North Carolina). Results are expressed as mean value ± SD for approximately normally distributed variables and median (interquartile range) for skewed variables, after applying Shapiro-Wilk test for normality. Qualitative data are presented as numbers (%). Differences between 2 groups were tested by the unpaired t test for approximately normally distributed variables and by Fisher exact test for categorical variables. To compare the lumen area, the intimal area, the medial area, maximum intimal thickness, and maximum medial thickness throughout the provocation test within each group, we adopted the Friedman test. A p value <0.05 was considered statistically significant.
Results
Baseline-OCT imaging
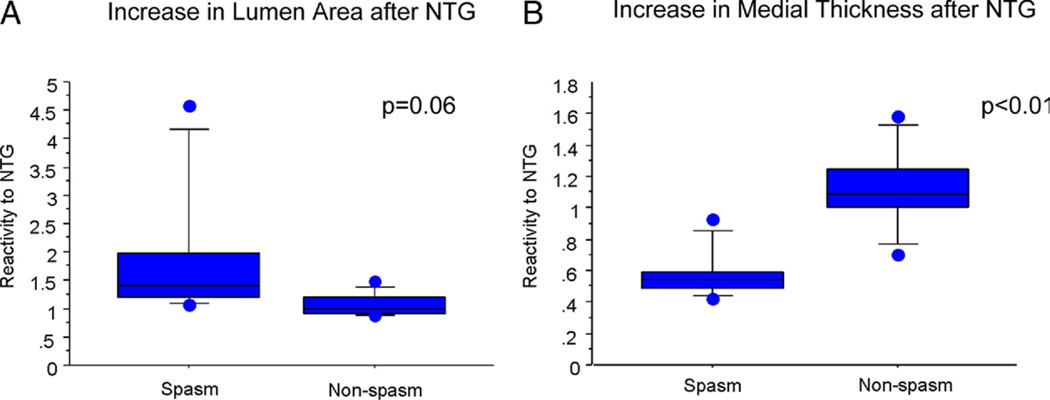
Clinical and lesion characteristics are summarized in Table 1. Coronary spasm was provoked in 10 of 19 coronary arteries during the provocation test. Baseline-OCT successfully imaged all spasm sites as well as NTG–OCT. The intimal bump was more frequently observed in the spasm lesion group, compared with the nonspasm lesion group (spasm 80% vs. nonspasm 0%, p < 0.01). The intimal bump site was correlated with the sites showing maximum intima thickness in 70% of the spasm lesion group. Baseline- and NTG-OCT measurements are summarized in Table 2. Reactivity to NTG tended to be larger in the spasm lesion group than in the nonspasm lesion group (spasm 1.92 ± 1.23 vs. nonspasm 1.07 ± 0.20, p = 0.06) (Fig. 3). Reactivity to NTG demonstrated by maximum medial thickness was smaller in the spasm group than in the nonspasm group (spasm 0.59 ± 0.16 vs. nonspasm 1.13 ± 0.27, p < 0.01) (Fig. 3).
Table 1.
Patient Characteristics
| Spasm (n = 10) |
Nonspasm (n = 9) |
p Value |
|
|---|---|---|---|
| Age (yrs) | 64.8 ± 8.0 | 68.2 ± 4.5 | 0.27 |
| Male | 7 (70%) | 6 (67%) | 0.99 |
| Hypertension | 4 (40%) | 6 (67%) | 0.37 |
| Diabetes mellitus | 2 (20%) | 2 (22%) | 0.99 |
| Hypercholesterolemia (>220 mg/dl) | 4 (40%) | 6 (67%) | 0.37 |
| Smoking | 5 (50%) | 2 (22%) | 0.35 |
| Acetylcholine/ergonovine | 6/4 | 4/5 | 0.66 |
| Target coronary artery (LAD/RCA/LCx) | 5/0/5 | 4/1/4 | 0.56 |
| Medication | |||
| Long-acting Ca channel blocker | 3 (30%) | 4 (44%) | 0.65 |
| Long-acting nitrates | 1 (10%) | 0 | 0.99 |
| Statin | 2 (20%) | 3 (33%) | 0.63 |
| Nicorandil | 2 (20%) | 3 (33%) | 0.63 |
| Beta- blocker | 0 | 2 (22%) | 0.22 |
| Angiotensin receptor blocker | 3 (30%) | 4 (44%) | 0.65 |
| Time from latest chest pain to OCT (days) | 11.9 ± 11.2 | 10.1 ± 10.3 | 0.81 |
Values are mean ± SD or n (%).
Ca = calcium; LAD = left anterior descending; LCx = left circumflex; OCT = optical coherence tomography; RCA = right coronary artery.
Table 2.
OCT Measurements
| Spasm (n = 10) |
Nonspasm (n = 9) |
p Value | |
|---|---|---|---|
| Intimal bump | 8 (80%) | 0 | <0.01 |
| Baseline-OCT | |||
| Maximum intimal thickness (mm) | 0.35 ± 0.29 | 0.25 ± 0.13 | 0.35 |
| Maximum medial thickness (mm) | 0.24 ± 0.04 | 0.12 ± 0.03 | <0.01 |
| Lumen area (mm2) | 4.02 ± 1.39 | 7.34 ± 1.88 | <0.01 |
| Intimal area (mm2) | 2.15 ± 1.13 | 2.60 ± 1.24 | 0.42 |
| Medial area (mm2) | 2.39 ± 1.04 | 1.36 ± 0.45 | 0.01 |
| NTG-OCT | |||
| Maximum intimal thickness (mm) | 0.22 ± 0.14 | 0.19 ± 0.09 | 0.66 |
| Maximum medial thickness (mm) | 0.13 ± 0.03 | 0.13 ± 0.02 | 0.65 |
| Lumen area (mm2) | 6.38 ± 1.27 | 7.71 ± 2.19 | 0.12 |
| Intimal area (mm2) | 1.89 ± 0.88 | 2.34 ± 0.85 | 0.27 |
Values are mean ± SD or n (%).
NTG = nitroglycerine; OCT = optical coherence tomography.
Figure 3. Reactivity of Lumen Area and Maximum Medial Thickness to NTG.
(A) Reactivity of lumen area to nitroglycerine (NTG). The spasm lesion group tends to be more sensitive to NTG. (B) Reactivity of maximum medial thickness to NTG. The reactivity of maximum medial thickness to NTG in the spasm lesion is significantly reduced, compared with the nonspasm lesion.
The conformational change of vascular structure throughout the provocation test
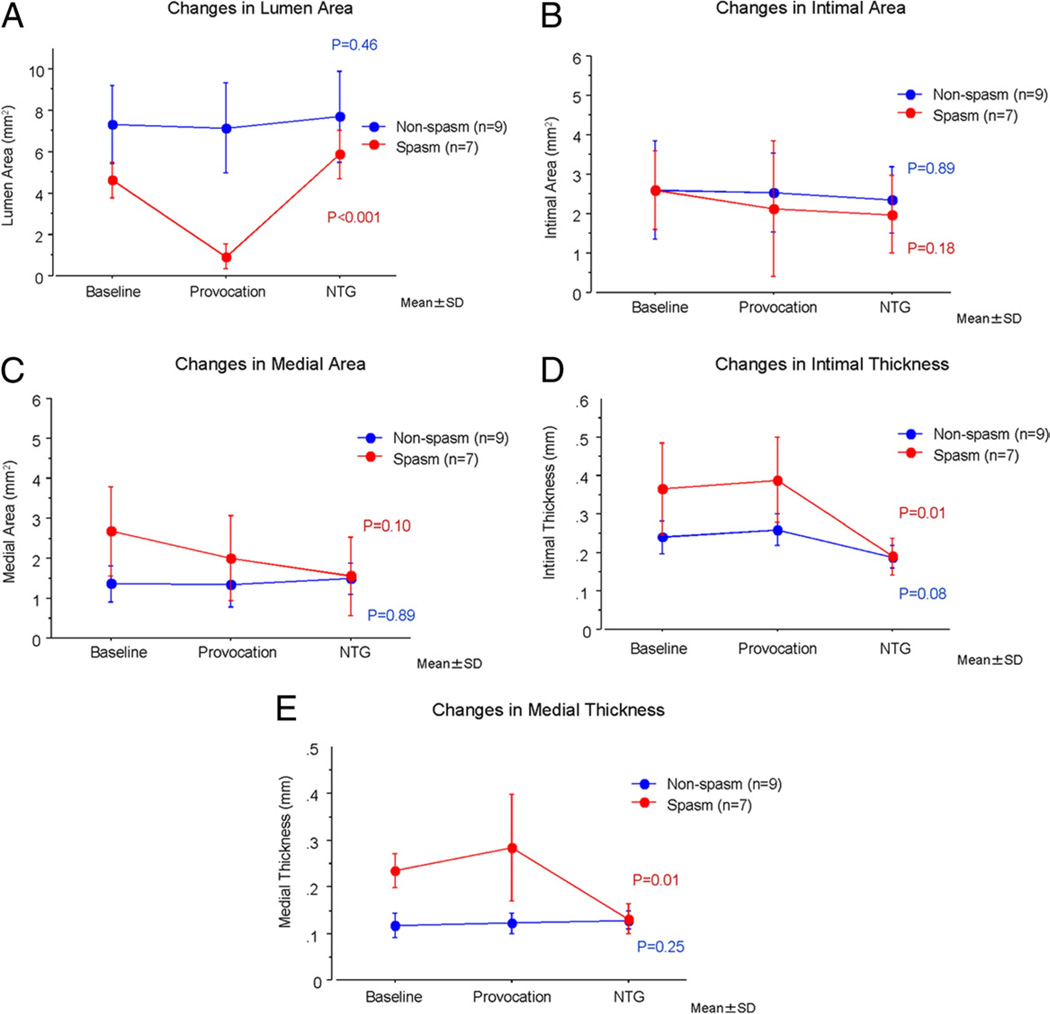
Provocation-OCT imaging could not be performed due to spontaneous spasm during preparation for the provocation in 1 case and due to a tight stenosis during spasm in 2 cases. Ultimately the change in coronary artery wall morphology could be analyzed in 7 of 10 (70%) spasm lesions and 9 of 10 (100%) of the nonspasm lesions. All spasm lesions showed intimal gathering during spasm (spasm 100% vs. nonspasm 0%, p < 0.01). Although no morphological changes were observed in the nonspasm lesions throughout the provocation test, the lumen area and the medial thickness of the spasm lesion group were changed throughout the provocation test (Fig. 4).
Figure 4. Changes of the Spasm and the Nonspasm Lesion Groups.
(A) Coronary lumen is changed in the spasm lesion group during the provocation test (p < 0.001), whereas there is no change in the nonspasm lesion group (p = 0.46). (B) No change is observed in the intimal area throughout the provocation test in either group. (C) No significant change is observed in the medial area throughout the provocation test in either group. (D) The maximum intimal thickness is changed in the spasm lesion during the provocation test (p = 0.01), whereas no significant change is noted in the nonspasm lesion. (E) The maximum medial thickness is changed in the spasm lesion during the provocation test (p = 0.01), whereas no significant change is noted in the nonspasm lesion. NTG = nitroglycerine.
Discussion
Morphological features of spasm lesion at baseline
Previous intravascular ultrasound (IVUS) studies have reported that the morphological features of vasospastic lesions are diffuse intimal thickening (12), negative remodeling (13), and increased area of sonolucent zone (14). Previous IVUS studies, however, could not precisely explain what happens to the vascular structure of the spasm lesion at baseline. Our OCT study revealed that the spasm lesion shows a larger medial area and medial thickness at baseline, whereas no other morphological difference is observed between spasm lesions and nonspasm lesions. In addition to the changes in the media, the intimal bump that is associated with the maximum intimal thickening site is frequently associated with spasm lesions at baseline. These OCT data suggested that abnormal medial contraction is already present even when the artery is at an asymptomatic state and this medial constriction squeezes the intima and facilitates the intimal bump or the intimal thickening in vasospastic angina patients. If this mild spasm occurred focally, it could appear by IVUS like negative remodeling. Increased medial area also contributes to the increased intima plus media area and might be involved in the increased sonolucent zone in IVUS.
Previous human angiography studies have reported increased basal vascular tone of vasospasm lesions (15,16). Animal studies by Shimokawa et al. (3) reported that increased activities of ρ-kinase causes hypercontraction of vascular smooth muscle cells and this might play a central role for the etiology of vasospasm (17). Kugiyama et al. (18) used NG-monomethyl-L-arginine, which is an antagonist for nitric oxide, and reported that a deficiency in endothelial nitric oxide activity in spasm lesions might cause hyperconstriction of the vasospastic artery. These previous results are consistent with our observations that the media of spasm lesion constricts even when spasm has not yet occurred.
Moreover, we speculate that the presence of these early asymptomatic lesions that precede actual spasm may explain why various and trivial stimulations can provoke spasm in humans (2,3,5). After the administration of NTG, such arterial contraction is completely released, and no difference in medial thickness was observed between spasm lesions and nonspasm lesions. These findings are consistent with the observations from pathology that no abnormalities could be seen in the medial layer of spasm lesions (15). Precursor medial contraction might be one of the pathophysiological features of spasm.
Conformational change of vascular structure during spasm
Previous human studies used luminal diameter for the assessment of vascular constriction, assuming that medial constriction affected the intima (15,16). Due to the lack of an appropriate imaging method for studying the intima and media separately, it has been unclear how each arterial layer changes in accordance with the drastic luminal narrowing that occurs in the human vasospasm lesion. For example, an OCT article discussed the possibility that the intimal layer itself contracted and contributed to the formation of intimal thickening in vasospastic angina patients (19). However, our study shows that the medial thickness increases as a result of medial contraction and facilitates the intimal gathering without an alteration of intimal area during vasospasm in the human coronary artery.
Previous studies reported that hypersensitivity to NTG is observed in the spasm lesion (15,16). Since the change of maximum medial thickness is so large in spastic lesions, it is reasonable to ascribe the luminal narrowing to increased basal vascular tone, instead of hypersensitivity. Our results indicate that direct measurements of the media by OCT could be used to better predict the reactivity of drugs administered to mitigate spasm.
Study limitations
First, we only analyzed a small number of patients with resting chest pain. To avoid endothelial injury, we inserted the OCT image wire without using the balloon occlusion catheter in this study. This technique made it difficult to explore tortuous lesions or distal lesions. In 3 lesions, we could not assess lesion morphology during spasm. We could not perform OCT imaging in 3 vessels, because we exceeded the total amount of OCT flushing media allowed.
Acknowledgments
Dr. Tearney is a consultant for Samsung; has performed sponsored research for the Terumo Corp.; and has received patent licensing and royalty income from the Terumo Corp. Dr. Bouma has a sponsored research agreement with and is an inventor on patents licensed to the Terumo Corp.; and is an inventor on patents licensed to LightLab Imaging.
Abbreviations and Acronyms
- Erg
ergonovine
- IVUS
intravascular ultrasound
- NTG
nitroglycerine
- OCT
optical coherence tomography
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Prinzmetal M, Ekmekci A, Kennamer R, et al. Variant form of angina pectoris, previously undelineated syndrome. JAMA. 1960;174:1794–1800. doi: 10.1001/jama.1960.03030140016004. [DOI] [PubMed] [Google Scholar]
- 2.Yasue H, Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760–765. doi: 10.2169/internalmedicine.36.760. [DOI] [PubMed] [Google Scholar]
- 3.Shimokawa H. Cellular and molecular mechanisms of coronary artery spasm: lessons from animal models. Jpn Circ J. 2000;64:1–12. doi: 10.1253/jcj.64.1. [DOI] [PubMed] [Google Scholar]
- 4.Kawano H, Ogawa H. Endothelial function and coronary spastic angina. Intern Med. 2005;44:91–99. doi: 10.2169/internalmedicine.44.91. [DOI] [PubMed] [Google Scholar]
- 5.Stern S, Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009;119:2531–2534. doi: 10.1161/CIRCULATIONAHA.108.843474. [DOI] [PubMed] [Google Scholar]
- 6.Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka A, Imanishi T, Kitabata H, et al. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non- ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur Heart J. 2009;30:1348–1355. doi: 10.1093/eurheartj/ehp122. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Imanishi T, Kitabata H, et al. Morphology of exertion-triggered plaque rupture in patients with acute coronary syndrome: an optical coherence tomography study. Circulation. 2008;118:2368–2373. doi: 10.1161/CIRCULATIONAHA.108.782540. [DOI] [PubMed] [Google Scholar]
- 9.Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary intima–media thickness by optical coherence tomography: comparison with intravascular ultrasound. Circ J. 2005;69:903–907. doi: 10.1253/circj.69.903. [DOI] [PubMed] [Google Scholar]
- 10.Kataiwa H, Tanaka A, Kitabata H, Imanishi T, Akasaka T. Safety and usefulness of non-occlusion image acquisition technique for optical coherence tomography. Circ J. 2008;72:1536–1537. doi: 10.1253/circj.cj-08-0406. [DOI] [PubMed] [Google Scholar]
- 11.Tearney GJ, Waxman S, Shishkov M, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. J Am Coll Cardiol Img. 2008;1:752–761. doi: 10.1016/j.jcmg.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyao Y, Kugiyama K, Kawano H, et al. Diffuse intimal thickening of coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 2000;36:432–437. doi: 10.1016/s0735-1097(00)00729-4. [DOI] [PubMed] [Google Scholar]
- 13.Hong YJ, Jeong MH, Choi YH, et al. Plaque components at coronary sites with focal spasm in patients with variant angina: virtual histology-intravascular ultrasound analysis. Int J Cardiol. 2010;144:367–372. doi: 10.1016/j.ijcard.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 14.Yamagishi M, Miyatake K, Tamai J, Nakatani S, Koyama J, Nissen SE. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J Am Coll Cardiol. 1994;23:352–357. doi: 10.1016/0735-1097(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 15.Kugiyama K, Murohara T, Yasue H, et al. Increased constrictor response to acetylcholine of the isolated coronary arteries from patients with variant angina. Int J Cardiol. 1995;52:223–233. doi: 10.1016/0167-5273(95)02478-6. [DOI] [PubMed] [Google Scholar]
- 16.Kaski JC, Tousoulis D, Gavrielides S, et al. Comparison of epicardial coronary artery tone and reactivity in prinzmetal’s variant angina and chronic stable angina pectoris. J Am Coll Cardiol. 1991;17:1058–1062. doi: 10.1016/0735-1097(91)90830-3. [DOI] [PubMed] [Google Scholar]
- 17.Kandabashi T, Shimokawa H, Miyata K, et al. Inhibition of myosin phosphatase by upregulated rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1beta. Circulation. 2000;101:1319–1323. doi: 10.1161/01.cir.101.11.1319. [DOI] [PubMed] [Google Scholar]
- 18.Kugiyama K, Yasue H, Okumura K, et al. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94:266–271. doi: 10.1161/01.cir.94.3.266. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa Y, Uemura Y, Ishigami KI, et al. Morphological features of coronary arteries in patients with coronary spastic angina: Assessment with intracoronary optical coherence tomography. Int J Cardiol. 2011;146:334–340. doi: 10.1016/j.ijcard.2009.07.011. [DOI] [PubMed] [Google Scholar]