Abstract
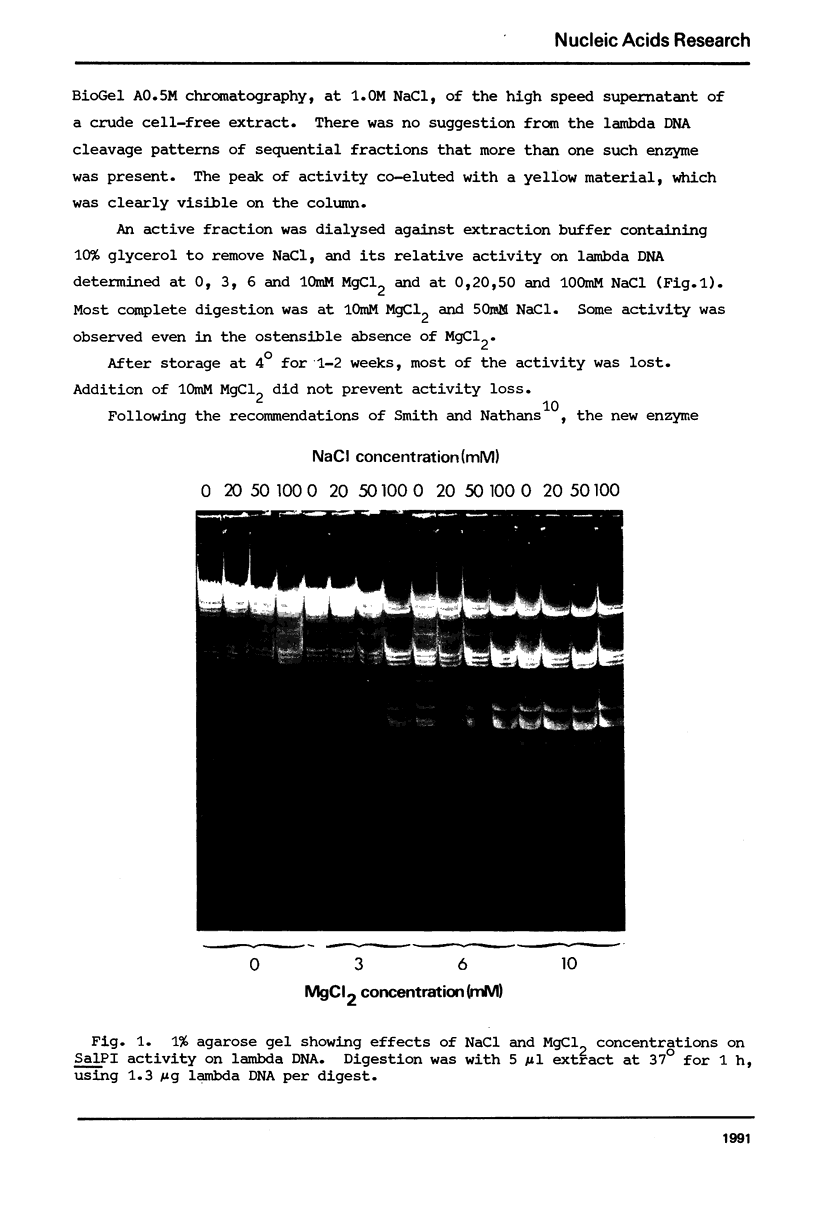
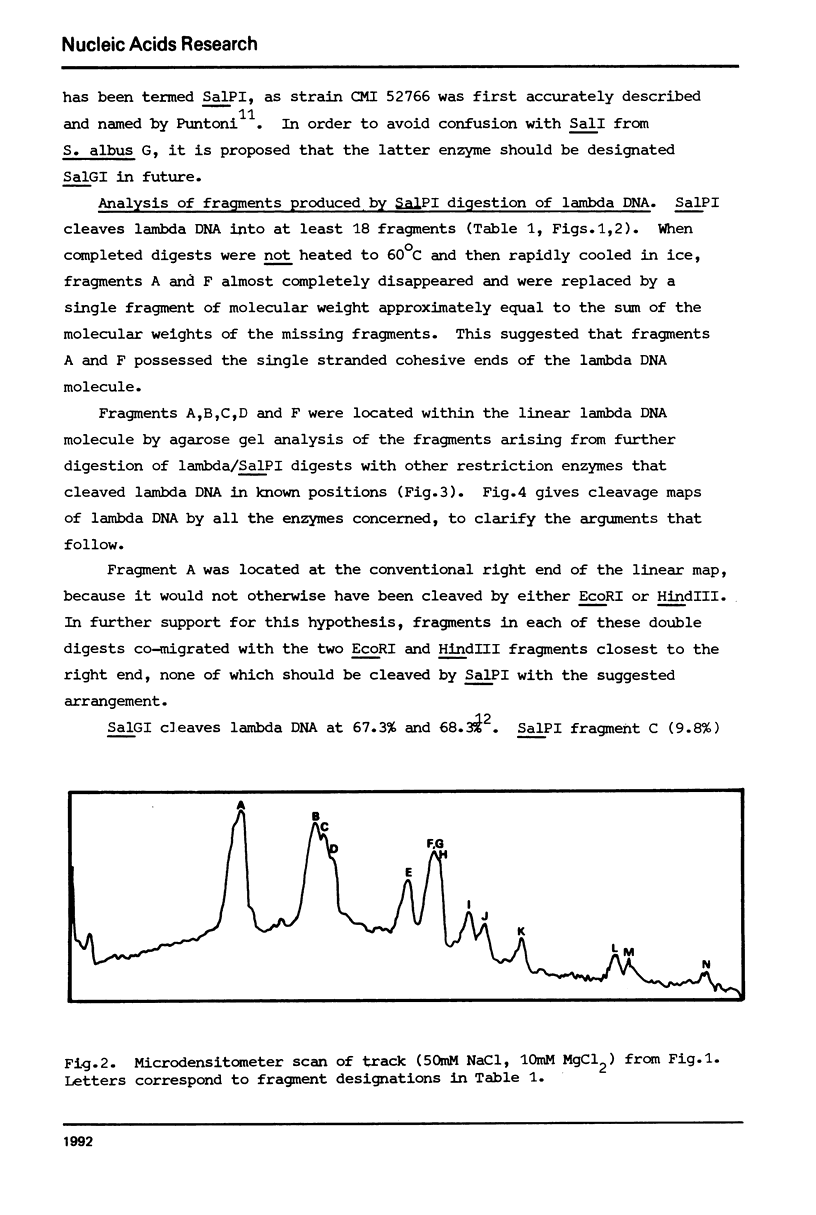
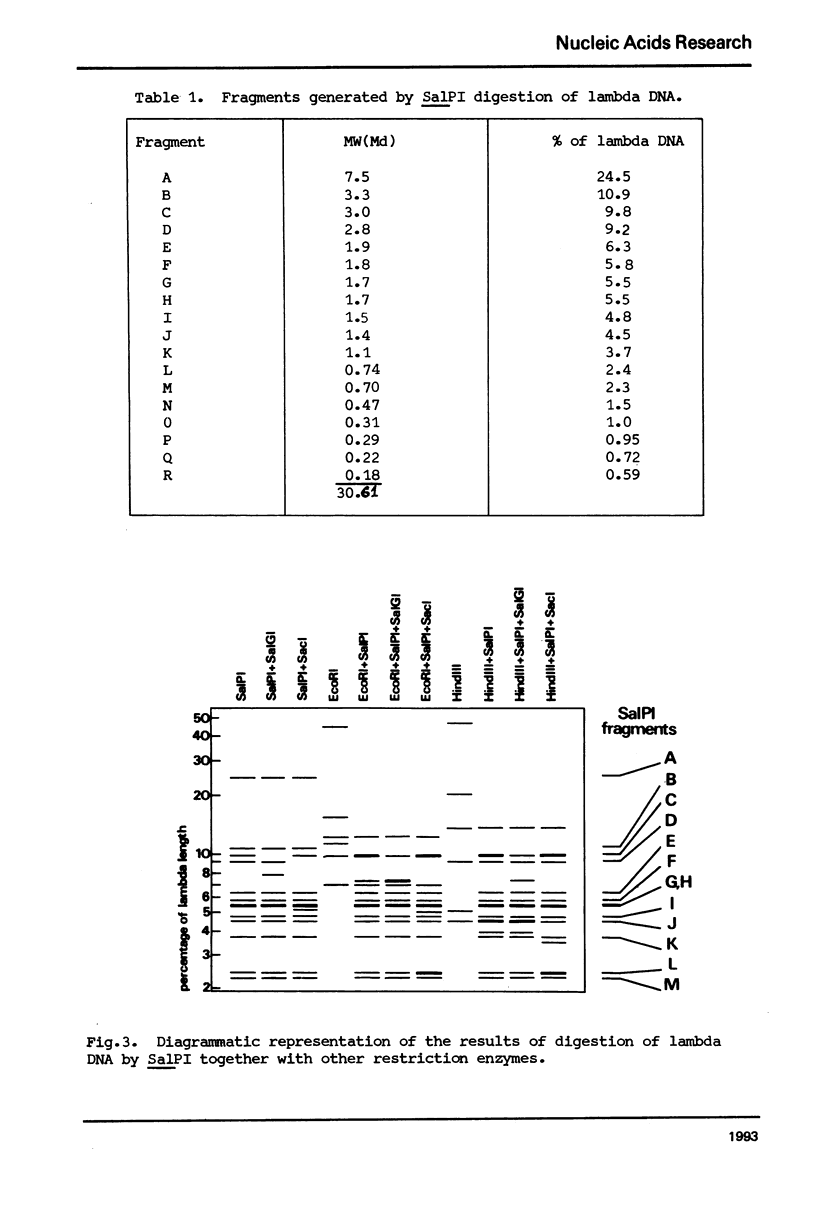
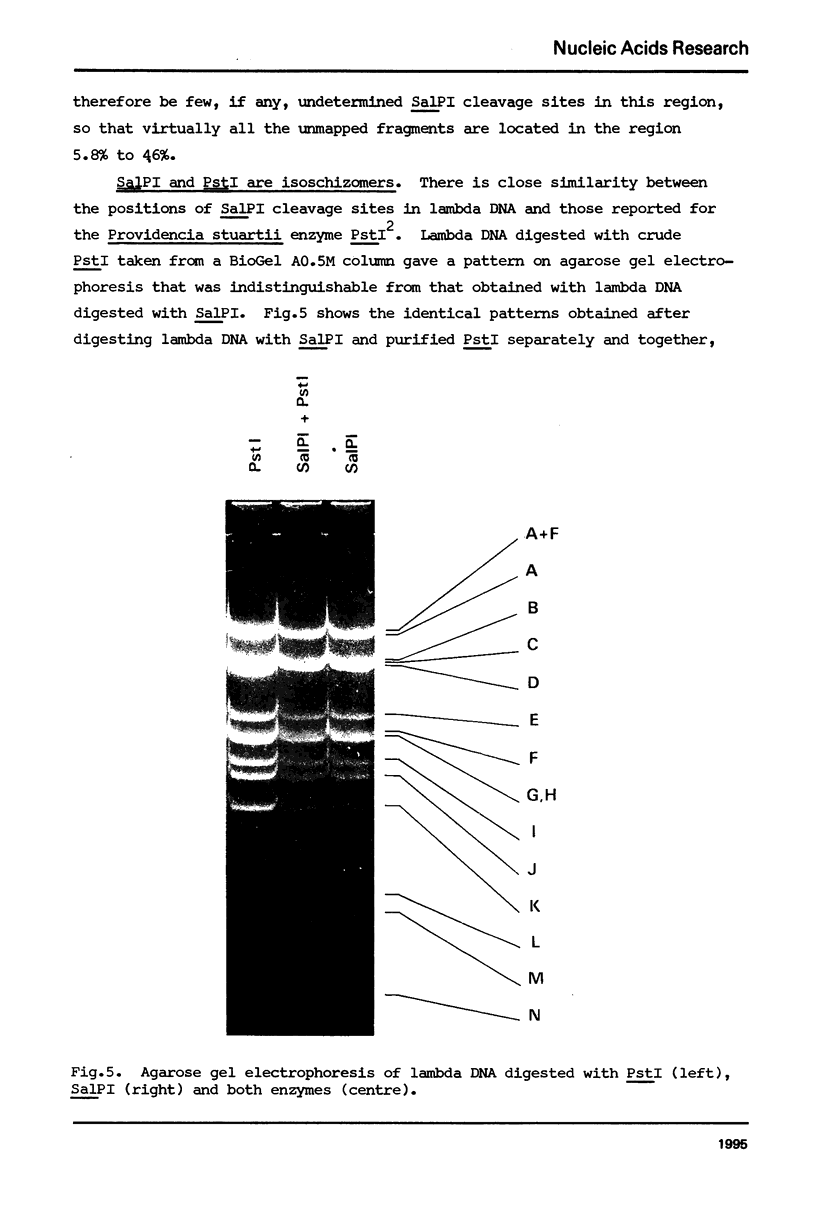
A class II site-specific endodeoxyribonuclease (SalPI) was identified in cell-free extracts of Streptomyces albus CMI 52766 after high speed centrifugation and fractionation through Bio Gel AO.5M. SalPI cleaves lambda DNA into at least 18 fragments. Five cleavage sites were located in the linear lambda map by the use of double and triple restriction enzyme digests involving EcoRI, HindIII, SalGI and another new Streptomyces enzyme, SacI. The results were indistinguishable from those previously obtained for a Providencia stuartii enzyme, PstI, by Smith, Blattner & Davies (Nucleic Acids Res. 1976 3, 343). SalPI and PstI were shown by a double digest test to have the same site specificity. None of 34 phages tested was obviously restricted by S. albus CMI 52766, and correspondingly DNA from two of them was not cleaved in vitro by SalPI. DNA from Streptomyces phage that does not form plaques on S. albus CMI 52766, and plasmid SCP2 DNA from S. coelicolor A3 (2), were both cleaved.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan P. An electron microscopic comparison of transcription on linear and superhelical DNA. J Mol Biol. 1976 Jul 25;105(1):161–176. doi: 10.1016/0022-2836(76)90201-1. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Wilde L. C. Restriction of a bacteriophage of Streptomyces albus G involving endonuclease SalI. J Bacteriol. 1976 Nov;128(2):644–650. doi: 10.1128/jb.128.2.644-650.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowding J. E. Characterization of a bacteriophage virulent for Streptomyces coelicolor A3(2). J Gen Microbiol. 1973 May;76(1):163–176. doi: 10.1099/00221287-76-1-163. [DOI] [PubMed] [Google Scholar]
- Freeman R. F., Bibb M. J., Hopwood D. A. Chloramphenicol acetylransferase-independent chloramphenicol resistance in Streptomyces coelicolor A3(2). J Gen Microbiol. 1977 Feb;98(2):453–465. doi: 10.1099/00221287-98-2-453. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Emeijanova L. K., Alikhanian S. I. The genetic location of prophage on the chromosome of Streptomyces coelicolor. Genetics. 1971 Jul;68(3):341–347. doi: 10.1093/genetics/68.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Schrempf H., Bujard H., Hopwood D. A., Goebel W. Isolation of covalently closed circular deoxyribonucleic acid from Streptomyces coelicolor A3(2). J Bacteriol. 1975 Feb;121(2):416–421. doi: 10.1128/jb.121.2.416-421.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Wilcockson J., Hull R. The rapid isolation of plant virus RNAs using sodium perchlorate. J Gen Virol. 1974 Apr;23(1):107–111. doi: 10.1099/0022-1317-23-1-107. [DOI] [PubMed] [Google Scholar]