Abstract
Background
Currently no prospective randomized trial has measured the efficacy of radiation therapy for resected retroperitoneal sarcomas (RPS). Our objective was to determine the effect of radiation therapy on disease-specific and overall survival between propensity score-matched surgically resected RPS patients using the Surveillance, Epidemiology, and End Results (SEER) database.
Patients and methods
The study population consisted of patients with histologically confirmed RPS who underwent surgical resection between 1988 and 2006. Exclusion criteria included multiple malignancies, distant metastasis, and unknown grade or stage. Cox modeling was used to determine covariate associations with disease-specific survival. Propensity score methods were used to perform survival analysis in patients who received radiation matched with patients who underwent surgery alone.
Results
Prior to matching, there were 762 patients (558 surgery only, 204 surgery with radiation). Factors independently associated with radiation therapy were age (P = 0.037), geographic region (P = 0.041), grade (P = 0.047), stage (P = 0.003), and surgery type (P = 0.01). Cox modeling demonstrated that age, sex, grade, and stage were independently associated with survival. Propensity scoring (309 matched pairs) and survival analysis using Kaplan–Meier methods demonstrated no difference between propensity score-matched patients receiving radiation therapy and those who did not (P = 0.35).
Conclusion
At present, SEER patients with surgically resected RPS who received radiation therapy did not demonstrate survival benefit.
Keywords: Kaplan–Meier, propensity score, radiation therapy, retroperitoneal sarcoma, survival
introduction
Retroperitoneal sarcomas (RPS) are an uncommon and heterogenous group of neoplasms that comprise ∼15% of adult soft tissue sarcomas (STS). The treatment of RPS poses a challenge due to anatomical constraints that often hinder complete resection, local recurrence of ∼50% despite complete resection [1], and limited effectiveness of adjuvant chemotherapy. Today the question of whether radiation therapy impacts disease-specific and overall survival in patients with resected RPS is still debated, as there has never been a completed randomized control trial comparing radiation therapy plus surgery to surgery alone. In lieu of the results of a randomized control trial, we have carried out the first analysis using a propensity score matching methodology on a large population-based study sample to determine whether addition of radiation therapy to surgical resection was associated with differences in disease-specific and overall survival.
In order to determine the effect of radiation on disease-specific survival in resected RPS patients, we used two different methods of analysis. First, we used Cox proportional hazards modeling to determine whether radiation therapy was independently associated with reduced hazard of death. Second, we created a subset of patients who were matched based upon propensity score to reduce covariate differences between groups that could bias survival outcome and compared the matched pairs of patients using the Kaplan–Meier method and the censored-data version of the sign test [2]. Propensity scores, defined as the conditional probability of that individual receiving a certain treatment [3], provide a statistical basis for matching a pair of patients with similar probabilities of receiving either treatment (surgery and radiation) or control (surgery alone) [4]. Propensity score matching balances the baseline covariates between treated and untreated subjects [5], such that analysis of differences between pairs can be attributed to the effect of the treatment and is not likely due to measured covariate differences. This study is the first to report the effect of radiation therapy on survival of patients after RPS resection using patient populations matched by propensity score methods.
methods
study population
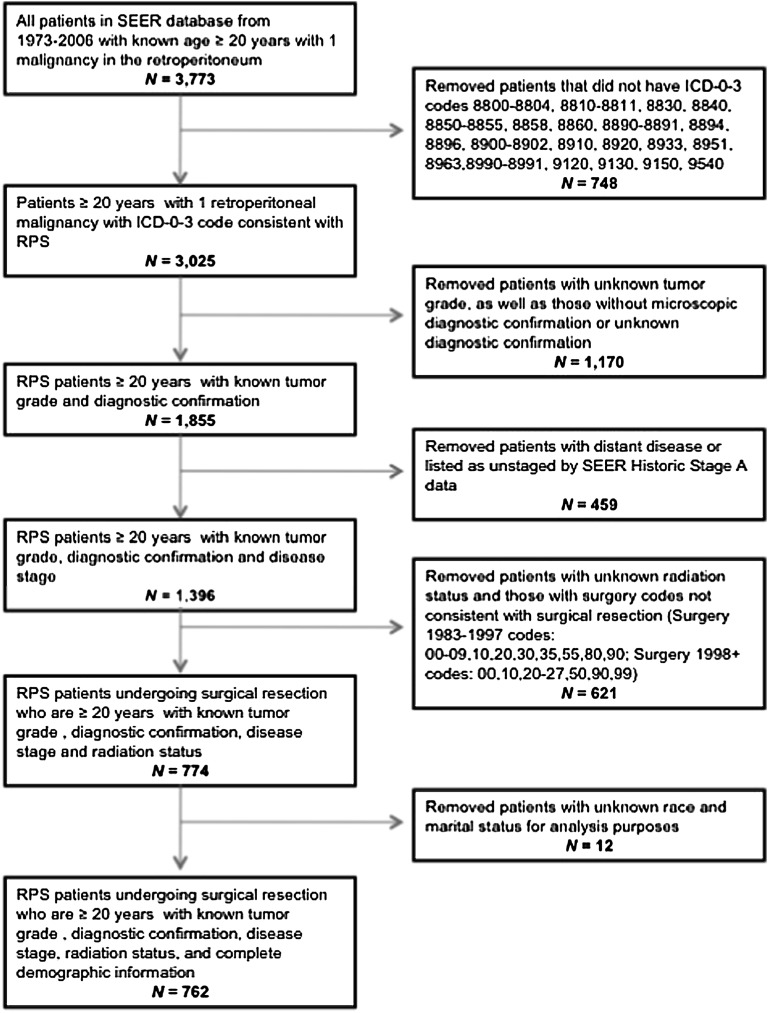
Patients included in the study were selected from all 17 registries included in the publically available Surveillance, Epidemiology and End Results (SEER) database. Patients were ≥ 20 years of age, diagnosed with RPS between 1988 and 2006, and had a single primary histologically confirmed malignancy of the retroperitoneum (ICD-0–3 code C480). Only patients with curative-intent surgical resections coded as simple/partial and total/radical were included. Those undergoing biopsy only, local tumor destruction only, debulking and palliative procedures were excluded. Further exclusion criteria included metastatic disease at diagnosis, unstaged disease, unknown radiation status, unknown grade, and unknown demographic information needed for matching (Figure 1).
Figure 1.
Patient selection flowchart.
variables of interest
The histology ICD-0-3 codes were consolidated into five tumor histology groups: leiomyosarcoma, liposarcoma, malignant fibrous histiocytoma, sarcoma not otherwise specified (NOS) for codes in the ‘soft tissue and sarcoma category’, and other for the remaining histologies. The disease stage covariate included two categories from SEER historic stage A. Localized is defined as disease confined to the organ of origin, while regional is defined as a neoplasm that has extended beyond the organ of origin to adjacent organs, lymph nodes, or combination thereof [6]. Radiation types documented in SEER are external beam radiation, radioactive implants, combination of beam and radioactive implants/isotopes, and NOS, while radiation sequences are preoperative only, intra-operative only, postoperatively only, intra-operative with either pre- or postoperative, and pre- plus postoperative. Tumor size was not selected as a covariate due to incomplete patient records in our patient population and previous analyses that showed tumor size did not impact survival [7–11]. Age and diagnosis period were converted from continuous variables into groups and analyzed as categorical variables. Individual SEER registries were classified as geographic regions: East (Connecticut, New Jersey), Midwest (Iowa, Detroit), South (Atlanta, Kentucky, Louisiana), and West (California excluding San Francisco/San Jose/Los Angeles, Hawaii, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle, Utah). Surgery was categorized as simple/partial (codes 40 and 50 from 1983 to 1997; code 30 from 1998 + ) or total/radical (code 60 from 1983 to 1997; codes 40 and 60 from 1998 + ).
statistical analysis
Frequency counts for the radiation characteristic analysis were carried out using table analyses. Chi-square tests were used to determine the association between radiation status and the study covariates. For the multivariate logistic regression, reported results originated from a full effects model in which covariates with a P value > 0.2 in the full model were removed in a stepwise fashion unless the covariate was clinically significant. Cox proportional hazards modeling was used to determine associations of variables of interest, including radiation status, with disease-specific survival. Results of the Cox model are from a full model with all covariates. Survival analysis was carried out by Kaplan–Meier method and a censored-data version of the sign test described by Klein and Moeschberger [2]. All statistical analyses were carried out using SAS 9.2 (Cary, NC).
propensity score estimation and matching
Ten variables were chosen for the propensity score matching based on the univariate and multivariate analyses described above: age by decade, sex, race, marital status, geographic region, diagnosis period, tumor histology, tumor grade, disease stage, and surgery type.
Propensity scores were created using logistic regression modeling the likelihood of a patient receiving radiation therapy based on the 10 study covariates [12]. While determining possible interactions between covariates, a significant interaction between grade and stage was found. Therefore, an interaction term grade × stage was also included in the logistic regression code in SAS to create the propensity scores.
A 1 : 2 match without replacement was used to pair each patient who received radiation in addition to surgical resection with at least one patient who received surgical resection only whose propensity score was within the designated caliper size [13]. The caliper size used was 0.6 of the standard error of the logits of the propensity scores, which was equal to 0.0081 [14]. Many radiation therapy patients were matched with two surgery only patients, but some were only matched with one if a second suitable match within the caliper did not exist.
assessing covariate balance
The method of standardized differences was used to assess balance of covariates before and after matching. This method is preferred to methods of hypothesis testing as the standardized difference does not depend on sample size [5]. In general, an absolute value of the standardized difference < 10% is desirable, as a level greater may indicate serious imbalance between the covariates [14]. The equation below gives the standardized difference for a continuous variable, where x is the sample mean and s is the sample variance [15]:
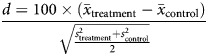 |
The equation below gives the standardized difference for a categorical variable, where p is the proportion [15]:
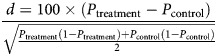 |
results
univariate and multivariate analyses of factors associated with radiation therapy
In order to determine factors that were associated with receiving radiation therapy, we carried out univariate and multivariate analyses on the study population of 762 patients. The univariate analysis demonstrated that geographic region (P = 0.02), tumor histology (P = 0.0007), tumor grade (P < 0.0001), and disease stage (P = 0.0005) were associated with receiving radiation therapy. Table 1 shows the factors independently associated with receiving radiation therapy using a multivariate full effects logistic regression model. Patient age and geographic region were independently associated with receiving radiation therapy. With respect to tumor grade, patients with grade 1 tumors were less likely to receive radiation therapy compared with patients with grade 4 tumors [odds ratio (OR) 0.36, 95% confidence interval (CI) 0.23 to 0.57]. Patients with localized RPS were less likely to get radiation than patients with tumors that involved adjacent organs (OR 0.58, 95% CI 0.41 to 0.83). In the process of assessing all covariates for interactions, a significant interaction between grade and surgery type was found (P = 0.027). Sex, diagnosis period, and histology were not found to be independently associated with receipt of radiation therapy.
Table 1.
Multivariate analysis of factors associated with adjuvant radiation therapy
| Covariate | P value | OR | 95% CI |
|---|---|---|---|
| Age decade (years) | 0.037 | ||
| 20–29 | 0.406 | 2.16 | 0.35–13.32 |
| 30–39 | 0.040 | 3.20 | 1.05–9.71 |
| 40–49 | 0.001 | 4.87 | 1.89–12.59 |
| 50–59 | 0.005 | 3.81 | 1.49–9.74 |
| 60–69 | 0.001 | 4.59 | 1.82–11.62 |
| 70–79 | 0.016 | 3.17 | 1.24–8.15 |
| 80 + | Reference | – | – |
| Geographic region | 0.040 | ||
| East | 0.112 | 0.64 | 0.37–1.11 |
| Midwest | 0.052 | 1.57 | 1.00–2.47 |
| South | 0.515 | 0.81 | 0.42–1.54 |
| West | Reference | – | – |
| Tumor grade | 0.047 | ||
| Grade 1 | 0.020 | 0.36 | 0.23–0.57 |
| Grade 2 | 0.738 | 0.81 | 0.51–1.28 |
| Grade 3 | 0.988 | 0.88 | 0.56–1.40 |
| Grade 4 | Reference | – | – |
| Disease stage | |||
| Localized | 0.003 | 0.58 | 0.41–0.83 |
| Regional | Reference | – | – |
| Surgery type | |||
| Simple/partial | 0.411 | 1.17 | 0.81–1.69 |
| Total/radical | Reference | – | – |
| Grade × surgery interaction | 0.027 | ||
| Simple/partial | |||
| Grade 1 versus grade 2 | 0.30 | 0.12–0.74 | |
| Grade 1 versus grade 3 | 0.19 | 0.07–0.49 | |
| Grade 1 versus grade 4 | 0.12 | 0.05–0.29 | |
| Grade 2 versus. grade 3 | 0.63 | 0.24–1.63 | |
| Grade 2 versus grade 4 | 0.40 | 0.17–0.96 | |
| Grade 3 versus grade 4 | 0.64 | 0.25–1.61 | |
| Total/radical | |||
| Grade 1 versus grade 2 | 0.48 | 0.27–0.87 | |
| Grade 1 versus grade 3 | 0.53 | 0.29–0.97 | |
| Grade 1 versus grade 4 | 0.53 | 0.31–0.91 | |
| Grade 2 versus grade 3 | 1.10 | 0.58–2.06 | |
| Grade 2 versus grade 4 | 1.10 | 0.63–1.93 | |
| Grade 3 versus grade 4 | 1.00 | 0.57–1.78 | |
OR, odds ratio; CI, confidence interval.
multivariate analysis of factors associated with disease-specific survival
In addition to analyzing factors associated with receiving radiation therapy, we also analyzed the same set of covariates for association with disease-specific survival by Cox proportional hazards modeling (Table 2). Age, sex, certain histological subtypes, tumor grade, and disease stage were independently associated with disease-specific survival in patients with resected RPS. Females demonstrated reduced hazard of death compared with males [hazard ratio (HR) 0.58, 95% CI 0.45 to 0.75], which has been observed in previous SEER [10] and non-SEER studies [16]. Although the histology covariate as a whole did not reach significance, leiomyosarcoma was independently associated with increased hazard of death compared with liposarcoma (HR 1.59, 95% CI 1.15 to 2.20). Both grade 1 (HR 0.27, 95% CI 0.18 to 0.40) and grade 2 tumors (HR 0.56, 95% CI 0.40 to 0.79) were associated with reduced hazard of death compared with grade 4 tumors. Localized stage of disease was associated with reduced hazard of death compared with regional disease (HR 0.77, 95% CI 0.59 to 0.99). Finally, radiation therapy was not significantly associated with reduced hazard of death in the Cox model (P = 0.30).
Table 2.
Multivariate Cox proportional hazards model for disease-specific survival
| Covariate | HR | 95% CI | P value |
|---|---|---|---|
| Age decade (years) | 0.0009 | ||
| 20–29 | 0.39 | 0.13–1.22 | |
| 30–39 | 0.42 | 0.22–0.83 | |
| 40–49 | 0.41 | 0.24–0.71 | |
| 50–59 | 0.40 | 0.24–0.68 | |
| 60–69 | 0.61 | 0.37–1.00 | |
| 70–79 | 0.77 | 0.47–1.27 | |
| 80+ | Reference | – | |
| Sex | <0.0001 | ||
| Female | 0.58 | 0.44–0.75 | |
| Male | Reference | – | |
| Race | 0.08 | ||
| Black | 1.50 | 0.89–2.51 | |
| Other | 1.47 | 0.98–2.21 | |
| White | Reference | – | |
| Marital status | 0.53 | ||
| Married | 0.91 | 0.68–1.22 | |
| Unmarried | Reference | – | |
| Geographic region | 0.61 | ||
| East | 1.00 | 0.68–1.47 | |
| Midwest | 1.04 | 0.75–1.45 | |
| South | 1.39 | 0.85–2.26 | |
| West | Reference | – | |
| Diagnosis period | 0.27 | ||
| 1988–1992 | 1.58 | 0.98–2.56 | |
| 1993–1997 | 1.41 | 0.91–2.19 | |
| 1998–2002 | 1.24 | 0.82–1.87 | |
| 2003–2006 | Reference | – | |
| Tumor histology | 0.08 | ||
| Leiomyosarcoma | 1.59 | 1.15–2.20 | |
| Liposarcoma | Reference | – | |
| Malignant fibrous histiocytoma | 1.44 | 0.94–2.20 | |
| Other | 1.35 | 0.72–2.55 | |
| Sarcoma NOS | 1.23 | 0.71–2.13 | |
| Tumor grade | <0.0001 | ||
| 1 | 0.27 | 0.18–0.40 | |
| 2 | 0.56 | 0.40–0.79 | |
| 3 | 0.95 | 0.68–1.33 | |
| 4 | Reference | – | |
| Disease stage | 0.04 | ||
| Localized | 0.77 | 0.59–0.99 | |
| Regional | Reference | – | |
| Surgery type | 0.49 | ||
| Simple/partial | 0.90 | 0.67–1.21 | |
| Total/radical | Reference | – | |
| Radiation | 0.30 | ||
| No | 1.17 | 0.87–1.56 | |
| Yes | Reference | – |
HR, hazard ratio; CI, confidence interval.
characteristics of the study population before and after propensity score matching
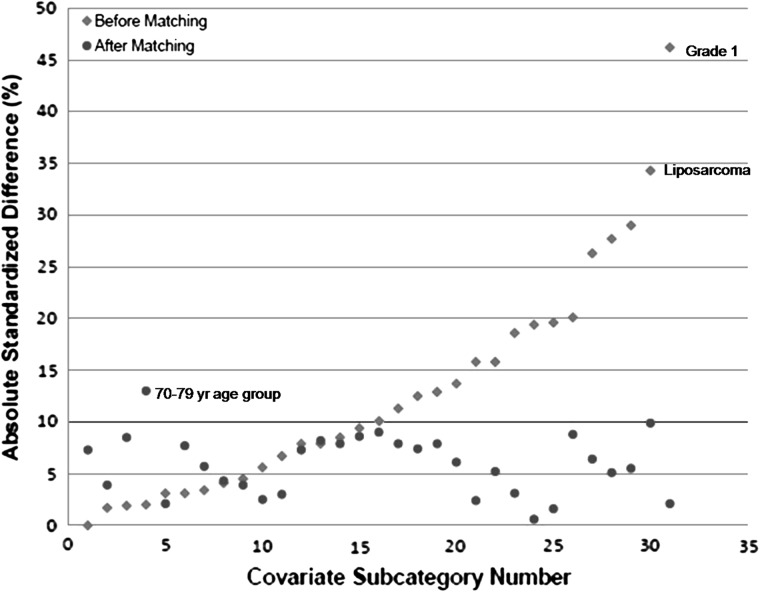
As previously mentioned, the goal of this study was not only to investigate the effect of radiation therapy on disease-specific survival by Cox proportional hazards modeling but also to approach the same question from a different angle using a propensity score matching analysis [3, 4]. Prior to propensity score matching, the study population contained 762 patients (204 patients who received radiation plus surgery and 558 patients who received surgery alone). After propensity score matching, the study population consisted of 471 patients (162 patients who received radiation plus surgery and 309 patients who received surgery alone). Table 3 lists the covariate differences between the two groups both before and after matching. Before matching, significant imbalances between the two groups were observed with respect to age, race, diagnosis period, geographic region, tumor histology, tumor grade, and disease stage categories. As anticipated, patients who received radiation therapy tended to have higher grade tumors and more advanced stage compared with patients who received surgery alone. Propensity score matching was effective in reducing the absolute standardized difference to < 10% for all covariates except the 70–79 years subcategory within the age covariate. Although age by decade was independently associated with survival as a whole, it is important to note that the 70–79 years group did not reach statistical significance in the Cox proportional hazards model. Most importantly, matching had equalized significant imbalances in the grade and stage covariates, which were both associated with increased hazard of death in the Cox proportional hazards model (Table 2). The absolute standardized difference for each covariate before and after propensity score matching is represented in Figure 2, which is a visual representation of the beneficial effect of matching on the balance of covariates [17]. Individual subcategories of each covariate are listed as a covariate subcategory number along the x-axis of Figure 2. We also analyzed the type of radiation received and the sequence in which it was received, with very similar results both before and after matching. After matching, 151 of 162 (93.2%) patients received external beam radiation and 130 of 162 (80.3%) of patients received radiation postoperatively. Information regarding radiation dose, field, and toxicity is not available in SEER.
Table 3.
Patient characteristics before and after propensity score matching
| Covariate | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| Surgery alone (N = 558) | Surgery and radiation (N = 204) | da (%) | Surgery alone (N = 309) | Surgery and radiation (N = 162) | da (%) | |
| Age | ||||||
| Mean ± SD | 59.9 ± 14.7 years | 58.5 ± 12.7 years | −0.7 | 58.7 ± 13.2 years | 56.9 ± 12.9 years | −1.1 |
| Median | 60 years | 60 years | 60 years | 57 years | ||
| Age group (years) | ||||||
| 20–29 | 9 (1.6%) | 2 (1.0%) | −5.6 | 3 (1.0%) | 2 (1.2%) | 2.5 |
| 30–39 | 39 (7.0%) | 12 (5.9%) | −4.5 | 18 (5.8%) | 11 (6.8%) | 3.9 |
| 40–49 | 95 (17.0%) | 41 (20.1%) | 7.9 | 65 (21.0%) | 39 (24.1%) | 7.3 |
| 50–59 | 126 (22.6%) | 46 (22.6%) | 0 | 65 (21.0%) | 39 (24.1%) | 7.3 |
| 60–69 | 127 (22.8%) | 58 (28.4%) | 12.9 | 87 (28.2%) | 40 (24.7%) | −7.9 |
| 70–79 | 111 (19.9%) | 39 (19.1%) | −2.0 | 63 (20.4%) | 25 (15.4%) | 13.0 |
| 80+ | 51 (9.1%) | 6 (2.9%) | −26.3 | 8 (2.6%) | 6 (3.7%) | 6.4 |
| Sex | ||||||
| Female | 322 (57.7%) | 111 (54.4%) | −6.7 | 180 (58.3%) | 92 (56.8%) | −3.0 |
| Male | 236 (42.3%) | 93 (45.6%) | – | 129 (41.7%) | 70 (43.2%) | – |
| Race | ||||||
| White | 469 (84.0%) | 164 (80.4%) | −9.4 | 260 (84.1%) | 131 (80.9%) | −8.6 |
| Black | 35 (6.3%) | 12 (5.9%) | −1.7 | 18 (5.8%) | 12 (6.8%) | 3.9 |
| Other | 54 (9.7%) | 28 (13.7%) | 12.5 | 31 (10.0%) | 20 (12.3%) | 7.4 |
| Diagnosis period | ||||||
| 1988–1992 | 69 (12.4%) | 23 (11.3%) | −3.4 | 38 (12.3%) | 17 (10.5%) | −5.7 |
| 1993–1997 | 108 (19.3%) | 37 (18.1%) | −3.1 | 52 (16.8%) | 26 (16.0%) | −2.1 |
| 1998–2002 | 199 (35.7%) | 62 (30.4%) | −11.3 | 99 (32.1%) | 58 (35.8%) | 7.9 |
| 2003–2006 | 182 (32.6%) | 82 (40.2%) | 15.8 | 120 (38.8%) | 61 (37.7%) | −2.4 |
| Marital status | ||||||
| Married | 385 (69.0%) | 139 (68.1%) | −1.9 | 220 (71.2%) | 109 (67.3%) | −8.5 |
| Unmarried | 173 (31.0%) | 65 (31.9%) | – | 89 (28.8%) | 53 (32.7%) | – |
| Geographic region | ||||||
| East | 91 (16.3%) | 20 (9.8%) | −19.4 | 33 (10.7%) | 17 (10.5%) | −0.6 |
| Midwest | 83 (14.9%) | 45 (22.1%) | 18.6 | 63 (20.4%) | 31 (19.1%) | −3.1 |
| South | 53 (9.5%) | 15 (7.3%) | −7.9 | 30 (9.7%) | 12 (7.4%) | −8.2 |
| West | 331 (59.3%) | 124 (60.8%) | 3.1 | 183 (59.2%) | 102 (63.0%) | 7.7 |
| Tumor histology | ||||||
| Leiomyosarcoma | 128 (22.9%) | 61 (29.9%) | 15.8 | 88 (28.5%) | 50 (30.9%) | 5.2 |
| Liposarcoma | 351 (62.9%) | 94 (46.1%) | −34.3 | 166 (53.7%) | 79 (48.8%) | −9.9 |
| Malignant fibrous histiocytoma | 38 (6.8%) | 26 (12.7%) | 20.1 | 28 (9.1%) | 19 (11.7%) | 8.8 |
| Other | 18 (3.2%) | 10 (4.9%) | 8.5 | 12 (3.9%) | 9 (5.5%) | 7.9 |
| Sarcoma NOS | 23 (4.1%) | 13 (6.4%) | 10.1 | 15 (4.8%) | 5 (3.1%) | −9.0 |
| Tumor grade | ||||||
| Grade 1 | 231 (41.4%) | 42 (20.6%) | −46.2 | 83 (26.9%) | 42 (26.0%) | −2.1 |
| Grade 2 | 109 (19.5%) | 46 (22.5%) | 19.6 | 76 (24.6%) | 41 (25.3%) | 1.6 |
| Grade 3 | 88 (15.8%) | 43 (21.1%) | 13.7 | 61 (19.7%) | 36 (22.2%) | 6.1 |
| Grade 4 | 130 (23.3%) | 73 (35.8%) | 27.7 | 89 (28.8%) | 43 (26.5%) | −5.1 |
| Disease stage | ||||||
| Localized | 296 (53.0%) | 79 (38.7%) | −29.0 | 140 (45.3%) | 69 (42.6%) | −5.5 |
| Regional | 262 (47.0%) | 125 (61.3%) | – | 169 (54.7%) | 93 (57.4%) | – |
| Surgery type | ||||||
| Partial/simple | 180 (32.3%) | 62 (30.4%) | −4.1 | 88 (28.5%) | 43 (26.5%) | 4.3 |
| Radical/total | 378 (67.7%) | 142 (69.6%) | – | 221 (71.5%) | 119 (73.5%) | – |
ad, standardized difference.
Figure 2.
Absolute standardized differences before and after propensity score matching. Note that individual subcategories for each covariate are listed as a covariate subcategory number along x-axis.
effect of radiation therapy on disease-specific survival using matched cohort
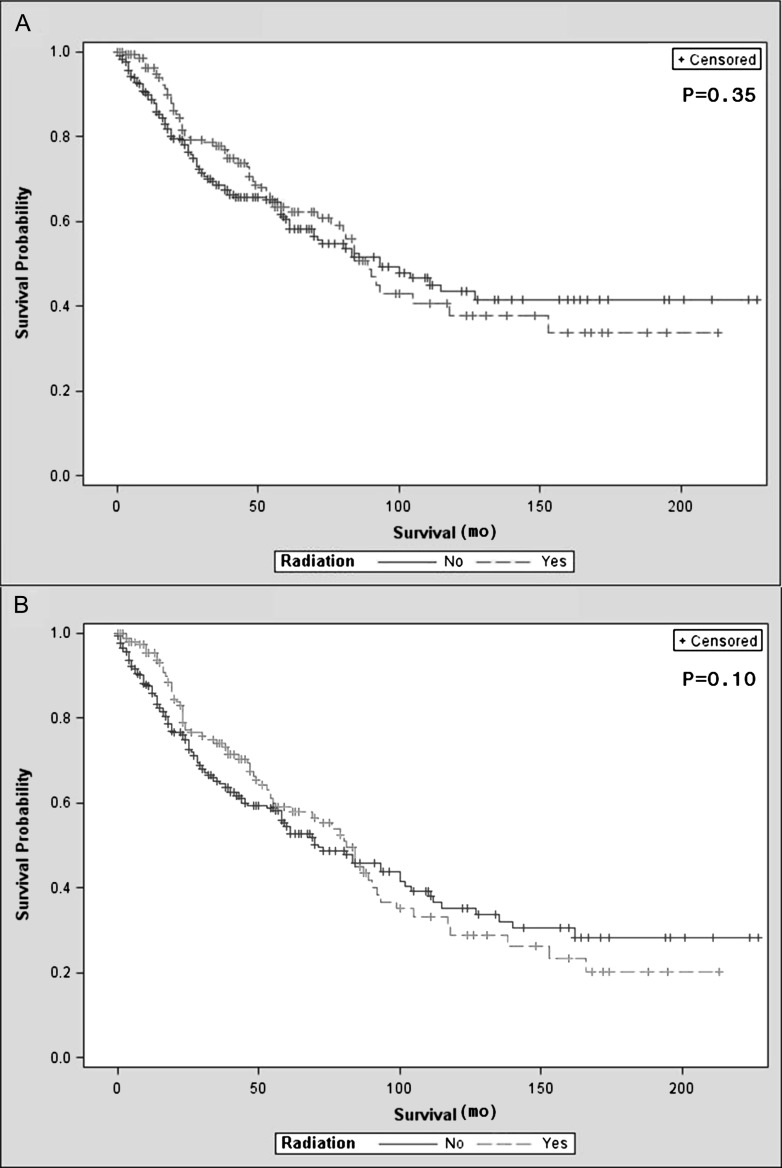
In order to determine the effect of radiation therapy in addition to surgery on disease-specific survival, we analyzed the matched cohorts by Kaplan–Meier method and the censored-data version of the sign test described by Klein and Moeschberger [2]. Propensity score matching generated 309 matched pairs with 162 of the radiation therapy patients being unique. Of these 162 radiation therapy patients, some were matched to two surgery alone patients if a suitable surgery alone patient existed within the propensity score caliper. There was no observed difference in disease-specific (P = 0.35) or overall survival (P = 0.10) between the group who had surgery plus radiation and the group who underwent surgery alone (Figure 3). In a subanalysis of the 309 matched pairs, 234 pairs contained a radiation therapy patient who received postoperative beam radiation matched with a control patient who underwent surgery alone. Analysis of these two groups by Kaplan–Meier method and the censored-data version of the sign test also showed no observed difference in disease-specific survival (P = 0.23). No formal sensitivity analysis was carried out due to the lack of association between receiving radiation therapy and disease-specific survival.
Figure 3.
Kaplan–Meier survival curves comparing surgery plus radiation to surgery alone in matched pairs (309 pairs, 162 unique radiation patients): (A) disease-specific survival; (B) overall survival.
discussion
In two recent SEER studies published by Tseng et al. [18] and Nathan et al. [10], the authors investigated predictors of overall survival after RPS resection using SEER data from 1988 to 2004 and 1988 to 2005, respectively. Both multivariate Cox proportional hazards models found younger age, female sex, liposarcoma histology, and lower grade tumors to be associated with reduced hazard of death. Although we analyzed only a subset of the total cohort of patients within that of Tseng and Nathan et al., our multivariate Cox proportional hazards model related to disease-specific survival demonstrates similar results with respect to patient and tumor factors which influence survival. Tumor grade has been consistently associated with overall survival in multiple prior studies [7–11] as well as localized versus regional extent of disease. One of the interesting differences between our current study and those of Tseng and Nathan et al. was related to the impact of tumor size on survival. Nathan et al. analyzed tumor size using several different size cutpoints as well as size as a continuous variable and found that tumor size did not have a significant impact on survival. Meanwhile, Tseng et al. subdivided tumor size into < 5 cm, 5–10 cm, 10–20 cm, and > 20 cm and reported that when using 10–20 cm tumor size as the referent group, tumors in the 5–10 cm range demonstrated reduced hazard of death in both all cause mortality and sarcoma-specific mortality. Our study did not use tumor size as a covariate due to insufficient patient records regarding tumor size in SEER. Of the 762 patients included in our study, only ∼25% (200/762) had recorded tumor size, making tumor size very difficult to incorporate into the propensity scores. Furthermore, of the patients with recorded tumor size, ∼75% (156/200) of them had tumor size ≥ 10 cm. Although sample sizes of each group were not given, it is possible that the significance of the 5–10 cm group in the Tseng study is due to selection bias, as the same finding did not hold true for the < 5 cm group and the fact that many retroperitoneal sarcomas are of large size when initially diagnosed.
Porter et al. [19] have previously investigated factors associated with receiving radiation therapy in patients with resected RPS using the SEER database. In their multivariate logistic regression, they found age, race, and geographic region to be independently associated with receiving radiation therapy. The results of our multivariate logistic regression coincided with their findings with respect to age and region. Our analysis did not find race to be associated with use of radiation therapy, which may be due to the fact that we used SEER data from 1988 to 2006 compared with the Porter study, which included data from 1973 to 2001. The reason we used data beginning in 1988 was related to the completeness of SEER surgery codes. In addition to the demographic factors analyzed by Porter et al., we also included disease characteristics in our logistic regression and found that disease stage and tumor grade were also independently associated with receiving radiation therapy.
While there was a noted trend toward decreased survival for the radiation group in the survival analysis after ∼80 months, we did not feel this observation warranted further discussion as the findings of the Kaplan–Meier analysis were not statistically significant. Although one could argue that the proportional hazards assumption may not hold true, we carried out a second independent analysis using propensity scoring to confirm whether radiation therapy was associated with increased survival with same overall finding.
Currently no prospective randomized trial has measured the efficacy of neoadjuvant or adjuvant radiation therapy in patients with resected RPS. Recently, Zhou et al. [20] carried out an analysis of SEER data looking at treatment factors that were associated with survival in patients with retroperitoneal sarcoma. Their analysis demonstrated an improvement in unadjusted 2-year overall survival in patients with locoregional disease who underwent surgery and radiotherapy as compared with surgery alone. However, this type of analysis does not take into consideration differences that may exist between groups in terms of covariates which could significantly influence survival, and the follow-up period is limited. Of note, the study did demonstrate a reduced hazard of death in the subgroup of American Joint Committee on Cancer (AJCC) stage I patients who underwent surgery and radiotherapy as compared with surgery alone, but the authors failed to provide data on the effect of radiation therapy in all patients who underwent surgical resection to determine whether radiation therapy was truly independently associated with improved survival. In contrast to the multivariate analysis reported by Zhou et al., our analysis focused only on patients who underwent surgical resection and used grade and tumor stage as covariates within the model instead of covariates used to stratify groups. In addition, our use of propensity scoring provided a method to compare disease-specific and overall survival between groups of matched patients.
It is important to note that our analysis did not evaluate the effect of adjuvant or neoadjuvant radiation therapy on locoregional recurrence or distant metastases following surgical resection. Indeed, there are several studies that have reported excellent local control using a variety of radiation therapy techniques [21–25]. The only randomized controlled trial ever carried out with RPS patients compared intraoperative radiation therapy (IORT) plus low-dose postoperative external beam radiation therapy (EBRT) with high-dose postoperative EBRT [26]. The investigators found the group with IORT demonstrated significantly fewer local recurrences, but no difference was observed between the groups with respect to overall survival. The planned randomized control trial ACOSOG Z9031 examining the effect of preoperative EBRT plus resection versus surgery alone was closed due to slow patient accrual. At the time of writing, the European Organization for Research and Treatment of Cancer (EORTC) protocol 62092 is preparing to accrue patients for a phase III randomized control trial comparing preoperative radiation therapy plus surgery to surgery alone for patients with RPS. However, the results of this study will not be available for many years to come.
The results of our study suggest that patients with surgically resected RPS presently entered into the SEER database who have received radiation therapy have not demonstrated a survival benefit. While the use of databases like SEER can provide a large patient population and long-term survival data, there are also limitations inherent in this type of population-based study. As previously mentioned, tumor size was not included as a covariate for the propensity score matching due to limited tumor size information in SEER. While tumor size has been associated with survival in extremity sarcoma, numerous retrospective studies have suggested that tumor size is not associated with survival in RPS [7–11]. Information regarding margin status after resection and details related to radiation dose, field, and toxicity are also not available in the SEER database, and it could be argued that these might significantly influence survival. However, as the primary role of radiation is to reduce locoregional recurrence, the lack of impact on disease-specific survival may relate to a lack of impact on the development of distant metastatic disease. In the absence of results from a randomized control trial, it is difficult to demonstrate that radiation therapy favorable impacts disease-specific or overall survival in patients with resected RPS.
funding
National Institutes of Health (K23 CA109115-01A5 to J.A.K).
disclosure
The authors declare no conflict of interest.
references
- 1.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Klein J, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer-Verlag; 1997. [Google Scholar]
- 3.Rosenbaum P, Rubin D. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 4.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 6. Surveillance, Epidemiology, and End Results (SEER) Program. Seer Limited-Use Record Description Cases Diagnosed in 1973–2006. Bethesda, MD: National Cancer Institute 2008.
- 7.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 8.Chiappa A, Zbar AP, Bertani E, et al. Primary and recurrent retroperitoneal soft tissue sarcoma: prognostic factors affecting survival. J Surg Oncol. 2006;93:456–463. doi: 10.1002/jso.20446. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan H, Raut CP, Thornton K, et al. Predictors of survival after resection of retroperitoneal sarcoma: a population-based analysis and critical appraisal of the AJCC staging system. Ann Surg. 2009;250:970–976. doi: 10.1097/SLA.0b013e3181b25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Leslie S, Thiebaud P. Using propensity scores to adjust for treatment selection bias. Proceedings of the SAS® Global Forum 2007 Conference. Cary, NC: SAS Institute Inc. 2007. [Google Scholar]
- 13.Coca-Perraillon M. Matching with Propensity Scores to Reduce Bias in Observational Studies. Philadelphia, PA: North East SAS Users Group (NESUG); 2006. [Google Scholar]
- 14.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierie JP, Betensky RA, Choudry U, et al. Outcomes in a series of 103 retroperitoneal sarcomas. Eur J Surg Oncol. 2006;32:1235–1241. doi: 10.1016/j.ejso.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng WH, Martinez SR, Do L, et al. Lack of survival benefit following adjuvant radiation in patients with retroperitoneal sarcoma: a SEER analysis. J Surg Res. 2011;168:e173–e180. doi: 10.1016/j.jss.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer. 2006;106:1610–1616. doi: 10.1002/cncr.21761. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z, McDade TP, Simons JP, et al. Surgery and radiotherapy for retroperitoneal and abdominal sarcoma: both necessary and sufficient. Arch Surg. 2010;145:426–431. doi: 10.1001/archsurg.2010.70. [DOI] [PubMed] [Google Scholar]
- 21.Alektiar KM, Hu K, Anderson L, et al. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2000;47:157–163. doi: 10.1016/s0360-3016(99)00546-5. [DOI] [PubMed] [Google Scholar]
- 22.Krempien R, Roeder F, Oertel S, et al. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2006;65:773–779. doi: 10.1016/j.ijrobp.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Tepper JE, Suit HD, Wood WC, et al. Radiation therapy of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1984;10:825–830. doi: 10.1016/0360-3016(84)90383-3. [DOI] [PubMed] [Google Scholar]
- 24.Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 25.Zagar TM, Shenk RR, Kim JA, et al. Radiation therapy in addition to gross total resection of retroperitoneal sarcoma results in prolonged survival: results from a single institutional study. J Oncol. 2008;2008:824036. doi: 10.1155/2008/824036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128:402–410. doi: 10.1001/archsurg.1993.01420160040005. [DOI] [PubMed] [Google Scholar]