Abstract
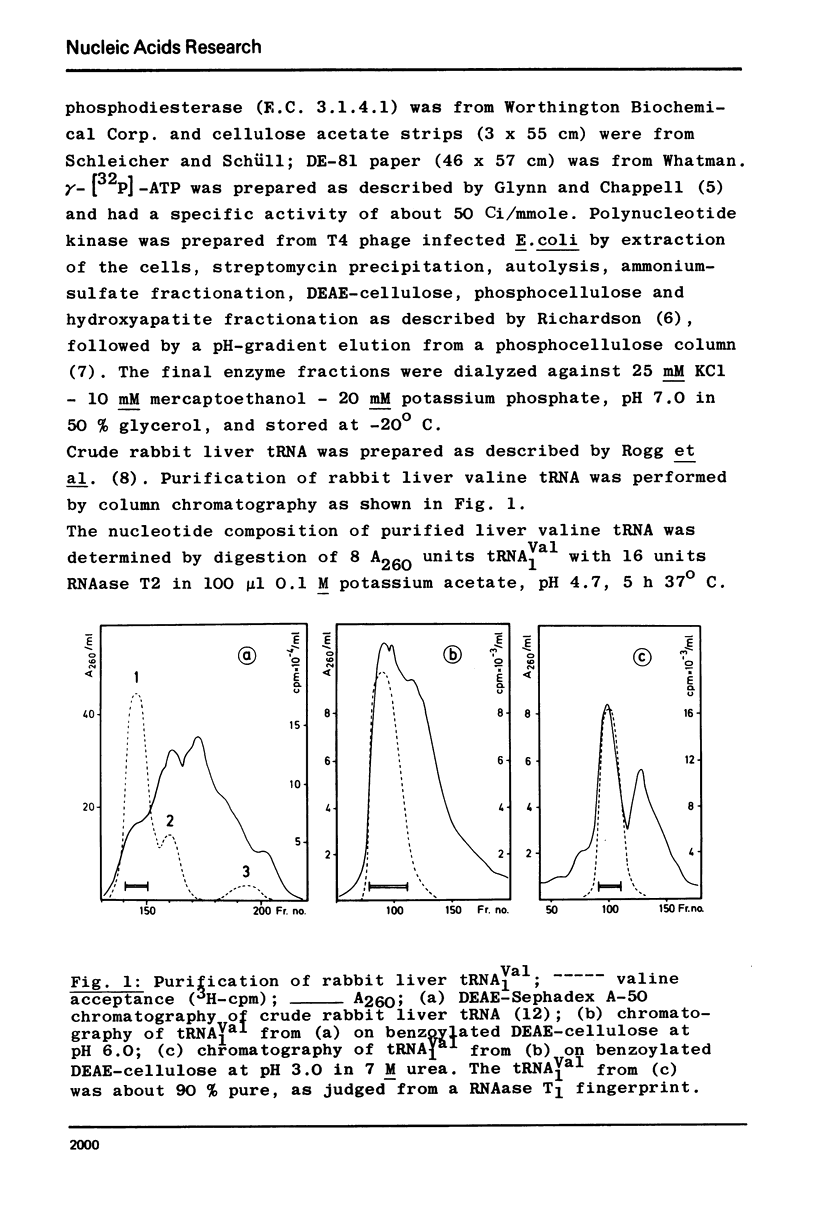
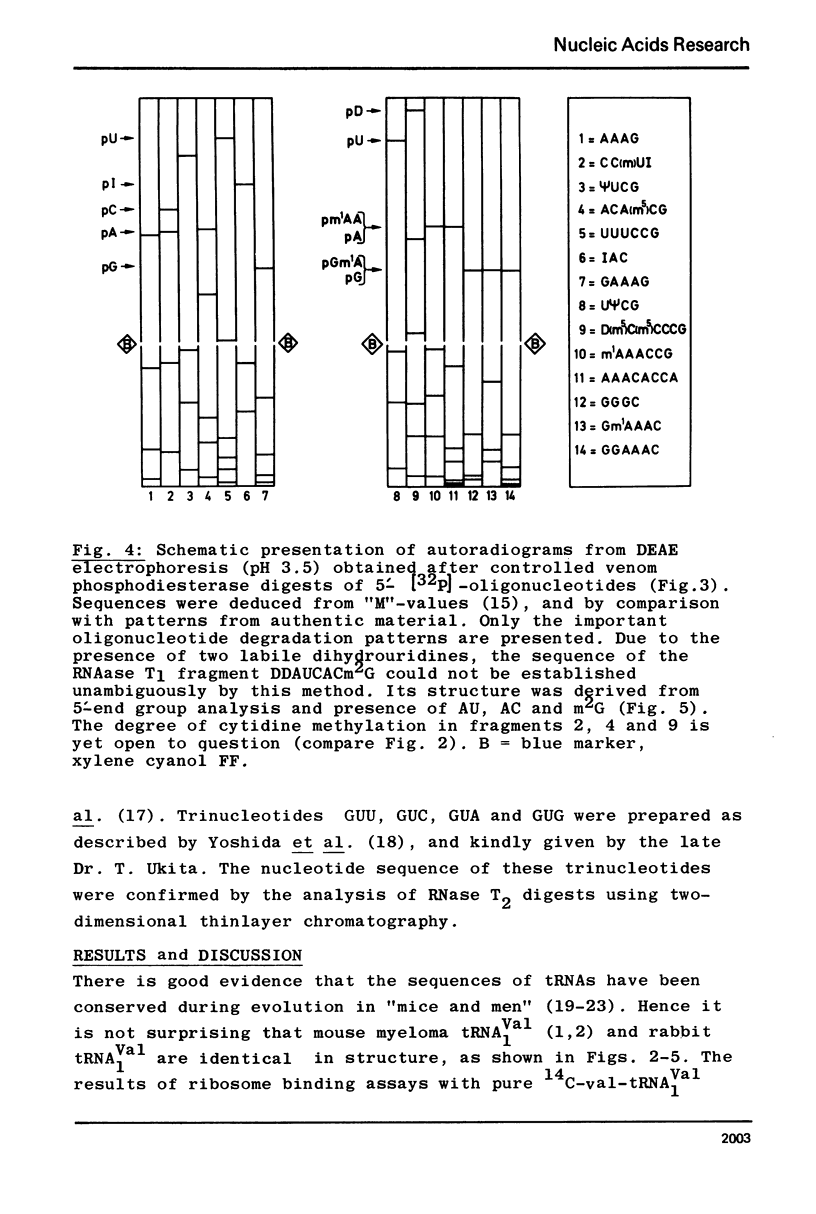
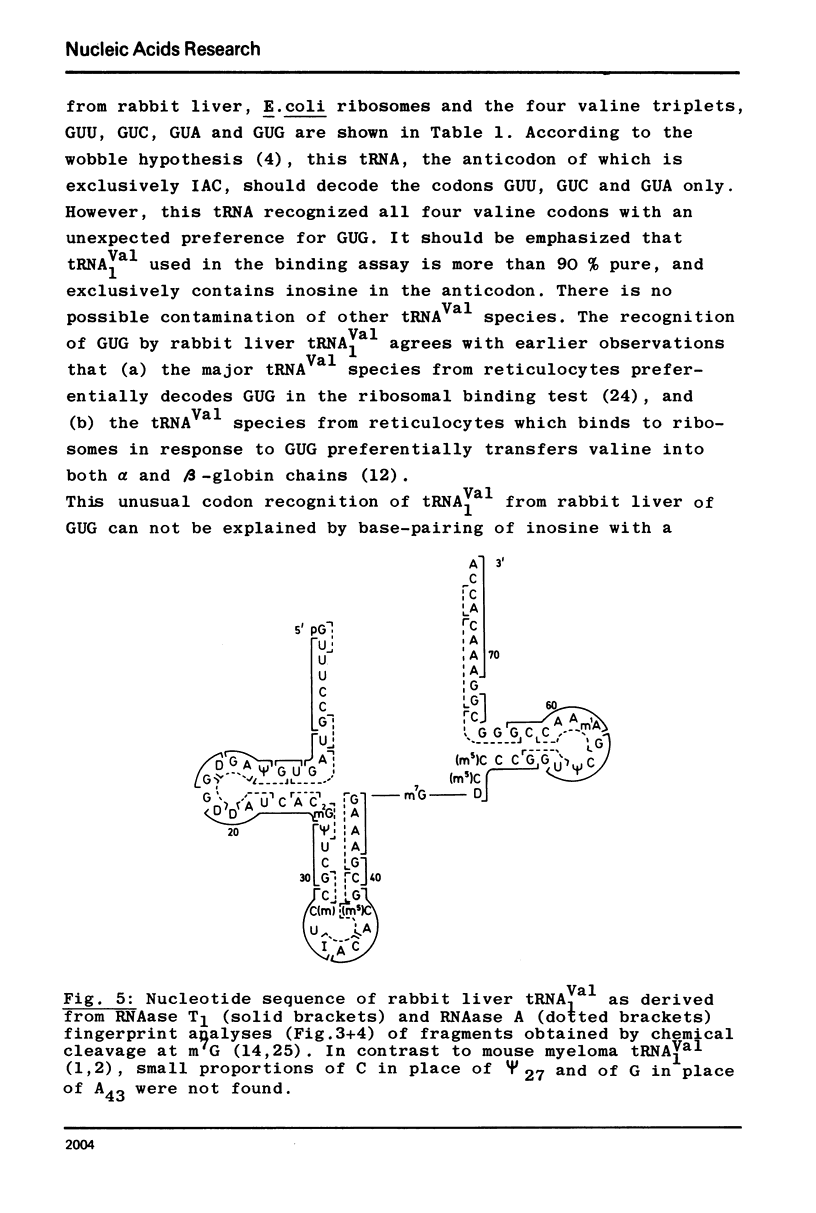
The major valine acceptor tRNA1Val from rabbit liver was purified and its nucleotide sequence determined by in vitro [32P] - labeling with T4 phage induced polynucleotide kinase and finger-printing techniques. Its primary structure was found to be identical with the major valine tRNA from mouse myeloma cells. According to the wobble hypothesis this tRNA, which exclusively has an IAC anticodon, should decode the valine codons GUU, GUC and GUA only. However, this tRNA recognizes all four valine codons with a surprising preference for GUG. It is unknown whether this is due to the lack of A37 modification next to the 3' end of the anticodon IAC. The nature of the inosine-guanosine interaction remains to be clarified.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brambilla R., Rogg H., Staehelin M. Unexpected occurrence of an aminoacylated nucleoside in mammalian tRNATyr. Nature. 1976 Sep 9;263(5573):167–169. doi: 10.1038/263167a0. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Files J. G., Weber K., Miller J. H. Translational reinitiation: reinitiation of lac repressor fragments at three internal sites early in the lac i gene of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Mar;71(3):667–670. doi: 10.1073/pnas.71.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank P., Gross H. J. Methyl-deficient mammalian transfer RNA: II. Homologous methylation in vitro of liver tRNA from normal and ethionine-fed rats: ethionine effect on 5-methyl-cytidine synthesis in vivo. Nucleic Acids Res. 1974 Oct;1(10):1259–1267. doi: 10.1093/nar/1.10.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank P., Riesner D., Gross H. J. Rabbit liver tRNA1Val:II. unusual secondary structure of T psi C stem and loop due to a U54:A60 base pair. Nucleic Acids Res. 1977 Jun;4(6):2009–2200. doi: 10.1093/nar/4.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith G., Ebel J. P., Dirheimer G. The primary structure of two mammalian tRNAs Phe: identity of calf liver and rabbit liver tRNAs Phe. FEBS Lett. 1974 Nov 1;48(1):50–52. doi: 10.1016/0014-5793(74)81059-8. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutzky R., Peterssen-Borstel H., Flosdorf J. Large scale enzymatic synthesis of nucleoside-5'-monophosphates using a phosphotransferase from carrots. Biotechnol Bioeng. 1974 Nov;16(11):1449–1458. doi: 10.1002/bit.260161103. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Lagerkvist U. Codon-acticodon recognition in the valine codon family. J Biol Chem. 1977 Jan 25;252(2):471–478. [PubMed] [Google Scholar]
- Nirenberg M., Caskey T., Marshall R., Brimacombe R., Kellogg D., Doctor B., Hatfield D., Levin J., Rottman F., Pestka S. The RNA code and protein synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:11–24. doi: 10.1101/sqb.1966.031.01.008. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Zachau G. Partial degradation of transfer RNAs and transfer RNA fragments by spleen phosphodiesterase as studied by disc electrophoretic methods. Biochim Biophys Acta. 1972 Sep 14;277(3):523–538. doi: 10.1016/0005-2787(72)90095-0. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. Primary structure of a mouse myeloma cell initiator transfer RNA. Nature. 1974 Feb 22;247(5442):516–518. doi: 10.1038/247516a0. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Clark B. F. The nucleotide sequences of cytoplasmic methionine and valine tRNAs from mouse myeloma cells. FEBS Lett. 1974 Oct 1;47(1):56–59. doi: 10.1016/0014-5793(74)80425-4. [DOI] [PubMed] [Google Scholar]
- Piper P. W. The primary structure of the major cytoplasmic valine tRNA of mouse myeloma cells. Eur J Biochem. 1975 Feb 3;51(1):295–304. doi: 10.1111/j.1432-1033.1975.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Anandaraj M. P., Chia L. S., Randerath E., Gupta R. C., Randerath K. Sequence studies on tRNAPhe from placenta: comparison with known sequences of tRNAPhe from other normal mammalian tissues. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1097–1105. doi: 10.1016/0006-291x(75)90470-2. [DOI] [PubMed] [Google Scholar]
- Rogg H., Wehrli W., Staehelin M. Isolation of mammalian transfer RNA. Biochim Biophys Acta. 1969 Nov 19;195(1):13–15. doi: 10.1016/0005-2787(69)90597-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., Petrissant G., Rajbhandary U. L. Replacement of the sequence G-T-phi-C-G(A)- by G-A-U-C-G- in initiator transfer RNA of rabbit-liver cytoplasm. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2600–2604. doi: 10.1073/pnas.70.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L., Boisnard M., Petrissant G. Nucleotide sequence of rabbit liver and sheep mammary gland cytoplasmic initiatory transfer RNAs. Nature. 1974 Feb 22;247(5442):518–520. doi: 10.1038/247518a0. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söll D., Jones D. S., Ohtsuka E., Faulkner R. D., Lohrmann R., Hayatsu H., Khorana H. G. Specificity of sRNA for recognition of codons as studied by the ribosomal binding technique. J Mol Biol. 1966 Aug;19(2):556–573. doi: 10.1016/s0022-2836(66)80023-2. [DOI] [PubMed] [Google Scholar]
- Takemoto T., Takeishi K., Nishimura S., Ukita T. Transfer of valine into rabbit haemoglobin from various isoaccepting species of valyl-tRNA differing in codon recognition. Eur J Biochem. 1973 Oct 18;38(3):489–496. doi: 10.1111/j.1432-1033.1973.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976 Sep 23;263(5575):289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- Wildenauer D., Gross H. J. Methyldeficient mammalian 4s RNA: evidence for L-ethionine-induced inhibition of N6-dimethyladenosine synthesis in rat liver tRNA. Nucleic Acids Res. 1974 Feb;1(2):279–288. doi: 10.1093/nar/1.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. A specific chemical chain scission of tRNA at 7-methylguanosine. FEBS Lett. 1970 Dec;11(3):160–164. doi: 10.1016/0014-5793(70)80518-x. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Furuichi Y., Kaziro Y., Ukita T. The modification of nucleosides and nucleotides. IX. Inactivation of coding response of yeast tRNA containing inosine residue by cyanoethylation. Biochim Biophys Acta. 1968 Oct 29;166(3):636–645. [PubMed] [Google Scholar]
- van Calker D., Hilse K. Properties of isoaccepting tRNA-Val from rabbit reticulocytes; fractionation and codon recognition. FEBS Lett. 1974 Feb 1;39(1):56–60. doi: 10.1016/0014-5793(74)80016-5. [DOI] [PubMed] [Google Scholar]


