Abstract
Background
Bone metastases are a significant and undertreated clinical problem in patients with advanced lung cancer.
Design
We reviewed the incidence of bone metastases and skeletal-related events (SREs) in patients with lung cancer and examined the burden on patients' lives and on health care systems. Available therapies to improve survival and lessen the impact of SREs on quality of life (QoL) were also investigated.
Results
Bone metastases are common in lung cancer; however, owing to short survival times, data on the incidences of SREs are limited. As with other cancers, the costs associated with treating SREs in lung cancer are substantial. Bisphosphonates reduce the frequency of SREs and improve measures of pain and QoL in patients with lung cancer; however, nephrotoxicity is a common complication of therapy. Denosumab, a recently approved bone-targeted therapy, is superior to zoledronic acid in increasing the time to first on-study SRE in patients with solid tumours, including lung cancer. Additional roles of bone-targeted therapies beyond the prevention of SREs are under investigation.
Conclusions
With increasing awareness of the consequences of SREs, bone-targeted therapies may play a greater role in the management of patients with lung cancer, with the aim of delaying disease progression and preserving QoL.
Keywords: bone metastases, lung neoplasms, neoplasm metastases, skeletal-related events, quality of life
introduction
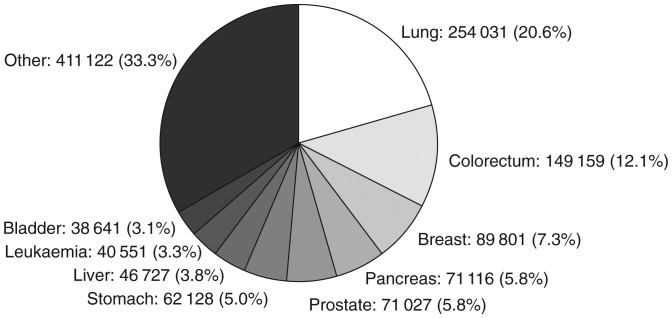
Lung cancer is the most common neoplasm worldwide, with an estimated 1.61 million new cases reported in 2008 [1]. In the European Union alone, lung cancer was responsible for ∼254 000 deaths, equating to 20.6% of cancer mortality (Figure 1). Overall survival rates are poor, with data from 2000 to 2002 indicating 1- and 5-year relative survival expectations for ∼37% and 12% of patients, respectively [2]. Non-small-cell lung cancer (NSCLC) accounts for 80%–85% of all lung cancer diagnoses [3], the majority of which present as late-stage disease [4], primarily owing to the asymptomatic nature of early disease.
Figure 1.
Cancer-related mortality in the European Union in 2008. Data represent estimated numbers of cancer deaths in females and males across all ages (total deaths = 1 234 303). Data from GLOBOCAN 2008 v1.2 [1].
Platinum-based combination chemotherapy prolongs survival in patients with NSCLC who have a good performance status and remains the first-line standard of care [3]. Both pemetrexed, for those with non-squamous NSCLC, and erlotinib maintenance treatment prolong overall survival in patients with advanced NSCLC whose disease has not progressed immediately following platinum-based chemotherapy [5, 6]. Other ‘individualised’ first-line treatments [e.g. monoclonal antibodies, such as bevacizumab, which targets vascular endothelial growth factor (VEGF) [7], and cetuximab, the epidermal growth factor receptor (EGFR) [8], or the tyrosine kinase inhibitors (TKIs) erlotinib and gefitinib [9–12]] have shown promise in some patients but have not significantly improved survival in overall populations.
As the life expectancy of individuals with lung cancer increases, symptom control measures are growing in importance. Therefore, physicians require an increased awareness of bone metastases and the need for their early management to prevent potentially debilitating and costly skeletal complications.
We present an overview of the prevalence, impact and treatment of bone metastases in lung cancer. In recent years, bisphosphonates have been the mainstay of pharmacological intervention for reducing the symptoms associated with bone metastases and the impact of the disease on quality of life (QoL). Bisphosphonates target the underlying cause of skeletal morbidity by binding to the bone surface and inhibiting osteoclast-mediated bone resorption. Bisphosphonates are, however, associated with nephrotoxicity, which requires monitoring and may necessitate initial dose adjustment and withholding of doses. Therefore, combining bisphosphonates with commonly used platinum-based chemotherapy as first-line treatment is complicated by the overlapping renal safety profiles of the two therapies.
Denosumab is a new treatment option with the potential to improve QoL for patients with bone metastases secondary to lung cancer. This agent binds to and neutralises receptor activator of nuclear factor κB (RANK) ligand (RANKL), a key molecule involved in osteoclast differentiation and survival [13–15], thereby inhibiting bone resorption [16]. In metastatic cancers involving the bone, denosumab has been shown to suppress markers of bone resorption [17–19]. This fully human monoclonal antibody, which targets the bone-remodelling pathway, is not cleared by the kidneys and is therefore not associated with the same problems as bisphosphonates in patients with renal impairment.
bone metastases and skeletal-related events
Lung cancer frequently spreads to bone, with metastases evident at post-mortem in up to 36% of patients [20] and bone marrow micrometastases found in 22%–60% of individuals [21]. The bone microenvironment is exposed to many growth factors and cytokines that provide a fertile ‘soil’ for cancer cells, making bone a preferred site of metastasis in advanced cancer. Individuals with lung cancer and bone metastases have poor prognoses with median survival times from detection of metastases typically measured in months [20]. Most patients who develop bone metastases experience complications such as hypercalcaemia, severe bone pain requiring palliative radiotherapy or analgesics, pathological fractures, spinal cord compression and bone instability requiring orthopaedic surgery. The last four of these complications are collectively known as skeletal-related events (SREs), although some historical studies also included hypercalcaemia in this grouping.
SREs are a complication of the unrestricted resorption of mineralised bone by osteoclasts and result in significant morbidity, requiring frequent hospitalisation, outpatient visits and reduced QoL [22, 23]. Unfortunately, screening and treatment of asymptomatic bone metastases are not considered necessary in clinical practice. Consequently, bone metastases are often not diagnosed in individuals with NSCLC until they cause substantial pain or an SRE [24]. It is therefore important to raise both patient and physician awareness of bone metastases in lung cancer. Furthermore, therapy should be considered at the time of bone metastasis detection, before debilitating pain develops and SREs are experienced. Positron-emission tomography scans may be useful for early detection of asymptomatic bone metastases [25–27]; however, recent European Society for Medical Oncology (ESMO) guidelines recommend a bone scan only if there is bone pain, hypercalcaemia or elevated alkaline phosphatase levels [3].
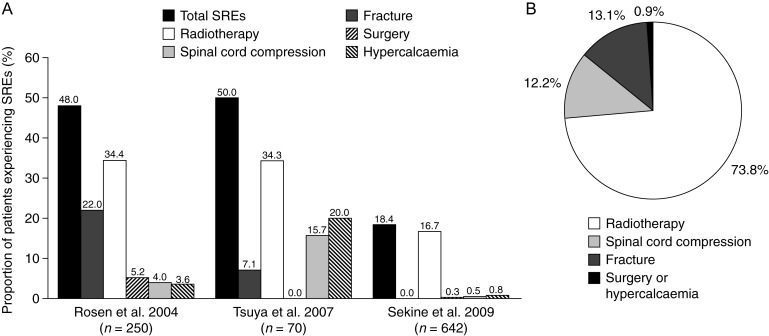
Owing to the historically short survival time in patients with NSCLC, reports of SRE frequency in this population are limited to data from the placebo arm of a large clinical trial [28, 29], retrospective studies from Asia [30–32] (Figure 2) and a Serbian bone scintigraphy study [33].
Figure 2.
Overview of the occurrence of SREs in patients with NSCLC. (A) Data from the placebo arm of a large clinical trial including patients with NSCLC (Rosen et al. 2004 [28]) and two retrospective audits of patients with NSCLC in Japan (Tsuya et al. 2007 [32] and Sekine et al. 2009 [30]). (B) Data from a retrospective audit of 273 patients with NSCLC and bone metastases in Korea [31]. NSCLC, non-small-cell lung cancer; SRE, skeletal-related event.
In a large multinational, randomised, double-blind phase III trial of zoledronic acid versus placebo in patients with bone metastases secondary to lung cancer and other solid tumours (except carcinomas of the breast and prostate) [28, 29], 46% of individuals treated with placebo experienced at least one SRE during the 21-month study, with an overall average of 2.71 SREs per year in the placebo arm [28]. A breakdown of the types of SREs experienced is shown in Figure 2A. A retrospective exploratory analysis revealed that before study entry, 69% of all randomised patients had experienced at least one SRE, and that these individuals had a higher risk of a subsequent SRE than those with no previous SREs (odds ratio: 1.41). During the study, the median time to first SRE among the subgroup of patients who had previously experienced an SRE and were subsequently randomised to placebo was ∼3.5 months [34].
The Serbian study retrospectively analysed 100 patients with lung cancer who underwent bone scintigraphy during a 3-year period (2003–2005) [33]. Bone metastases were confirmed in 57% of patients, with suspicious findings recorded in 11% of individuals [33].
Results from retrospective studies from Japan [30, 32] and Korea [31] confirmed these findings. In one Japanese study, the charts of all patients with NSCLC treated from February 2002 to January 2005 at a single hospital were analysed for disease stage [using the tumour–node–metastasis (TNM) staging system], presence of bone metastases, frequency of SREs and survival [32]. Of 230 assessable individuals, 70 (30.4%) had bone metastases during their treatment, consistent with the frequency reported from autopsy studies [20]. Bone metastases were evident at the time of initial diagnosis in 46 of these 70 patients (65.7%) [32]. Moreover, of patients with bone metastases, 50.0% experienced SREs, the most common of which were radiotherapy to bone (34.3%) and hypercalcaemia (20.0%; Figure 2A). Among 135 individuals with stage IV NSCLC, 41.5% had bone metastases; 44.6% of those with bone metastases experienced SREs (Table 1). Median survival time was shorter (187 days) for patients with SREs than for those without (366 days; Table 1), although this difference was not statistically significant.
Table 1.
Comparison of median survival times of patients with stage III or stage IV NSCLC, with or without bone metastases and SREs
| NSCLC stage | Patients, n | Median survival time (days) |
|---|---|---|
| Stage III | ||
| No bone metastases | 81 | 314 |
| ≥1 bone metastases | 14 | 298 |
| Stage III + bone metastasesa | ||
| No SRE | 4 | 255 |
| ≥1 SRE | 10 | 240 |
| Stage IV | ||
| No bone metastases | 79 | 268 |
| ≥1 bone metastases | 56 | 237 |
| Stage IV + bone metastases | ||
| No SRE | 31 | 366 |
| ≥1 SRE | 25 | 187 |
Data from a retrospective audit of patients with NSCLC in Japan [32].
aFollowing initial treatment.
NSCLC, non-small-cell lung cancer; SRE, skeletal-related event.
[Copyright (2007), with permission from Elsevier]
The second Japanese study retrospectively analysed 642 patients with metastatic NSCLC treated from December 2000 to June 2006 and showed that median survival was 15.4 months [30]. First-line platinum-based chemotherapy was given to 73.1% of patients, and 18.2% of patients were treated with gefitinib. Only 6.6% of patients received the bisphosphonate zoledronic acid. In total, 118 (18.4%) patients experienced SREs (Figure 2A), 40.7% of which were within 6 months of starting first-line antitumour therapy. A further 27.1% of individuals experienced an SRE 6–12 months after commencing treatment. Multivariate analysis revealed that men, patients with a performance status of 2–3 and those with multiple bone metastases were at greatest risk of a first SRE.
Finally, a more recent Korean retrospective study of 273 patients with bone metastases secondary to NSCLC treated from January 2006 to March 2009 showed that 62.6% had at least one SRE and 16.8% experienced multiple SREs [31]. Radiotherapy to bone was by far the most common SRE reported (Figure 2B). Analysis of risk factors for SREs suggested that long-term smoking, non-adenocarcinoma tumours, poor performance status and no history of treatment with EGFR TKIs were predictors for SREs [31]. Surprisingly, only 20.9% of patients with bone metastases were receiving bisphosphonates and only 6.6% received a bone-targeted agent before experiencing an SRE.
why treat bone metastases and SREs?
Bone metastases are a significant cause of morbidity in patients with advanced cancer. The frequency of SREs varies across tumour types, but on average, individuals experience an SRE every 3–6 months [20]. These events typically occur around periods of disease progression, becoming more frequent as the disease becomes more extensive [20]. Owing to recent advances in systemic treatment of NSCLC, the median survival of patients with advanced disease has increased to ∼1 year. This may give tumours more time to metastasise to bone, so SREs may become a more common problem. Even with the relatively short survival time for patients with NSCLC, a large percentage will experience SREs. Moreover, once an individual experiences a first SRE, they are likely to experience subsequent events, leading to a spiral of debilitating and costly bone problems. Therefore, there is a need to consider treatments that can reduce the risk of SREs.
pain and reduced QoL
Bone metastases and SREs are associated with significant pain and reduced QoL, with negative effects on day-to-day functioning. Indeed, pain from bone metastases is the most frequent form of pain reported by patients with cancer and is often disproportionate to the extent of bone involvement [35]. The pain associated with bone metastases frequently necessitates strong analgesia or palliative radiation. Strong narcotics, such as morphine, are stigmatised by connotations of addiction and their association with death [36], and therefore, patients may be reluctant to use them. Furthermore, narcotics may suppress respiration, which is unwelcome in individuals with lung cancer.
The pathophysiological mechanisms of pain in patients with bone metastases have not been fully elucidated but may include tumour-induced osteolysis, production of tumour growth factors and cytokines, nerve infiltration, ion channel stimulation and production of endothelins and nerve growth factors in local tissues [20]. Bone metastases commonly occur at the base of the skull, the vertebral column or pelvic and femoral areas. Metastases at the base of the skull are associated with cranial nerve palsies, neuralgias and headaches, while vertebral metastases produce neck and back pain, with or without neurological complications secondary to epidural extension. Pelvic and femoral lesions produce pain in the lower back and limbs, often associated with mechanical instability and incident pain. Bone metastases can also result in pathological fractures (most commonly of the ribs and vertebrae), which cause pain and have a detrimental effect on QoL [20]. Pathological fractures and spinal cord compression impair mobility and functional independence.
Despite the prospect of severe pain and reduced QoL, there are few data on patient-reported outcomes [37]. In an Italian study of 1021 individuals enrolled in three randomised trials of chemotherapy for NSCLC, QoL (using the European Organisation for Research and Treatment of Cancer core questionnaire and the lung cancer-specific module), analgesic use and adequacy of pain management (using the Pain Management Index) were assessed. Bone metastases were present in 22% of patients and were associated with some degree of pain in almost 75% of these cases [22]. Almost 50% of participants reported that pain affected their daily activities (30% a little, 16% quite a bit and 3% very much) and the severity of pain was linearly correlated with decreases in QoL scores; mean global QoL decreased from 64.9 for patients without pain to 36.4 for those with severe pain (P < 0.001). The study revealed that 82% of individuals reporting pain received inadequate analgesia. Although analgesics may be used to treat the symptoms of bone pain, they do not address the underlying cause, and side-effects of opiates, such as nausea and constipation, may be worse than the pain itself [38]. Therefore, delaying the need to use strong analgesics may be advantageous in patients with lung cancer.
A Norwegian study evaluated pain, QoL, depression, physical functioning and social functioning at the time of enrolment into a randomised clinical trial of 157 oncology outpatients with pain from bone metastases [39]. One aim of the study was to determine which of the following were key predictors of QoL: pain characteristics (i.e. severity, duration, meaning of pain and perceived availability and efficacy of pain relief), psychological distress (e.g. depression), physical functioning and social functioning. Another aim was to determine the extent to which all of these variables were correlated. The results showed that ‘meaning of pain’ (patients' perception of pain [40]) was significantly correlated with all the other variables, particularly pain intensity and duration. The key factors that predicted QoL were depression, social functioning and physical functioning, with depression proving to be the most important of these [39].
cost
Cancer-related bone disease contributes significantly to health care costs. In 2004, the cost of metastatic bone disease in the USA estimated by the National Institutes of Health was US$12.6 billion or 17% of total oncology expenditure [41]. In this analysis, the estimated number of patients with lung cancer was 237 469, with an estimated metastatic bone disease rate of 15.6%. The increase in expenditure following metastatic bone disease diagnosis was ∼US$36 000 in commercially insured patients with lung cancer when compared with a matched-control group of patients who did not have metastatic disease (P < 0.001).
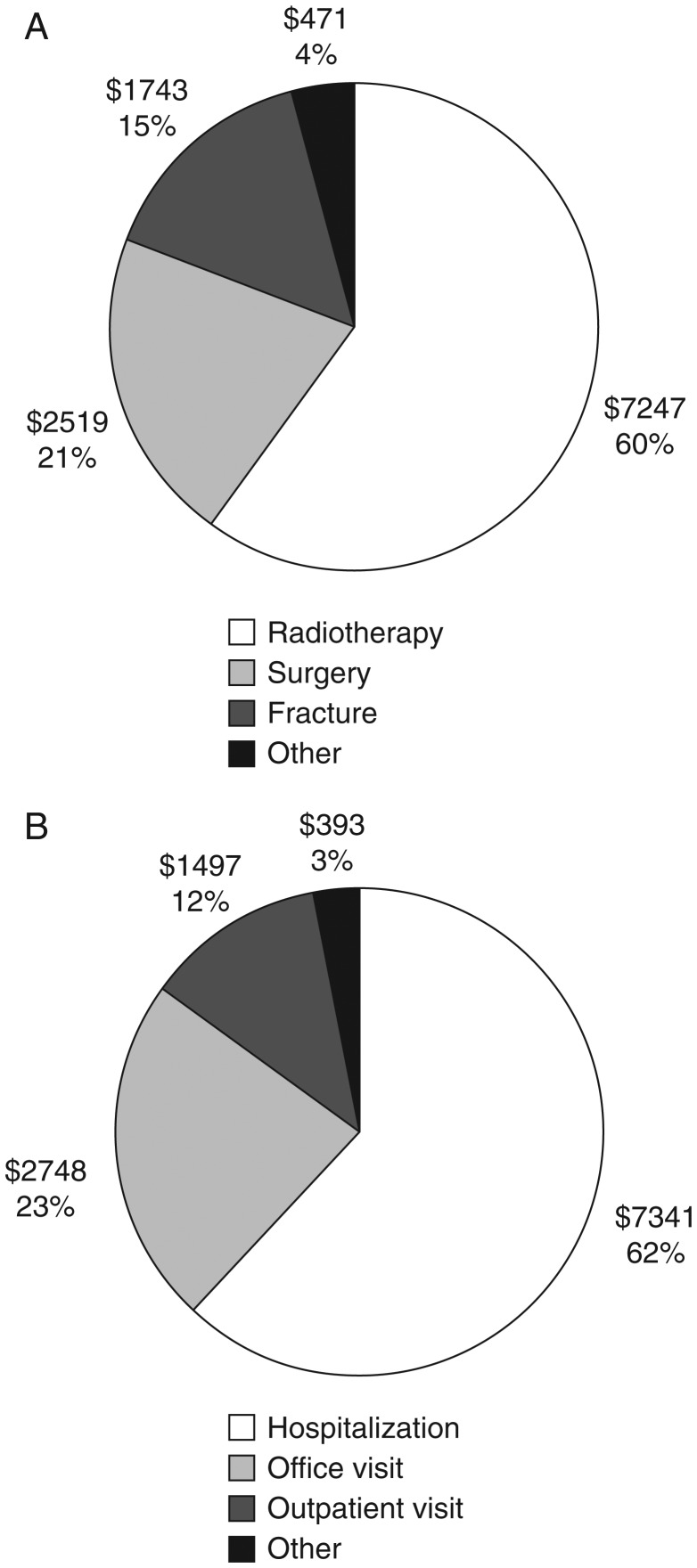
An earlier retrospective analysis of patients identified in a USA insurance claims database from 1995 to 2002 showed that 55% of 534 individuals with lung cancer and bone metastases experienced one or more SRE, with a median survival time after the first SRE of only 4.1 months. On average, the first SRE-related claim was only 1.2 months after the diagnosis of bone metastasis, again indicating that early intervention may yield the most benefit in terms of morbidity and cost saving [42]. The estimated lifetime cost of treating patients who experienced one or more SREs was ∼US$12000 per patient, with most of the cost (80%) incurred in the 2 months after the first claim for an SRE. Most costs were associated with radiotherapy to bone (60%; US$7247), but surgery to bone (21%; US$2519) and treatment of fractures (15%; US$1743) were also a significant burden (Figure 3A). The breakdown of the costs according to the types of services used by patients is shown in Figure 3B. This study therefore shows that the economic burden of treating SREs associated with bone metastases secondary to lung cancer is substantial. Importantly, this study focused on costs that could be directly attributed to SREs and did not consider peripheral costs such as physician visits and outpatient procedures. Therefore, it is likely to underestimate the true cost associated with SREs. Further analysis of this study revealed that total medical costs were US$48 173 greater for patients with an SRE versus those without [43].
Figure 3.
Estimated costs of skeletal complications in patients with lung cancer by (A) type of SRE and (B) type of service. Values are in US$. Data are based on Kaplan–Meier-estimated costs of SRE-related care in patients with bone metastases arising from lung cancer who experienced at least one SRE (n = 295) [42]. In (A), ‘other’ includes opiods/nonsteroidal anti-inflammatory drugs (US$133), physical medicine (US$115), spinal cord compression (US$77) and non-operative treatment of fractures (US$71). In (b), ‘other’ includes skilled nursing (US$174), outpatient pharmacy (US$133), emergency room visit (US$60), home health (US$25) and laboratory-associated costs (US$1). Figure reproduced from Delea et al. 2004 [42] with permission from S. Karger AG, Basel.
Finally, a more recently reported European study evaluated the burden imposed by SREs on Spanish hospital resources [23]. Admission rates (3-year incidence from 2003) due to bone metastases were 156 per 1000 for patients with lung cancer, increasing to 260 per 1000 following an SRE. This study also examined admission rates and costs associated with breast and prostate cancer; for these neoplasms, first-admission costs increased with the development of bone metastases and SREs. The cost associated with a first admission for lung cancer (€4994) was higher than for metastatic bone disease (€4227) or for an SRE (€4298); this may reflect the severity of lung cancer at initial presentation, again highlighting the need for increased awareness leading to earlier diagnosis. Nevertheless, the cost of an SRE in patients with lung cancer remains high.
current treatments for NSCLC and links with renal and bone health
Combination chemotherapy is the standard of care for NSCLC. Third-generation agents such as pemetrexed, vinorelbine, gemcitabine and taxanes (e.g. paclitaxel and docetaxel), as well as TKIs (e.g. erlotinib and gefitinib) or monoclonal antibodies (e.g. bevacizumab), are often combined with cisplatin or carboplatin in selected patients [3]. However, doses may be limited by the cumulative nephrotoxicity of the platinum-based component [44]. A study in 400 patients with advanced solid tumours treated with weekly high-dose cisplatin showed that ∼40% of patients experienced some level of nephrotoxicity [45]. The cisplatin analogue carboplatin is less nephrotoxic than cisplatin. However, a meta-analysis of nine NSCLC trials (2968 patients) found that carboplatin was less effective than cisplatin in terms of survival [46]. If platinum-based therapy is contraindicated, single or combination use of third-generation agents or TKIs may be considered, although studies generally show lower response rates with these regimens than with platinum-based therapies [3].
In contrast to treatment-induced bone loss associated with the early treatment of patients with breast and prostate cancer, the components of first-line therapy for NSCLC may have beneficial effects on bone resorption. In vitro, cisplatin has been shown to be effective against hypercalcaemia of malignancy by exerting inhibitory effects on osteoclasts and bone resorption [47]. Results from a recent study suggest that, in addition to antitumour effects, gefitinib has inhibitory effects on bone resorption [48]. In addition, treatment with the anti-human VEGF antibody bevacizumab (with zoledronic acid) inhibited the number of experimental bone metastases, including osteoblastic changes in a murine model of human lung cancer [49]. Clinical studies have also shown benefits. In a study examining the effect of chemotherapy on bone metabolism in 30 individuals with stage III NSCLC, two courses of combination mitomycin C, cisplatin and vinblastine resulted in a significant reduction in bone resorption (P < 0.05) [50]. In patients with spinal metastases from lung cancer, gefitinib was effective in reducing pain and inducing normal bone formation [51].
Radiotherapy is also an important treatment to ease symptoms in NSCLC [3]. As discussed above, it also plays a key role in the management of bone metastases. Radiotherapy can be considered following spinal cord compression and for stabilisation following surgery associated with pathological fractures.
In treatment of lung cancer, there is also precedence that early palliative intervention is beneficial. A recent study demonstrated the value of early palliative care in the course of metastatic NSCLC [52]. As well as improvements in QoL and a reduced need for aggressive end-of-life care, early palliative intervention prolonged survival by ∼2 months.
role of bone-targeted therapies in lung cancer
Despite improvements in the primary treatment of lung cancer, SREs still affect many patients and complicate the clinical picture. Recent ESMO guidelines for staging and treatment of NSCLC do not consider the use of bone-targeted therapies for the prevention of SREs [3]. However, recent National Comprehensive Cancer Network guidelines recommend therapy with either bisphosphonates or denosumab for patients with NSCLC [53]. Notably, a panel of European expert physicians recommend that individuals with NSCLC are screened for asymptomatic bone metastases at initial disease staging and treated with bone-targeted therapy if bone metastases are confirmed [54].
Bisphosphonates have shown efficacy in randomised placebo-controlled trials for preventing and delaying SREs and improving QoL in patients with solid tumours, including NSCLC, while concomitantly preventing the increase in pain that accompanies progression of malignant bone disease [28, 55–58]. Zoledronic acid is the bisphosphonate most widely used for the prevention of SREs in patients with bone metastases from advanced cancer and can be used to treat advanced NSCLC [59]. A long-term study of 773 individuals with NSCLC and other solid tumours (excluding breast and prostate) revealed that fewer patients treated with zoledronic acid 4 mg experienced at least one SRE than those taking placebo (zoledronic acid, 39%; placebo, 46%). Furthermore, zoledronic acid significantly delayed the median time to first SRE (236 versus 155 days; P = 0.009), significantly reduced the annual incidence of SREs (1.74 versus 2.71; P = 0.012) and significantly reduced the risk of experiencing an SRE by 31% [hazard ratio (HR) = 0.693; P = 0.003] [28]. When used in conjunction with platinum-based chemotherapy in 32 patients with NSCLC and bone metastases, zoledronic acid 4 mg was recently reported to significantly reduce pain scores and analgesic use [60].
Clinical experience suggests that bisphosphonates are rarely used in treatment regimens for NSCLC [61] despite evidence that they are an effective treatment of bone metastases and prevention of SREs in this population [28]. One of the reasons for this may be the potential adverse effects of these agents. Orally administered bisphosphonates are associated with gastrointestinal intolerance, while nephrotoxicity and osteonecrosis of the jaw are usually linked with i.v. agents [62–64]. Owing to the potential for renal damage, careful monitoring of renal function is required before and during treatment with zoledronic acid [65]. Given the additional potential for platinum-induced nephrotoxicity associated with first-line chemotherapy, renal monitoring may be a particularly important consideration when using bisphosphonates in patients with NSCLC.
An alternative bone-targeted therapy with reduced nephrotoxicity could assist with reducing the potential cumulative nephrotoxicity when combined with platinum-based agents. Denosumab, administered monthly as a subcutaneous injection (120 mg), was non-inferior to zoledronic acid in delaying the time to first on-study SRE [HR = 0.84; 95% confidence interval (CI) 0.71–0.98; P = 0.0007 (non-inferiority); P = 0.06 (superiority)] in patients with bone metastases arising from solid tumours including NSCLC (excluding breast and prostate) [66]. Denosumab also showed a trend of delayed time to first and subsequent SREs over zoledronic acid [rate ratio = 0.90; 95% CI 0.77–1.04; P = 0.14 (superiority)]. Overall survival was similar between groups. The incidence of osteonecrosis of the jaw was similarly low in both treatment groups; however, adverse events potentially associated with acute phase reactions and nephrotoxicity occurred more frequently in patients treated with zoledronic acid than denosumab. Overall, denosumab may be more suitable than zoledronic acid for combination treatment with platinum-based therapy in patients with NSCLC. Recently, denosumab has been approved for prevention of SREs in patients with solid tumours by the Australian, Canadian, European, Japanese, Russian, Swiss and USA regulatory authorities.
additional roles of bone-targeted therapies
Results from recent studies suggest that bone-targeted therapies may have actions beyond bone resorption and in reducing SREs. In a retrospective audit of patients diagnosed with NSCLC between 2004 and 2009, 42% presented with metastatic bone disease [61]. Patients treated with zoledronic acid in combination with platinum-based chemotherapy had increased overall survival (34 weeks) relative to those treated with chemotherapy alone (19 weeks; P = 0.01). Improvements in survival have yet to be demonstrated prospectively.
RANKL may also trigger tumour cell proliferation [67] and promote migration of RANK-expressing tumour cells to bone [68]. Denosumab may therefore also have direct antitumour effects. An exploratory analysis of data from a randomised phase III trial in patients with bone metastases arising from solid tumours (including NSCLC) was conducted to examine overall survival [69]. In 702 individuals with NSCLC, overall median survival was increased by 1.4 months in those treated with denosumab compared with patients treated with zoledronic acid (9.5 months denosumab, 8.1 months zoledronic acid; HR = 0.78; 95% CI 0.65–0.94; P = 0.01). These hypothesis-generating data suggest further prospective evaluation is warranted.
conclusions
Bone metastases are common in lung cancer but, owing to lack of awareness of the adverse consequences and possibly to the relatively short survival time associated with lung cancer, they are rarely treated until late-stage disease when patients experience potentially debilitating SREs. Bisphosphonates reduce the frequency of SREs and improve pain and QoL scores in various tumour types. However, concern over cumulative nephrotoxicity when used with first-line platinum-based chemotherapies may have contributed to the limited use of bisphosphonates in patients with lung cancer. Denosumab is a new bone-targeted therapy that is as effective as the most widely used bisphosphonate, zoledronic acid, for reducing the frequency of SREs in patients with lung cancer. Denosumab may therefore be more compatible than zoledronic acid for combination with first-line chemotherapy for lung cancer because dose adjustment for impaired renal function is not required. Additional roles for bone-targeting therapies beyond reducing SREs are under investigation.
disclosure
TB has received honoraria for speaking on behalf of Amgen, the makers of XGEVA, and has participated in Advisory Boards for this company. KOB has participated in Advisory Boards for Amgen, the makers of XGEVA and AstraZeneca, the makers of Iressa, and has received grants for translational research from these companies. CM has no conflicts of interest to declare.
acknowledgements
The authors take full responsibility for the content of this publication and confirm that it reflects their viewpoint and medical expertise. Writing and editorial support was provided by Oxford PharmaGenesis™ Ltd. Funding for this support was provided by Amgen (Europe) GmbH.
references
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; http://globocan.iarc.fr (29 November 2011, date last accessed) [Google Scholar]
- 2.Brenner H, Francisci S, de Angelis R, et al. ng-term survival expectations of cancer patients in Europe in 2000–2002. Eur J Cancer. 2009;45:1028–1041. doi: 10.1016/j.ejca.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.D'Addario G, Fruh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 4.Stinchcombe TE, Lee CB, Socinski MA. Current approaches to advanced-stage non-small-cell lung cancer: first-line therapy in patients with a good functional status. Clin Lung Cancer. 2006;7(Suppl 4):S111–S117. doi: 10.3816/clc.2006.s.002. [DOI] [PubMed] [Google Scholar]
- 5.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 6.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu Y-L, Chen G, et al. Efficacy results from the randomised phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine (GEM), in Chinese advanced non-small-cell lung cancer (NSCLC) patients (PTS) with EGFR activating mutations. Ann Oncol. 2010;21(Suppl 8):viii6. (Abstr LBA13) [Google Scholar]
- 10.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 13.Fuller K, Wong B, Fox S, et al. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998;188:997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 15.Lacey DL, Tan HL, Lu J, et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol. 2000;157:435–448. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 17.Lipton A, Steger GG, Figueroa J, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14:6690–6696. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- 18.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 19.Body JJ, Lipton A, Gralow J, et al. Effects of denosumab in patients with bone metastases with and without previous bisphosphonate exposure. J Bone Miner Res. 2010;25:440–446. doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 21.Coello MC, Luketich JD, Litle VR, Godfrey TE. Prognostic significance of micrometastases in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–225. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 22.Di Maio M, Gridelli C, Gallo C, et al. Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer. 2004;90:2288–2296. doi: 10.1038/sj.bjc.6601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pockett RD, Castellano D, McEwan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) 2010;19:755–760. doi: 10.1111/j.1365-2354.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iordanidou L, Trivizaki E, Saranti S, et al. Is there a role of whole body bone scan in early stages of non small cell lung cancer patients. J BUON. 2006;11:491–497. [PubMed] [Google Scholar]
- 25.Cheran SK, Herndon JE, II, Patz EF., Jr Comparison of whole-body FDG-PET to bone scan for detection of bone metastases in patients with a new diagnosis of lung cancer. Lung Cancer. 2004;44:317–325. doi: 10.1016/j.lungcan.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 26.MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50:287–293. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- 27.Irving L. Detection of bone metastases in non-small cell lung cancer (NSCLC) J Clin Oncol. 2004;22:7189. (Abstr 7189) [Google Scholar]
- 28.Rosen LS, Gordon D, Tchekmedyian NS, et al. ng-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 29.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 30.Sekine I, Nokihara H, Yamamoto N, et al. Risk factors for skeletal-related events in patients with non-small cell lung cancer treated by chemotherapy. Lung Cancer. 2009;65:219–222. doi: 10.1016/j.lungcan.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Sun JM, Ahn JS, Lee S, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71:89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229–232. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Jaukovic L, Ajdinovic B, Jankovic Z, Dugonjic S. Incidence and imaging characteristics of skeletal metastases detected by bone scintigraphy in lung cancer patients. Vojnosanit Pregl. 2006;63:1001–1005. doi: 10.2298/vsp0612001j. (Article in Serbian) [DOI] [PubMed] [Google Scholar]
- 34.Hirsh V, Tchekmedyian NS, Rosen LS, et al. Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clin Lung Cancer. 2004;6:170–174. doi: 10.3816/CLC.2004.n.030. [DOI] [PubMed] [Google Scholar]
- 35.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 36.Welshman A. Palliative care. Some organisational considerations. Minerva Anestesiol. 2005;71:439–443. [PubMed] [Google Scholar]
- 37.Costa L, Badia X, Chow E, et al. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 38.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Rustoen T, Moum T, Padilla G, et al. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manage. 2005;30:234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Ferrell BR, Ferrell BA, Rhiner M, Grant M. Family factors influencing cancer pain management. Postgrad Med J. 1991;67(Suppl 2):S64–S69. [PubMed] [Google Scholar]
- 41.Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007;109:2334–2342. doi: 10.1002/cncr.22678. [DOI] [PubMed] [Google Scholar]
- 42.Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390–396. doi: 10.1159/000082923. [DOI] [PubMed] [Google Scholar]
- 43.Delea T, McKiernan J, Brandman J, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol. 2006;4:341–347. [PubMed] [Google Scholar]
- 44.Kuhlmann MK, Burkhardt G, Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant. 1997;12:2478–2480. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- 45.de Jongh FE, van Veen RN, Veltman SJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardizzoni A, Boni L, Tiseo M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007;99:847–857. doi: 10.1093/jnci/djk196. [DOI] [PubMed] [Google Scholar]
- 47.Abramson EC, Chang J, Mayer M, et al. Effects of cisplatin on parathyroid hormone- and human lung tumor-induced bone resorption. J Bone Miner Res. 1988;3:541–546. doi: 10.1002/jbmr.5650030510. [DOI] [PubMed] [Google Scholar]
- 48.Okano Y, Nishio M. Efficacy of gefitinib in treatment of lung cancer patients with bone metastasis. Clin Calcium. 2008;18:527–533. (Article in Japanese) [PubMed] [Google Scholar]
- 49.Otsuka S, Hanibuchi M, Ikuta K, et al. A bone metastasis model with osteolytic and osteoblastic properties of human lung cancer ACC-LC-319/bone2 in natural killer cell-depleted severe combined immunodeficient mice. Oncol Res. 2009;17:581–591. doi: 10.3727/096504009789745511. [DOI] [PubMed] [Google Scholar]
- 50.Kolaczkowska M, Junik R, Rzymkowska M, Kramer L. The effect of chemotherapy on bone metabolism in patients with non-small cell lung cancer. Pneumonol Alergol Pol. 1998;66:283–289. (Article in Polish) [PubMed] [Google Scholar]
- 51.Zukawa M, Nakano M, Hirano N, et al. The effectiveness of gefitinib on spinal metastases of lung cancer—report of two cases. Asian Spine J. 2008;2:109–113. doi: 10.4184/asj.2008.2.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 53.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology (NCCN Guidelines™): Non-small cell lung cancer (version 3.2011). http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (27 June 2011, date last accessed) [Google Scholar]
- 54.De Marinis F, Eberhardt W, Harper PG, et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J Thorac Oncol. 2009;4:1280–1288. doi: 10.1097/JTO.0b013e3181b68e5a. [DOI] [PubMed] [Google Scholar]
- 55.Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 56.Saad F, Gleason DM, Murray R, et al. ng-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 57.Pavlakis N, Schmidt R, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2005;(3):CD003474. doi: 10.1002/14651858.CD003474.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Body JJ, Diel IJ, Bell R, et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain. 2004;111:306–312. doi: 10.1016/j.pain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 59.European Medicines Agency. Zometa® (Zoledronic Acid): Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000336/WC500051730.pdf (21 November 2011, date last accessed) [Google Scholar]
- 60.Hu XY, Zou QF, Jin C, et al. Efficacy of zoledronic acid combined with chemotherapy in treatment of skeletal metastases of non-small cell lung cancer and the bone metabolic markers. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:1343–1346. (Article in Chinese) [PubMed] [Google Scholar]
- 61.Calderone RG, Nimako K, Leary AN, et al. Use of zoledronic acid in lung cancer. J Thor Oncol. 2010;5:S99–100. (Abstr 253P) [Google Scholar]
- 62.Abrahamsen B. Adverse effects of bisphosphonates. Calcif Tissue Int. 2010;86:421–435. doi: 10.1007/s00223-010-9364-1. [DOI] [PubMed] [Google Scholar]
- 63.Gebara SN, Moubayed H. Risk of osteonecrosis of the jaw in cancer patients taking bisphosphonates. Am J Health Syst Pharm. 2009;66:1541–1547. doi: 10.2146/ajhp080251. [DOI] [PubMed] [Google Scholar]
- 64.Diel IJ, Bergner R, Grotz KA. Adverse effects of bisphosphonates: current issues. J Support Oncol. 2007;5:475–482. [PubMed] [Google Scholar]
- 65.Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med. 2003;349:1676–1679. doi: 10.1056/NEJM200310233491721. [DOI] [PubMed] [Google Scholar]
- 66.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 67.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 68.Jones DH, Nakashima T, Sanchez OH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 69.Scagliotti G, Hirsh V, Siena S, et al. Overall survival improvement in patients with lung cancer treated with denosumab versus zoledronic acid: results from a randomized phase 3 study. Presented at: 14th World Congress on Lung Cancer, July 2011, Amsterdam, The Netherlands. http://www.2011worldlungcancer.org/abstracts.html (27 June 2011, date last accessed) [DOI] [PubMed] [Google Scholar]