Abstract
Background
The two most common forms of non-Hodgkin lymphoma (NHL) exhibit different sex ratios: diffuse large B-cell lymphoma (DLBCL) occurs more frequently in men and follicular lymphoma (FL) more frequently in women. Looking among women alone, this pooled analysis explores the relationship between reproductive histories and these cancers.
Materials and methods
Self-reported reproductive histories from 4263 women with NHL and 5971 women without NHL were pooled across 18 case–control studies (1983–2005) from North America, Europe and Japan. Study-specific odd ratios (ORs) and confidence intervals (CIs) were estimated using logistic regression and pooled using random-effects meta-analyses.
Results
Associations with reproductive factors were found for FL rather than NHL overall and DLBCL. In particular, the risk of FL decreased with increasing number of pregnancies (pooled ORtrend = 0.88, 95% CI 0.81–0.96). FL was associated with hormonal contraception (pooled OR = 1.30, 95% CI 1.04–1.63), and risks were increased when use started after the age of 21, was used for <5 years or stopped for >20 years before diagnosis. DLBCL, on the other hand, was not associated with hormonal contraception (pooled OR = 0.87, 95% CI 0.65–1.16).
Conclusions
Hormonal contraception is associated with an increased risk of FL but not of DLBCL or NHL overall.
Keywords: case–control studies, diffuse large B-cell lymphoma, follicular lymphoma, hormonal contraceptives, non-Hodgkin lymphoma, reproductive history
introduction
Non-Hodgkin lymphoma (NHL) occurs more often in men than women, although within this heterogeneous group of malignancies, some subtypes are more common among women than men [1]. For the two most common NHL subtypes, the sex ratio for diffuse large B-cell lymphoma (DLBCL) is consistent with NHL overall, while follicular lymphoma (FL) has a slight female predominance. The reasons for the differential sex ratios, like the causes of most NHL subtypes, are unclear. NHL has been linked to severe immunosuppression and so factors that affect immune response, such as sex hormones [2], may be involved. For women, a relationship between reproductive history and NHL has been suggested.
Among women, production of sex hormones such as estrogen and progestogen changes with different reproductive stages such as menarche, pregnancy and menopause, or is altered exogenously by the use of hormonal contraception or other hormone treatments. Menstrual and reproductive factors as well as hormonal contraception have been examined with respect to NHL risk, but to date, findings have been equivocal [3–22]. Few studies have reported risks for NHL subtypes [3–7, 11, 22], and generally have been limited by small study size. To investigate the association between NHL and menstrual and reproductive factors, we conducted a pooled analysis of individual data from case–control studies involved in the International Lymphoma Epidemiology Consortium (InterLymph).
materials and methods
Case–control studies with data on reproductive factors were identified through the InterLymph Consortium. Table 1 outlines the studies' designs and more details have been published [4, 7, 11, 13, 15, 23–32]. Eighteen studies conducted between 1983 and 2005 in 10 countries across North America, Europe and Japan contributed data to this pooled analysis. Women with NHL were identified using rapid ascertainment techniques and female controls matched to cases on age were selected from population registers or from among hospital or clinic patients. The appropriate ethical committees approved each study and participants gave their informed consent.
Table 1.
Characteristics of case–control studies included in the pooled analysis
| Study (reference) | Location | Year of diagnosis | Age range | Cases (n = 4263) |
Controls (n = 5971) |
|||
|---|---|---|---|---|---|---|---|---|
| n | Participation (%) | Source | n | Participation (%) | ||||
| NCI-SEER [23] | Detroit, MI; Iowa; Los Angeles, CA; Seattle, WA, USA | 1998–2001 | 20–70 | 327 | 76 | If age <65 years selection by RDD; if age ≥ 65 years, random selection from Centers for Medicare and Medicaid Services, stratified by study area, age, sex and race | 269 | 52 |
| Connecticut [11] | Connecticut, USA | 1996–2000 | 21–84 | 600 | 72 | if age <65 years selection by RDD; if age ≥ 65 years, random selection from Centers for Medicare and Medicaid Services, stratified by age | 717 | age <65: 69; age ≥65: 47 |
| Nebraska NHL Study [24] | Nebraska, USA | 1999–2002 | 20–75 | 172 | 74 | RDD, frequency matched by age and sex | 254 | 78 |
| Mayo Clinic Phase 1 [25] | Iowa, Wisconsin, Minnesota, USA | 2002–2005 | 20+ | 310 | 66 | Random selection from patients at Mayo general medicine clinic, frequency matched by 5-year age group, sex and county of residence | 486 | 70 |
| UCSF [7] | San Francisco, CA, USA | 1988–1995 | 21–74 | 581 | 72 | RDD, frequency matched by age, sex and county of residence | 836 | 78 |
| Los Angeles Study [13] | Los Angeles County, CA, USA | 1989–1992 | 18–75 | 177 | 45 | Random neighbourhood control, individually matched on age, race and language | 177 | ∼69 |
| British Columbia Study [26] | Vancouver and Victoria, Canada | 2000–2004 | 20–82 | 346 | 78 | Random selection from Client Registry of the Ministry of Health, frequency matched by age, sex and region | 397 | 46 |
| UK [4] | Yorkshire, Lancashire, South Lakeland and parts of Southwest England | 1998–2003 | 16–69 | 393 | 70 | Random selection from general practice lists, individually matched by age, sex and region of residence | 397 | 69 |
| EpiLymph [27] | Parts of Ireland, Germany, France, Czech Republic, Spain and Italy | 1998–2004 | 18–80 | 744 | 88 | Population or hospital controls matched by age (±5 years), sex and study region | 1141 | 63 |
| Ireland [27] | Six hospitals on the east coast of the Republic of Ireland | 2001–2003 | 18–80 | 55 | 90 | Hospital controls matched by age (±5 years), sex and study region | 84 | 75 |
| Germany [28] | Ludwigshafen/Upper Palatinate, Heidelberg/Rhine-Neckar County, Würzburg/Lower Frankonia, Hamburg, Bielefeld and Munich | 1999–2002 | 18–80 | 232 | 88 | Random selection from population register, individually matched by sex, age and study region | 320 | 44 |
| France [27] | Amiens, Dijon and Montpellier | 2000–2003 | 18–80 | 96 | 91 | Hospital controls matched by age (±5 years), sex and study region | 139 | 74 |
| Czech Republic [27] | One centre in Czech Republic | 2001–2003 | 18–80 | 87 | 90 | Hospital controls individually matched by age (±5 years), sex and study region | 138 | 60 |
| Spain [29] | Barcelona, Tortosa, Reus and Madrid | 1998–2002 | 18–80 | 181 | 82 | Hospital controls matched by age (±5 years), sex and study region | 302 | 96 |
| Italy [27] | Sardinia | 1998–2004 | 18–80 | 93 | 93 | Random selection from population census list, matched by age (±5 years), sex and study region | 158 | 66 |
| Northern Italy [15] | Aviano and Milan | 1983–1992 | 17–79 | 181 | >97 | Patients admitted for acute, non-neoplastic, non-immunological conditions in the hospitals where cases diagnosed | 448 | >97 |
| Italy [30] | Aviano and Naples | 1999–2002 | 18–84 | 105 | 97 | Hospital controls, frequency matched by age (in 5-year bands), sex and study centre to cases of lymphohematopoietic neoplasms | 163 | 91 |
| HERPACC1 [31, 32] | Aichi Cancer Centre, Nagoya, Japan | 1988–2000 | 18–79 | 173 | ∼99 | Random sample of patients not diagnosed with cancer, individually matched by age and sex | 364 | ∼99 |
| HERPACC2 [32] | Aichi Cancer Centre, Nagoya, Japan | 2001–2004 | 18–79 | 154 | ∼99 | Random sample of patients not diagnosed with cancer, individually matched by age and sex | 322 | ∼99 |
RDD, random digit dialling.
NHL diagnoses were confirmed by pathology reports or samples. Lymphoma codes as described in the International Classification of Diseases for Oncology 3rd edition (ICD-O-3) were of interest in this analysis and included B-cell subtypes of NHL (DLBCL: ICD-O-3 codes 9679/3, 9680/3, 9684/3; FL: 9690/3, 9691/3, 9695/3, 9698/3; chronic lymphocytic leukaemia/small lymphocytic lymphoma: 9670/3, 9823/3; marginal zone lymphoma: 9689/3, 9699/3; mantle cell lymphoma: 9673/3; Burkitt lymphoma: 9687/3, 9826/3; and other unspecified B-cell lymphoma: 9671/3, 9728/3), and T-cell lymphomas as a whole (9700/3, 9701/3, 9702/3, 9705/3, 9708/3, 9709/3, 9714/3, 9716/3, 9717/3, 9718/3, 9719/3, 9729/3, 9827/3) as well as NHL in total (defined by the above ICD-O-3 codes and 9591/3, 9675/3 and 9727/3). These groupings have been used in other InterLymph pooled analyses, and methods to incorporate other classification schemes such as the Working Formulation (used in Connecticut, UCSF, Los Angeles and Northern Italy studies) have been described [33]. The majority of studies did not recruit cases with HIV-associated lymphoma, Hodgkin lymphoma or multiple myeloma, and so these exclusion criteria were applied across the pooled dataset.
Women were asked about their reproductive histories during in-person or telephone interviews, or through self-completed questionnaires. An anonymized dataset was supplied for each study and was checked for inconsistencies before harmonizing variables and coding data uniformly across studies. Details of reproductive histories collected varied by study: the number of children or births was asked in all 18 studies; whether women had ever been pregnant (13 studies); number of pregnancies (7); ages when periods started and stopped (8). Parity was defined as having one or more full-term pregnancies (Los Angeles), live births (Connecticut) or children (all other studies). The woman's age at first birth and the number of years between the last birth and date of diagnosis for cases and date of interview for controls were derived from the children's dates of birth or woman's age at her children's births. When examining the risk of NHL related to parity, analyses were restricted to women aged 40 or older, a group likely to have completed their families. Information on hormonal contraception was collected in 14 studies with all collecting years of use, 13 requested age or year at first use and 11 requested age at last use. Analysis of hormonal contraception was limited to women born in 1925 or later who would be of reproductive age when hormonal contraception first became available [34]. Control distributions of reproductive variables followed the patterns expected; for example, women in southern Europe and Ireland had a greater number of children, and Japanese women tended to be older at menarche than elsewhere. Accordingly, variable categories were initially defined by the interquartile ranges within each study, but since findings were similar to those based upon uniform categories across all studies, the latter are reported.
A two-stage meta-analysis was carried out. The first stage was to conduct logistic regression to estimate study-specific odds ratios (ORs) and 95% confidence intervals (CIs) adjusted for age as a continuous variable and ethnicity grouped as Caucasian or other as potential confounders. In order to include all studies, exact methods were employed where the number of cases and controls in any cell was five or less, and where there were no cases or controls, risks were estimated by adding a half to all cell frequencies. Study-specific risk estimates were then pooled in a meta-analysis using a fixed-effects model where there was no evidence of heterogeneity and a random-effects model when heterogeneity was present. Heterogeneity was tested using Cochrane's Q test, statistically significant at Pheterogeneity <0.10, and the amount of heterogeneity was described by the I2 statistic. Pooled risk estimates for trend were calculated by pooling the study-specific ORs for trend and were based upon the ordinal variables. Sensitivity analyses stratified by covariates such as study design were conducted; meta-analyses were repeated, including risks estimated from cell frequencies of more than five to confirm the stability of the pooled risk estimates. To assess whether findings were influenced by confounding factors, analyses were conducted adjusting study-specific risk estimates for socioeconomic status (high, medium, low), smoking status (never, ever), consumption of alcohol (never, ever) and body mass index (underweight, normal weight-for-height, overweight, obese [35]). Individuals with missing values for reproductive variables were excluded from the relevant analysis. All analyses were conducted using Stata 11.1 (StataCorp LP, College Station, TX, 2010).
results
Data on reproductive factors were pooled from 18 case–control studies and totalled 4263 women with NHL and 5971 controls. The majority of NHLs were B-cell in origin (n = 3461, 81%) and 5% (n = 221) were T-cell; for 14% (n = 581), immunophenotype was unknown (Table 2). DLBCL (32%) and FL (25%) were the most common subtypes, while chronic lymphocytic lymphoma/small lymphocytic lymphoma, marginal zone B-cell lymphoma and other specific subtypes each comprised ≤10% of all NHLs. Almost 85% of cases were Caucasian, ∼70% were born between 1920 and 1949 and the median age at diagnosis was 60 years. Compared with controls, cases tended to be older in age, of white race and of lower socioeconomic status.
Table 2.
Characteristics of women included in the pooled analysis
| Cases, n (%) | Controls, n (%) | |
|---|---|---|
| NHL subtype | 4263 (100) | – |
| Diffuse large B-cell lymphoma | 1354 (32) | – |
| Follicular lymphoma | 1055 (25) | – |
| Chronic lymphocytic lymphoma/small lymphocytic lymphoma | 432 (10) | |
| Marginal zone B-cell lymphoma | 388 (9) | |
| Other B-cell lymphoma | 232 (5) | – |
| T-cell lymphoma | 221 (5) | – |
| Unclassified | 581 (14) | – |
| Age | 4263 (100) | 5971 (100) |
| ≤55 | 1640 (38) | 2473 (41) |
| 56–65 | 1177 (28) | 1513 (25) |
| >65 | 1446 (34) | 1985 (33) |
| Year of birth | 4263 (100) | 5971 (100) |
| Before 1920 | 234 (5) | 324 (5) |
| 1920–1929 | 933 (22) | 1260 (21) |
| 1930–1939 | 1133 (27) | 1493 (25) |
| 1940–1949 | 979 (23) | 1347 (23) |
| 1950–1959 | 589 (14) | 784 (13) |
| 1960 or later | 395 (9) | 763 (13) |
| Ethnicity | 4263 (100) | 5971 (100) |
| Caucasian | 3698 (87) | 4974 (83) |
| Asian | 384 (9) | 765 (13) |
| Afro-Caribbean | 103 (2) | 147 (2) |
| Mixed, other or not known | 78 (2) | 85 (1) |
| Socioeconomic statusa | 3336 (100) | 4568 (100) |
| High | 849 (25) | 1293 (28) |
| Medium | 1138 (34) | 1642 (36) |
| Low | 1338 (40) | 1625 (36) |
| Not known | 11 (0.3) | 8 (0.2) |
aSocioeconomic status data were collected from 15 studies (NCI-SEER, Nebraska, Mayo, UCSF, Los Angeles, British Columbia, UK, EpiLymph studies, North Italy and Italy).
Table 3 shows the findings for age at menarche, whether menstrual periods had stopped and the age when periods stopped. Compared with women who reached menarche between the ages of 12 and 14, women who were younger or older at menarche did not have an increased risk of NHL. Pooled risks of NHL were also close to 1 for periods having stopped compared with not, and for periods stopping at younger or older ages relative to stopping between the ages of 45 and 51. Similarly, no associations were found for the two most common subtypes DLBCL and FL (Table 3).
Table 3.
Associations between non-Hodgkin lymphoma, diffuse large B-Cell Lymphoma, and follicular lymphoma and menstrual histories
| Controls | NHL | Pooled ORa | 95% CI | I2 | Phet | DLBCL | Pooled ORa | 95% CI | I2 | Phet | FL | Pooled ORa | 95% CI | I2 | Phet | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at menarcheb | Number of studies = 8 | |||||||||||||||
| Totalc | 3733 | 2497 | 847 | 647 | ||||||||||||
| <12 | 627 | 424 | 0.96 | 0.83–1.10 | 0% | 0.45 | 159 | 1.04 | 0.85–1.28 | 0% | 0.62 | 114 | 1.02 | 0.81–1.28 | 0% | 0.86 |
| 12–14 | 2417 | 1627 | 1 | ref | 555 | 1 | ref | 426 | 1 | ref | ||||||
| ≥15 | 639 | 408 | 1.00 | 0.87–1.16 | 0% | 0.49 | 122 | 0.84 | 0.67–1.06 | 0% | 0.44 | 96 | 0.98 | 0.76–1.27 | 4% | 0.40 |
| Periods stoppedb | Number of studies = 8 | |||||||||||||||
| Totalc | 3074 | 2091 | 674 | 493 | ||||||||||||
| No | 918 | 555 | 1 | ref | 189 | 1 | ref | 144 | 1 | ref | ||||||
| Yes | 2126 | 1511 | 1.15 | 0.91–1.44 | 21% | 0.26 | 479 | 1.18 | 0.78–1.77 | 41% | 0.11 | 345 | 1.02 | 0.61–1.70 | 49% | 0.06 |
| Age at which periods stoppedb | Number of studies = 8 | |||||||||||||||
| Totalc | 2126 | 1511 | 479 | 345 | ||||||||||||
| <45 | 512 | 420 | 1.16 | 0.98–1.37 | 0% | 0.90 | 144 | 1.28 | 1.00–1.65 | 0% | 0.81 | 101 | 1.28 | 0.96–1.72 | 0% | 0.77 |
| 45–51 | 980 | 651 | 1 | ref | 203 | 1 | ref | 143 | 1 | ref | ||||||
| ≥52 | 550 | 380 | 1.05 | 0.89–1.24 | 0% | 0.91 | 115 | 1.05 | 0.81–1.36 | 0% | 0.87 | 87 | 1.13 | 0.84–1.52 | 0% | 0.90 |
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; OR, odds ratio; CI, confidence interval.
aPooled ORs adjusted for age and ethnicity were estimated in meta-analysis using a random-effects model; pooled ORs and CIs were similar from a fixed-effects model where the amount of between-study variation in risk (I2) was low.
bStudies with data on periods starting and stopping were Connecticut, Mayo, UK, North Italy, Italy, HERPACC1 and HERPACC2; UCSF had data on age at menarche only, while Los Angeles had information on periods stopping.
cFrequencies do not sum to the total due to missing values.
The majority of women aged ≥40 had had at least one pregnancy, and NHL was not associated with ever having been pregnant (pooled risk estimate = 0.97, 95% CI 0.80–1.17) or the number of pregnancies (pooled risk estimate for trend = 0.97, 95% CI 0.91–1.03) when compared with women who had never been pregnant (Table 4). Parity, number of children, age at birth of their first child and number of years since their last birth were also not associated with total NHL. Heterogeneity in risks associated with the number of children was due to two studies showing significant trends in opposite directions; the majority of studies showed no trend. Findings for DLBCL and FL were on the whole similar to those for NHL, although some statistically significant risks of FL were found. For instance, FL risks decreased with increasing number of pregnancies (pooled risk estimate for trend = 0.88, 95% CI 0.81–0.96); however, there was no trend with increasing number of children either in all 18 studies or in the 7 studies which also had data on the number of pregnancies (pooled risk estimate for trend = 0.97, 95% CI 0.91–1.03; pooled risk estimate for trend = 0.95, 95% CI 0.88–1.03, respectively). FL risk was increased among women who had had a child in the 10 years before diagnosis when compared with women who had never had a child (pooled risk estimate = 1.87, 95% CI 1.02–3.40). The risk estimates for gravidity and parity changed little when adjusted for contraception use, socioeconomic status, smoking status, alcohol consumption and body mass index.
Table 4.
Associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma and reproductive histories among women aged ≥40
| Controls | NHL | Pooled ORa | 95% CI | I2 | Phet | DLBCL | Pooled ORa | 95% CI | I2 | Phet | FL | Pooled ORa | 95% CI | I2 | Phet | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ever pregnantb | Number of studies = 13 | |||||||||||||||
| Totalc | 3531 | 2396 | 793 | 537 | ||||||||||||
| No | 307 | 199 | 1 | ref | 73 | 1 | ref | 41 | 1 | ref | ||||||
| Yes | 3163 | 2137 | 0.97 | 0.80–1.17 | 0% | 0.59 | 702 | 0.81 | 0.59–1.13 | 15% | 0.29 | 481 | 0.94 | 0.66–1.33 | 0% | 0.78 |
| Number of pregnanciesd | Number of studies = 7 | |||||||||||||||
| Totalc | 2609 | 1736 | 590 | 417 | ||||||||||||
| None | 209 | 116 | 1 | ref | 41 | 1 | ref | 28 | 1 | ref | ||||||
| 1 | 249 | 166 | 1.01 | 0.65–1.56 | 28% | 0.22 | 60 | 1.25 | 0.70–2.22 | 14% | 0.32 | 46 | 1.07 | 0.59–1.92 | 0% | 0.95 |
| 2 | 605 | 438 | 1.20 | 0.91–1.58 | 0% | 0.97 | 131 | 0.95 | 0.63–1.44 | 0% | 0.93 | 130 | 1.49 | 0.94–2.36 | 0% | 0.85 |
| 3 | 585 | 398 | 1.09 | 0.82–1.44 | 0% | 0.80 | 139 | 1.00 | 0.64–1.58 | 6% | 0.38 | 79 | 0.93 | 0.57–1.52 | 0% | 0.64 |
| ≥4 | 885 | 547 | 0.98 | 0.75–1.28 | 0% | 0.72 | 195 | 0.92 | 0.62–1.36 | 0% | 0.52 | 117 | 0.82 | 0.51–1.31 | 0% | 0.97 |
| Trend | 0.97 | 0.91–1.03 | 20% | 0.28 | 0.95 | 0.89–1.03 | 0% | 0.68 | 0.88 | 0.81–0.96 | 0% | 0.89 | ||||
| Parouse | Number of studies = 18 | |||||||||||||||
| Totalc | 5151 | 3816 | 1162 | 985 | ||||||||||||
| No | 681 | 489 | 1 | ref | 160 | 1 | ref | 126 | 1 | ref | ||||||
| Yes | 4463 | 3322 | 1.04 | 0.92–1.18 | 0% | 0.58 | 1000 | 0.88 | 0.71–1.08 | 14% | 0.29 | 859 | 1.06 | 0.86–1.31 | 0% | 0.76 |
| Number of childrene | Number of studies = 18 | |||||||||||||||
| Totalc | 5151 | 3816 | 1162 | 985 | ||||||||||||
| None | 681 | 489 | 1 | ref | 160 | 1 | ref | 126 | 1 | ref | ||||||
| 1 | 603 | 510 | 1.20 | 0.99–1.45 | 12% | 0.32 | 147 | 0.97 | 0.71–1.33 | 22% | 0.19 | 137 | 1.33 | 1.00–1.77 | 0% | 0.89 |
| 2 | 1665 | 1225 | 1.06 | 0.92–1.22 | 0% | 0.56 | 348 | 0.84 | 0.65–1.08 | 18% | 0.24 | 343 | 1.13 | 0.90–1.42 | 0% | 0.51 |
| 3 | 1136 | 833 | 1.03 | 0.88–1.20 | 0% | 0.66 | 251 | 0.90 | 0.72–1.14 | 0% | 0.59 | 200 | 0.97 | 0.76–1.25 | 0% | 0.90 |
| ≥4 | 1055 | 749 | 1.00 | 0.82–1.22 | 28% | 0.13 | 254 | 0.99 | 0.78–1.26 | 0% | 0.55 | 179 | 1.02 | 0.78–1.33 | 0% | 0.80 |
| Trend | 0.98 | 0.93–1.02 | 37% | 0.06 | 0.98 | 0.93–1.04 | 0% | 0.72 | 0.97 | 0.91–1.03 | 0% | 0.90 | ||||
| Age at first childf | Number of studies = 15 | |||||||||||||||
| Totalc | 4341 | 3039 | 982 | 745 | ||||||||||||
| Nulliparous | 563 | 374 | 1 | ref | 132 | 1 | ref | 90 | 1 | ref | ||||||
| <25 | 2069 | 1533 | 1.10 | 0.94–1.27 | 0% | 0.48 | 483 | 0.91 | 0.70–1.20 | 24% | 0.19 | 372 | 1.08 | 0.83–1.39 | 0% | 0.82 |
| 25–29 | 1161 | 776 | 1.02 | 0.87–1.21 | 0% | 0.54 | 245 | 0.86 | 0.67–1.10 | 0% | 0.60 | 185 | 1.05 | 0.79–1.38 | 0% | 0.83 |
| ≥30 | 506 | 330 | 0.96 | 0.75–1.23 | 31% | 0.12 | 113 | 0.87 | 0.60–1.24 | 24% | 0.19 | 92 | 1.16 | 0.84–1.61 | 0% | 0.57 |
| Trend | 0.98 | 0.91–1.05 | 31% | 0.12 | 0.95 | 0.86–1.05 | 20% | 0.24 | 1.03 | 0.94–1.13 | 0% | 0.52 | ||||
| Years since last childg | Number of studies = 11 | |||||||||||||||
| Totalc | 2946 | 2057 | 667 | 521 | ||||||||||||
| Nulliparous | 410 | 260 | 1 | ref | 96 | 1 | ref | 63 | 1 | ref | ||||||
| ≥30 | 1427 | 997 | 1.11 | 0.88–1.41 | 26% | 0.20 | 302 | 0.83 | 0.62–1.10 | 4% | 0.41 | 222 | 1.10 | 0.79–1.54 | 0% | 0.63 |
| 10–29 | 990 | 717 | 1.10 | 0.84–1.44 | 37% | 0.10 | 240 | 0.90 | 0.56–1.43 | 51% | 0.02 | 211 | 1.23 | 0.90–1.68 | 0% | 0.91 |
| <10 | 70 | 51 | 1.26 | 0.82–1.94 | 0% | 0.86 | 15 | 1.13 | 0.60–2.12 | 0% | 1.00 | 19 | 1.87 | 1.02–3.40 | 0% | 0.68 |
| Trend | 1.04 | 0.94–1.15 | 17% | 0.28 | 0.99 | 0.84–1.17 | 31% | 0.15 | 1.14 | 0.99–1.30 | 0% | 0.94 | ||||
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; OR, odds ratio; CI, confidence interval.
aPooled ORs adjusted for age and ethnicity were estimated in meta-analysis using a random-effects model; pooled ORs and CIs were similar from a fixed-effects model where the amount of between-study variation in risk (I2) was low.
bStudies with data on ever being pregnant were Connecticut, UCSF, Los Angeles, EpiLymph-Ireland, EpiLymph-Germany, EpiLymph-France, EpiLymph-Czech Republic, EpiLymph-Spain, EpiLymph-Italy, Northern Italy, Italy, HERPACC1 and HERPACC2.
cFrequencies do not sum to the total due to missing values.
dStudies with data on the number of pregnancies were Connecticut, UCSF, Los Angeles, Northern Italy, Italy, HERPACC1 and HERPACC2.
eAll studies collected data on parity and number of children.
fStudies with data on age at first child were Connecticut, Mayo, UCSF, Los Angeles, UK, EpiLymph-Ireland, EpiLymph-Germany, EpiLymph-France, EpiLymph-Czech Republic, EpiLymph-Spain, EpiLymph-Italy, North Italy, Italy, HERPACC1 and HERPACC2.
gStudies with data on years since the last child were Mayo, UCSF, UK, EpiLymph-Ireland, EpiLymph-Germany, EpiLymph-France, EpiLymph-Czech Republic, EpiLymph-Spain, EpiLymph-Italy, North Italy and Italy.
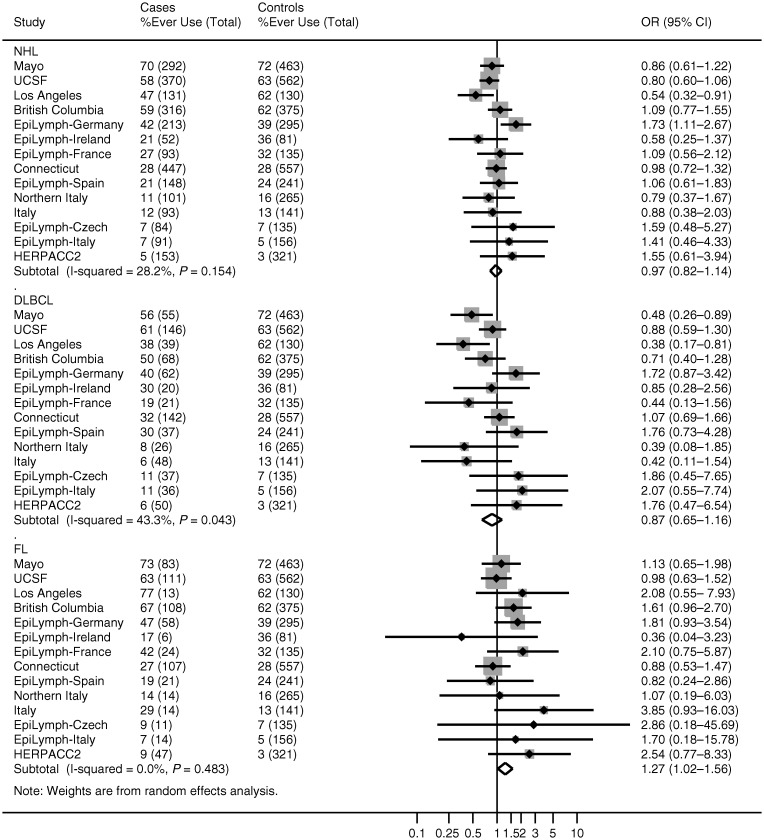
Among women born in 1925 or later, ∼40% reported having used hormonal contraception (Table 5). Use was not associated with NHL (pooled risk estimate = 0.98, 95% CI 0.83–1.16). Risks were also not increased among women who used hormonal contraception before or after the age of 22 or the year 1975; who used hormonal contraception for ≤5 years or >5 years; nor whose use was current or in the past 10, 20 or more years ago. Pooled risks for DLBCL were largely consistent with those for NHL overall (Figure 1). For FL, study-specific risk estimates mostly ranged from around one- to twofold, and when pooled, gave an increased risk of 1.30 with hormonal contraception use (95% CI 1.04–1.63). FL risk was also increased among women who were aged >22 years at first use (pooled risk estimate = 1.46, 95% CI 1.10–1.92); who first used contraception before 1975 (pooled risk estimate = 1.28, 95% CI 1.02–1.60); who used it for ≤5 years (pooled risk estimate = 1.56, 95% CI 1.19–2.03); and who last used it ≥20 years ago (pooled risk estimate = 1.55, 95% CI 1.02–2.35). Adjusting for the number of pregnancies, the number of children, socioeconomic status, smoking status, alcohol consumption and body mass index did not alter these findings.
Table 5.
Associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma and hormonal contraception use among women born in 1925 or later
| Hormonal contraception | Controls | NHL | Pooled ORa | 95% CI | I2 | Phet | DLBCL | Pooled ORa | 95% CI | I2 | Phet | FL | Pooled ORa | 95% CI | I2 | Phet |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contraceptionb | Number of studies = 14 | |||||||||||||||
| Totalc | 3857 | 2584 | 787 | 631 | ||||||||||||
| Never used | 2337 | 1567 | 1 | ref | 502 | 1 | ref | 327 | 1 | ref | ||||||
| Ever used | 1495 | 987 | 0.98 | 0.83–1.16 | 33% | 0.11 | 277 | 0.87 | 0.65–1.16 | 43% | 0.04 | 296 | 1.30 | 1.04–1.63 | 7% | 0.38 |
| Age first usedd | Number of studies = 13 | |||||||||||||||
| Totalc | 3536 | 2431 | 737 | 584 | ||||||||||||
| Never used | 2036 | 1426 | 1 | ref | 456 | 1 | ref | 284 | 1 | ref | ||||||
| ≤22 | 769 | 451 | 0.81 | 0.62– 1.05 | 44% | 0.05 | 129 | 0.72 | 0.48–1.11 | 50% | 0.02 | 130 | 1.09 | 0.82–1.44 | 0% | 0.89 |
| >22 | 699 | 510 | 1.05 | 0.86–1.28 | 32% | 0.13 | 139 | 0.91 | 0.70–1.18 | 9% | 0.36 | 157 | 1.46 | 1.10–1.92 | 13% | 0.31 |
| Trend | 1.01 | 0.92–1.10 | 26% | 0.18 | 0.95 | 0.83–1.10 | 20% | 0.24 | 1.18 | 1.04–1.35 | 9% | 0.36 | ||||
| Year first usedd | Number of studies = 13 | |||||||||||||||
| Totalc | 3536 | 2431 | 737 | 584 | ||||||||||||
| Never used | 2036 | 1426 | 1 | ref | 456 | 1 | ref | 284 | 1 | ref | ||||||
| >1975 | 456 | 277 | 1.06 | 0.84–1.34 | 14% | 0.30 | 94 | 1.14 | 0.80–1.62 | 13% | 0.32 | 71 | 1.25 | 0.80–1.95 | 22% | 0.22 |
| ≤1975 | 1012 | 684 | 0.92 | 0.73–1.18 | 52% | 0.02 | 174 | 0.78 | 0.55–1.10 | 41% | 0.06 | 216 | 1.28 | 1.02–1.60 | 0% | 0.47 |
| Trend | 0.96 | 0.87–1.06 | 38% | 0.08 | 0.91 | 0.77–1.08 | 43% | 0.05 | 1.13 | 1.01–1.27 | 2% | 0.42 | ||||
| Years of useb | Number of studies = 14 | |||||||||||||||
| Totalc | 3857 | 2584 | 787 | 631 | ||||||||||||
| Never used | 2337 | 1567 | 1 | ref | 502 | 1 | ref | 327 | 1 | ref | ||||||
| ≤5 | 797 | 581 | 1.13 | 0.89–1.43 | 51% | 0.01 | 161 | 0.99 | 0.68–1.45 | 52% | 0.01 | 175 | 1.56 | 1.19–2.03 | 11% | 0.33 |
| >5 | 663 | 386 | 0.86 | 0.73–1.02 | 0% | 0.49 | 111 | 0.83 | 0.64–1.06 | 0% | 0.60 | 116 | 1.12 | 0.86–1.47 | 0% | 0.72 |
| Trend | 0.94 | 0.87–1.02 | 0% | 0.50 | 0.91 | 0.80–1.04 | 9% | 0.36 | 1.09 | 0.96–1.24 | 0% | 0.47 | ||||
| Years since last usede | Number of studies = 11 | |||||||||||||||
| Totalc | 2943 | 2008 | 606 | 488 | ||||||||||||
| Never used | 1860 | 1271 | 1 | ref | 377 | 1 | ref | 260 | 1 | ref | ||||||
| ≥20 | 408 | 312 | 1.06 | 0.74–1.51 | 56% | 0.01 | 77 | 1.00 | 0.66–1.53 | 33% | 0.14 | 110 | 1.55 | 1.02–2.35 | 33% | 0.15 |
| 10–19 | 282 | 205 | 1.10 | 0.88–1.36 | 0% | 0.77 | 65 | 1.07 | 0.77–1.48 | 0% | 0.93 | 63 | 1.34 | 0.96–1.88 | 0% | 0.79 |
| 1–9 | 184 | 118 | 1.08 | 0.82–1.42 | 0% | 0.59 | 48 | 1.39 | 0.94–2.05 | 0% | 0.70 | 31 | 1.34 | 0.78–2.31 | 15% | 0.30 |
| Current | 170 | 58 | 0.68 | 0.48–0.97 | 0% | 0.84 | 27 | 1.04 | 0.65–1.68 | 0% | 0.71 | 12 | 0.71 | 0.39–1.28 | 0% | 0.93 |
| Trend | 0.97 | 0.91–1.04 | 0% | 0.98 | 1.04 | 0.95–1.14 | 0% | 0.88 | 1.04 | 0.94–1.16 | 0% | 0.93 | ||||
NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; OR, odds ratio; CI, confidence interval.
aPooled ORs adjusted for age and ethnicity were estimated in meta-analysis using a random-effects model; pooled ORs and CIs were similar from a fixed-effects model where the amount of between-study variation in risk (I2) was low.
bStudies with data on hormonal contraception use and number of years hormonal contraception was used were Connecticut, Mayo, UCSF, Los Angeles, British Columbia, EpiLymph-Ireland, EpiLymph-Germany, EpiLymph-France, EpiLymph-Czech Republic, EpiLymph-Spain, EpiLymph-Italy, North Italy, Italy and HERPACC2.
cFrequencies do not sum to the total due to missing values.
dHERPACC2 did not have data on age or year first used contraception.
eMayo, Los Angeles and HERPACC2 did not have data on number of years since last used contraception.
Figure 1.
Study-specific associations between non-Hodgkin lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and hormonal contraception use among women born 1925 or later. Studies are ordered by the percentage of control women who had ever used hormonal contraceptives. NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma.
discussion
This pooled analysis of InterLymph case–control studies from 10 countries across North America, Europe and Japan found little evidence to support an association between reproductive factors and NHL. The examination of potential risk factors among women limited the number of subjects for most studies to under half those recruited and so when considering NHL subtypes, study-specific ORs were most robust for the two most common, DLBCL and FL. In general, pooled risk estimates for other subtypes, including other B-cell lymphomas and T-cell lymphoma, were similar to those for NHL overall in finding no effect (data not shown). As for exogenous hormones, hormonal contraception was found to increase the risk of FL, while no association was found for DLBCL or NHL overall. Findings were examined further in sensitivity analyses and were found to be consistent whether pooled by continent or population- or hospital-based study design; restricted to studies where the participation rates were ≥70%, or to Caucasians; or adjusted for socioeconomic status, other lifestyle or reproductive factors.
Four studies included in this meta-analysis have reported their findings for menstrual factors [4, 7, 11, 13], 11 for reproductive histories [4, 7, 11, 13, 15, 22] and 9 for hormonal contraception use [7, 11, 13, 22]; the remaining studies are included here for the first time. This dataset comprises most of the available information arising from case–control studies on NHL risk associated with reproductive histories; only four others have published their findings, two on reproductive histories [17, 18] and two on contraception use [19, 21]. Among cohorts or case–control studies nested within cohorts, findings have been reported for menstrual factors in four cohorts [3, 5, 6, 9, 12], reproductive histories in eight [5, 6, 8–10, 12, 14, 16] and contraception in three [3, 5, 20]. When examining the evidence, our findings are in agreement with those published previously for ages at menarche or menopause in showing no association with NHL overall or its subtypes [3, 5, 6, 9, 12]. As for reproductive histories, the gravidity and parity variables investigated here have shown little consistent effect in other independent studies [5, 6, 8–10, 12, 14, 16–18]. In one cohort, NHL risks were found to decrease with increasing gravidity and parity [6], with trends suggested not only for FL—as we found for gravidity—but for DLBCL as well. We also found an increased risk of FL among women who had had a child <10 years before diagnosis; no other data were available for direct comparison with one cohort reporting the risks of NHL overall, finding no association [10].
Hormonal contraception does not appear to be linked with the risk of NHL overall [3, 5, 19–21]; for NHL subtypes, associations have been examined less often [3, 5]. Findings varied, with one cohort suggesting decreasing risks of DLBCL with longer use of hormonal contraception but no association for FL [5], and the other reporting no association with either DLBCL or FL [3]. The US women followed in these cohorts may differ from the women studied here with regard to factors such as birth cohort and socioeconomic status, for instance. In our study, risks were increased for FL, particularly for older age or earlier time period at first use; shorter durations of use; and last use at least 20 years before diagnosis. Findings for shorter durations of use may relate to older women of earlier birth cohorts having started contraceptives at older ages. Unfortunately data on contraception formulation were not available, although the majority of women were probably using estrogen and progestogen rather than progestogen-only contraception. Hormonal contraception was also likely to be taken orally as contraception administered by other routes is rare in the countries of study [34]. As for investigating possible dose–response relationships, the time period of first use was chosen as a surrogate marker for hormone dose, although at around the same time, oral contraception changed from sequential administration of hormones to the combined pill. During the 1970s, estrogen and progestogen levels in the pill were reduced and our findings for FL are consistent with periods when hormone contraception doses were at their highest. Interestingly, we found that FL risk declined as time since last use got closer to diagnosis. As the studies included are contemporaneous, this finding may reflect use during the higher dose era. Nevertheless, to our knowledge, this is the first study of NHL that has considered the time before diagnosis that hormonal contraception was used. Its effect has been examined for breast cancer where a similar pattern has been reported among women diagnosed at ages akin to the majority of our FL cases (i.e. after the age of 50) [36].
The mechanisms by which hormonal contraception may lead to FL are uncertain but may involve the effects estrogen has on the immune system. Sex hormones are known to affect B-cell development, cytokine production and cytokine receptor expression, for instance [2]. Estrogen at physiological levels increases the production of cytokines associated with innate immunity [e.g. interleukin-2 (IL-2), interferon-γ (IFN-γ)] and suppresses the humoral response. With the pharmacological intake of estrogen from hormonal contraceptives, the immune system switches more towards the humoral response with the production of cytokines such as IFN-γ being reduced and IL-6 and IL-10 increased [37]. This environment may increase the number of B lymphocyte subpopulations perhaps via estrogen receptors and the estrogen-induced expression of the bcl-2 gene reducing B-cell apoptosis [38]. There is also the suggestion from mouse models that estrogen can increase sensitivity to prolactin and prolactin can cause more autoreactive B cells to mature to follicular B cells [39, 40]. However, estrogen effects vary between species and even strains of mice so the exact processes by which estrogen alters the immune system are not fully understood, and even less is known about its role in lymphomagenesis.
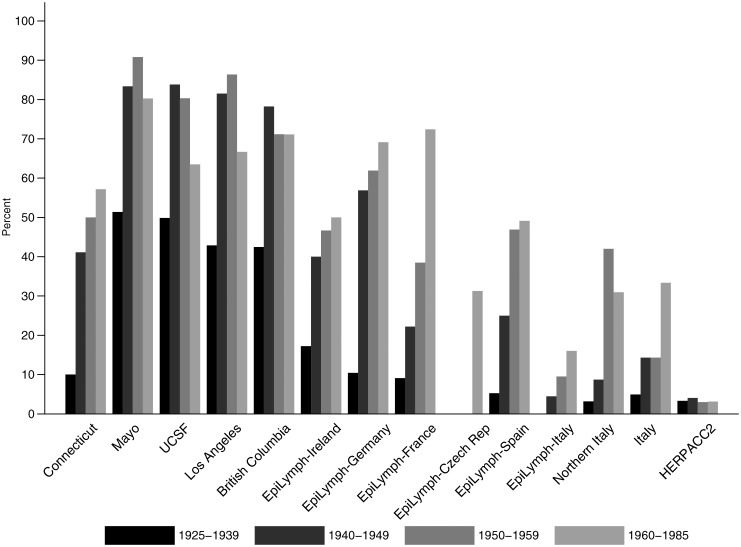
Oral contraception has been available in the United States since the early 1960s, from the mid to late 1960s in Europe and not until the 1990s in Japan. With regard to our investigation of NHL risk, the reliability of the findings depends on the accuracy of self-reported information—which for oral contraception has been shown to be high when compared with medical records [41–43]—and the representativeness of controls of the population from which cases arise. As a comparison, data on ever using oral contraception among 100 000 women participating as controls in studies of breast cancer were accessed [44]. Our control data were similar to the percentage of ever users among US, Canadian, German, French and Italian women born in 1925–1929 through to 1945–1949, and although not entirely consistent, differences may relate to factors such as region and socioeconomic status. Examination of data by study and birth cohort (Figure 2) indicates the variation in lifetime use of oral contraceptives among different generations of women living in a number of economically developed nations.
Figure 2.
Percent of control women who had ever used hormonal contraception by study and birth cohort. Shading of the bars reflects the birth cohort distribution, where >40% of women were born before 1940, >25% in the 1940s and ∼15% in each of the other two time periods.
In conclusion, this study found little evidence of an association between reproductive factors and NHL overall or its two most common subtypes, DLBCL and FL. The results suggest that the risk of FL was increased among women who had used hormonal contraception but that hormonal contraception was not related to NHL overall or DLBCL. FL risk was highest for use many years before diagnosis and may relate to oral contraceptives of higher hormone doses. This analysis has the advantage of a large sample size, detailed exposure information and information on potentially confounding factors and the consistency of NHL classification. One limitation, however, was it included women in economically developed nations and not other parts of the world where the incidence of FL may differ. In addition, since the majority of women studied were born before 1950, our findings may not be applicable to women of later birth cohorts and in particular, may not apply to lower dose contraceptives if a long latency is needed before FL onset. Future investigations among women of later birth cohorts may address whether lower dose contraceptives pose a risk to the development of FL.
funding
This work was supported by the National Cancer Institute (grants PC65064, PC67008, PC67009, PC67010 and PC71105 to the NCI-SEER study); National Cancer Institute (grant CA62006 to the Connecticut study); American Institute for Cancer Research (grant 99B083 to the Nebraska study); National Cancer Institute (grants CA92153 and CA97274 to the Mayo study); National Institute of Health (grants CA45614, CA89745, CA87014, CA150037 and CA143947 to the UCSF study); National Cancer Institute (grant CA50850 to the Los Angeles study); the Canadian Cancer Society through the National Cancer Institute of Canada, the Canadian Institutes for Health Research, and the Chan Sisters Foundation (the British Columbia study); the Leukaemia and Lymphoma Research (the UK study); European Commission (grant QLK4-CT-2000–00422 to the EpiLymph study); Association pour la Recherche contre le Cancer and Fondation de France (grants 5111 and 1999 008471 to the EpiLymph-France study); Compagnia di San Paolo di Torino, Programma Oncologia 2001 (the EpiLymph-Italy study); Health Research Board (the EpiLymph-Ireland study); Spanish Ministry of Health (FISS grant PI040091, PI081555, RCESP C03/09, RTICESP C03/10, RTIC RD06/0020/0095 and CIBERESP 06/06/0073) and Marato de TV3 Foundation (grant 051210 to the EpiLymph-Spain study); German Federal Office for Radiation Protection (grants StSch4261 and StSch4420 to the EpiLymph-Germany study); the European Regional Development Fund and the State Budget of the Czech Republic (RECAMO, CZ.1.05/2.1.00/03.0101 to EpiLymph-Czech Republic study); the National Research Council (CNR) Applied Project ‘Clinical Applications of Oncological Research’ and the Italian Association for Cancer Research (the Northern Italy study); the European Community (Europe Against Cancer Programme), the Lega Italiana per la Lotta Contro i Tumori and Italian Association for Research on Cancer (the Italy study); and Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan (HERPACC1 and 2 studies).
disclosures
The authors declare no conflicts of interest.
references
- 1.Smith A, Roman E, Howell D, et al. The Haematological Malignancy Research Network (HMRN): a new information strategy for population based epidemiology and health service research. Br J Haematol. 2010;148:739–753. doi: 10.1111/j.1365-2141.2009.08010.x. doi:10.1111/j.1365-2141.2009.08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. doi:10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Wang SS, Sullivan-Halley J, et al. Oral contraceptives, menopausal hormone therapy use and risk of B-cell non-Hodgkin lymphoma in the California Teachers Study. Int J Cancer. 2011;129:974–982. doi: 10.1002/ijc.25730. doi:10.1002/ijc.25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mildon KH, Ansell P, Roman E, et al. Reproductive factors, menopausal hormone therapy, and risk of non-Hodgkin, diffuse large B-cell and follicular lymphomas: a UK case-control study. Cancer Causes Control. 2010;21:2079–2083. doi: 10.1007/s10552-010-9626-2. doi:10.1007/s10552-010-9626-2. [DOI] [PubMed] [Google Scholar]
- 5.Morton LM, Wang SS, Richesson DA, et al. Reproductive factors, exogenous hormone use and risk of lymphoid neoplasms among women in the National Institutes of Health-AARP Diet and Health Study Cohort. Int J Cancer. 2009;124:2737–2743. doi: 10.1002/ijc.24248. doi:10.1002/ijc.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott J, Lu Y, Chang ET, et al. Reproductive factors and non-Hodgkin lymphoma risk in the California Teachers Study. PLoS One. 2009;4:e8135. doi: 10.1371/journal.pone.0008135. doi:10.1371/journal.pone.0008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Bracci PM, Holly EA. Non-Hodgkin lymphoma in women: reproductive factors and exogenous hormone use. Am J Epidemiol. 2008;168:278–288. doi: 10.1093/aje/kwn119. doi:10.1093/aje/kwn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakauchi F. Marital status and having children and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8(suppl.):123–128. [PubMed] [Google Scholar]
- 9.Sakauchi F. Reproductive history and health screening for women and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8(suppl.):129–134. [PubMed] [Google Scholar]
- 10.Frisch M, Pedersen BV, Wohlfahrt J, et al. Reproductive patterns and non-Hodgkin lymphoma risk in Danish women and men. Eur J Epidemiol. 2006;21:673–679. doi: 10.1007/s10654-006-9035-8. doi:10.1007/s10654-006-9035-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Holford TR, Leaderer B, et al. Menstrual and reproductive factors and risk of non-Hodgkin's lymphoma among Connecticut women. Am J Epidemiol. 2004;160:766–773. doi: 10.1093/aje/kwh278. doi:10.1093/aje/kwh278. [DOI] [PubMed] [Google Scholar]
- 12.Cerhan JR, Habermann TM, Vachon CM, et al. Menstrual and reproductive factors and risk of non-Hodgkin lymphoma: the Iowa women's health study (United States) Cancer Causes Control. 2002;13:131–136. doi: 10.1023/a:1014354105164. doi:10.1023/A:1014354105164. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RA, Levine AM, Bernstein L. Reproductive factors and risk of intermediate- or high-grade B-Cell non-Hodgkin's lymphoma in women. J Clin Oncol. 2001;19:1381–1387. doi: 10.1200/JCO.2001.19.5.1381. [DOI] [PubMed] [Google Scholar]
- 14.Adami HO, Tsaih S, Lambe M, et al. Pregnancy and risk of non-Hodgkin's lymphoma: a prospective study. Int J Cancer. 1997;70:155–158. doi: 10.1002/(sici)1097-0215(19970117)70:2<155::aid-ijc3>3.0.co;2-w. doi:10.1002/(SICI)1097-0215(19970117)70:2<155::AID-IJC3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Tavani A, Pregnolato A, La Vecchia C, et al. A case-control study of reproductive factors and risk of lymphomas and myelomas. Leuk Res. 1997;21:885–888. doi: 10.1016/s0145-2126(97)00062-3. doi:10.1016/S0145-2126(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 16.Kvale G, Heuch I, Nilssen S. Parity in relation to mortality and cancer incidence: a prospective study of Norwegian women. Int J Epidemiol. 1994;23:691–699. doi: 10.1093/ije/23.4.691. doi:10.1093/ije/23.4.691. [DOI] [PubMed] [Google Scholar]
- 17.Olsson H, Olsson ML, Ranstam J. Late age at first full-term pregnancy as a risk factor for women with malignant lymphoma. Neoplasma. 1990;37:185–190. [PubMed] [Google Scholar]
- 18.Miller AB, Barclay TH, Choi NW, et al. A study of cancer, parity and age at first pregnancy. J Chronic Dis. 1980;33:595–605. doi: 10.1016/0021-9681(80)90002-8. doi:10.1016/0021-9681(80)90002-8. [DOI] [PubMed] [Google Scholar]
- 19.Beiderbeck AB, Holly EA, Sturkenboom MC, et al. No increased risk of non-Hodgkin's lymphoma with steroids, estrogens and psychotropics (Netherlands) Cancer Causes Control. 2003;14:639–644. doi: 10.1023/a:1025698109991. doi:10.1023/A:1025698109991. [DOI] [PubMed] [Google Scholar]
- 20.Cerhan JR, Wallace RB, Folsom AR, et al. Medical history risk factors for non-Hodgkin's lymphoma in older women. J Natl Cancer Inst. 1997;89:314–318. doi: 10.1093/jnci/89.4.314. doi:10.1093/jnci/89.4.314. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin's lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res. 1992;52:5510s–5515s. [PubMed] [Google Scholar]
- 22.Costas L, Casabonne D, Benavente Y, et al. Reproductive factors and lymphoid neoplasms in Europe: findings from the EpiLymph case-control study. Cancer Causes Control. 2012;23:195–206. doi: 10.1007/s10552-011-9869-6. doi:10.1007/s10552-011-9869-6. [DOI] [PubMed] [Google Scholar]
- 23.Cerhan JR, Bernstein L, Severson RK, et al. Anthropometrics, physical activity, related medical conditions, and the risk of non-Hodgkin lymphoma. Cancer Causes Control. 2005;16:1203–1214. doi: 10.1007/s10552-005-0358-7. doi:10.1007/s10552-005-0358-7. [DOI] [PubMed] [Google Scholar]
- 24.Chiu BC, Kolar C, Gapstur SM, et al. Association of NAT and GST polymorphisms with non-Hodgkin's lymphoma: a population-based case-control study. Br J Haematol. 2005;128:610–615. doi: 10.1111/j.1365-2141.2004.05358.x. doi:10.1111/j.1365-2141.2004.05358.x. [DOI] [PubMed] [Google Scholar]
- 25.Cerhan JR, Ansell SM, Fredericksen ZS, et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007;110:4455–4463. doi: 10.1182/blood-2007-05-088682. doi:10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinelli JJ, Ng C, Weber JP, et al. Organochlorines and risk of non-Hodgkin lymphoma. Int J Cancer. 2007;121:2767–2775. doi: 10.1002/ijc.23005. doi:10.1002/ijc.23005. [DOI] [PubMed] [Google Scholar]
- 27.Besson H, Brennan P, Becker N, et al. Tobacco smoking, alcohol drinking and non-Hodgkin's lymphoma: a European multicenter case-control study (EpiLymph) Int J Cancer. 2006;119:901–908. doi: 10.1002/ijc.21913. doi:10.1002/ijc.21913. [DOI] [PubMed] [Google Scholar]
- 28.Becker N, Deeg E, Rudiger T, et al. Medical history and risk for lymphoma: results of a population-based case-control study in Germany. Eur J Cancer. 2005;41:133–142. doi: 10.1016/j.ejca.2004.08.028. doi:10.1016/j.ejca.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 29.de Sanjose S, Shah KV, Domingo-Domenech E, et al. Lack of serological evidence for an association between simian virus 40 and lymphoma. Int J Cancer. 2003;104:522–524. doi: 10.1002/ijc.10993. doi:10.1002/ijc.10993. [DOI] [PubMed] [Google Scholar]
- 30.Talamini R, Montella M, Crovatto M, et al. Non-Hodgkin's lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380–385. doi: 10.1002/ijc.20137. doi:10.1002/ijc.20137. [DOI] [PubMed] [Google Scholar]
- 31.Tajima K, Hirose K, Inoue M, et al. A model of practical cancer prevention for out-patients visiting a hospital: the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC) Asian Pac J Cancer Prev. 2000;1:35–47. [PubMed] [Google Scholar]
- 32.Suzuki T, Matsuo K, Ito H, et al. A past history of gastric ulcers and Helicobacter pylori infection increase the risk of gastric malignant lymphoma. Carcinogenesis. 2006;27:1391–1397. doi: 10.1093/carcin/bgi334. doi:10.1093/carcin/bgi334. [DOI] [PubMed] [Google Scholar]
- 33.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. doi:10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, France. International Agency for Research on Cancer; 1999. p. 72. Hormonal Contraception and Post-Menopausal Hormonal Therapy IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. World Health Org and International Agency for Research on Cancer. [Google Scholar]
- 35.WHO Expert Committee on Physical Status. Geneva, Switzerland: World Health Organization; 1995. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. p. 854. WHO Technical Report Series 1–415. [PubMed] [Google Scholar]
- 36.Hannaford PC, Selvaraj S, Elliott AM, et al. Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner's oral contraception study. Br Med J. 2007;335:651. doi: 10.1136/bmj.39289.649410.55. doi:10.1136/bmj.39289.649410.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karpuzoglu E, Zouali M. The multi-faceted influences of estrogen on lymphocytes: toward novel immuno-interventions strategies for autoimmunity management. Clin Rev Allergy Immunol. 2011;40:16–26. doi: 10.1007/s12016-009-8188-0. doi:10.1007/s12016-009-8188-0. [DOI] [PubMed] [Google Scholar]
- 38.Grimaldi CM, Cleary J, Dagtas AS, et al. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peeva E, Michael D, Cleary J, et al. Prolactin modulates the naive B cell repertoire. J Clin Invest. 2003;111:275–283. doi: 10.1172/JCI16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha S, Gonzalez J, Rosenfeld G, et al. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis Rheum. 2009;60:1743–1752. doi: 10.1002/art.24500. doi:10.1002/art.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nischan P, Ebeling K, Thomas DB, et al. Comparison of recalled and validated oral contraceptive histories. Am J Epidemiol. 1993;138:697–703. doi: 10.1093/oxfordjournals.aje.a116907. [DOI] [PubMed] [Google Scholar]
- 42.Hunter DJ, Manson JE, Colditz GA, et al. Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception. 1997;56:373–378. doi: 10.1016/s0010-7824(97)00172-8. doi:10.1016/S0010-7824(97)00172-8. [DOI] [PubMed] [Google Scholar]
- 43.Norell SE, Boethius G, Persson I. Oral contraceptive use: interview data versus pharmacy records. Int J Epidemiol. 1998;27:1033–1037. doi: 10.1093/ije/27.6.1033. doi:10.1093/ije/27.6.1033. [DOI] [PubMed] [Google Scholar]
- 44.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: further results. Contraception. 1996;54:1S–106S. doi: 10.1016/s0010-7824(15)30002-0. doi:10.1016/0010-7824(96)00111-4. [DOI] [PubMed] [Google Scholar]