Abstract
Background
Providing care to a spouse with Alzheimer’s disease (AD) may contribute to cardiovascular disease (CVD). The acute phase reactant C-reactive protein is a well-established biomarker of an increased CVD risk.
Objective
To investigate the hypothesis that dementia caregiving is associated with elevated circulating levels of CRP and possibly other biomarkers of CVD risk.
Methods
We examined 118 elderly spousal Alzheimer caregivers and 51 non-caregiving controls about once a year for up to three years. Random regression models with fixed and time-variant effects for a range of covariates known to affect biomarker levels were used to evaluate changes in CRP and in twelve additional measures of inflammation, cellular adhesion, endothelial function, and hemostasis in relation to caregiving status, years of caregiving, and major transitions in the caregiving situation.
Results
During the study period longer duration of caregiving was associated with elevated CRP levels (p=0.040) and caregivers showed greater tumor necrosis factor alpha (TNF-α) levels than controls (p=0.048). Additionally, three months after the death of the AD spouse, caregivers showed a significant drop in CRP levels (p=0.003) and also in levels of soluble intercellular adhesion molecule (sICAM)-1 (p=0.008).
Conclusion
Duration of caregiving, and being a caregiver per se, were both associated with chronic low-grade inflammation as indicated by elevated CRP and TNF-α levels, respectively. Conversely, death of the AD spouse was associated with lower CRP and sICAM-1 levels. The findings indicate that chronic caregiving of those with dementia may result in increased inflammation and thereby, possibly increased CVD risk.
Keywords: Alzheimer disease, biomarkers, cardiovascular disease, caregiver, cytokines, inflammation, psychological stress
INTRODUCTION
Evidence to date suggests that in addition to compromising mental health, providing informal care to a spouse with Alzheimer’s disease (AD) also takes its toll on caregivers’ physical health, particularly the cardiovascular system [1–4; for review]. Relative to their non-caregiving counterparts, AD caregivers have a higher risk of developing incident coronary heart disease (CHD) [5].
Stressors and distress associated with caregiving have been linked to key mechanisms of the initiation, propagation, and clinical manifestation of atherothrombotic diseases. For instance, duration of AD caregiving was associated with endothelial dysfunction [6] and carotid intima-media thickness [7]. Dementia severity of the care recipient was also related to impaired endothelial function and enhanced coagulation activity in the caregiver [6, 8]. High negative affect, including depression, low positive affect, sleeping difficulties, and low subjective health are commonly found in caregivers [2, 3, 9, 10], and these factors may also increase the risk of cardiovascular disease (CVD) [11]. In dementia caregivers, depressive symptoms shortened the time to incident CVD [12], and relative to non-caregiving controls, AD caregivers showed associations between poor sleep and inflammation (i.e., interleukin (IL)-6 and C-reactive protein (CRP)) [13] and between role overload and reduced fibrinolytic capacity over time [14].
In terms of sociodemographic factors, male AD caregivers showed higher risk to develop CHD than female caregivers [15]. Atherosclerotic burden seems particularly high in older AD caregivers, as there is an age-related increase in coagulation (i.e., fibrin D-dimer levels) [16] and inflammatory markers (i.e., IL-6) [17]. Low socioeconomic status, defined as low education level, was associated with greater dementia caregiver burden than higher education [18].
In addition, major transitions in the caregiving situation such as placement of the AD spouse in a long-term care facility or death of the AD spouse showed associations with a drop in caregivers’ D-dimer levels starting half a year after the transition; this effect was accompanied by a decrease in depressive symptoms and role overload [19]. Biological changes due to increased sympathoadrenal medullary (SAM) arousal might partially link caregiving stress with the atherosclerotic mechanisms delineated above [20, 21].
Although traditional risk factors are increased in AD caregivers [22, 23] and mediate some of the CHD risk in this group [15], many patients at-risk for CHD cannot be identified solely on the basis of traditional CVD risk factors alone [24]. This has prompted an intense search for circulating biomarkers to improve CVD risk prediction [25]. In this regard, the acute phase reactant CRP is probably the most established biomarker of increased CVD risk [26, 27], which may directly affect expression of cellular adhesion molecules, impact fibrinolysis and impair endothelial function [25]. Moreover, it has been demonstrated that high-sensitivity CRP (but no other cardiovascular biomarkers) adds prognostic information on future CVD risk above and beyond the Framingham CHD risk score [28], particularly in individuals aged 65 and older [29].
In addition to CRP, slightly elevated levels of several markers of inflammation [e.g., tumor-necrosis factor (TNF)-α, interferon-γ, IL-6, IL-8, IL-10, IL-12], endothelial dysfunction [e.g., endothelin-1, von Willebrand factor (VWF)], upregulated cellular adhesion [e.g., soluble intercellular adhesion molecule (sICAM)-1, soluble vascular cellular adhesion molecule (sVCAM)-1], and hemostasis [e.g., fibrin D-dimer, plasminogen activator inhibitor (PAI)-1)] have also been shown to be involved in atherosclerosis and to predict the risk of incident CHD and poor prognosis in patients with established CHD [30–41]. Notably, the predictive value of biomarkers for CVD risk is largely independent of sociodemographic, life style and traditional CVD risk factors.
Given this information, the primary aim of this study was to investigate the longitudinal relationship between measures of AD caregiving and the most established biomarker of increased CVD risk, high-sensitive CRP. Our specific hypothesis was that CRP would be higher in spousal AD caregivers compared to non-caregiving controls, show a direct association with the duration of caregiving, and a drop following a major transition in the caregiving situation. Secondary analyses were performed with several additional cardiovascular biomarkers as outcomes to explore whether these would add important information above and beyond that obtained from the CRP analysis. In the analysis, and because these covariates may variously affect the concentrations of biomarkers including CRP [28, 42], we a priori controlled for sociodemographic factors (i.e., age. gender, education), traditional cardiovascular risk factors, medication, and diseases, as well as physical symptomatology, life style factors (i.e., alcohol consumption, physical activity), role overload, affect, and subjective sleep quality. Some of these covariates were also found to partially account for the relationship between caregiving stress and biomarker levels, such as age [15, 16] and sleep [13]. Therefore, in case of a significant association between biomarkers and caregiver status, duration of caregiving, or transitions in the caregiving situation, we explored whether covariates, which differentiated caregivers from controls at study entry, would moderate or mediate these relationships.
MATERIALS AND METHODS
Study Participants and Design
We recruited community-dwelling spousal AD caregivers and non-caregiving married controls into the University of California, San Diego (UCSD) “Alzheimer’s Caregiver Study” which is investigating health consequences of dementia caregiving stress. Participants were referred from the UCSD Alzheimer’s Disease Research Center, agencies serving caregivers, local senior citizen health fairs, community support groups, and other participants. Caregivers and controls were matched in terms of age (≥55 years) and gender. Exclusion criteria were current major illnesses (e.g., cancer), severe hypertension (blood pressure exceeding 200/120 mm Hg), and medications affecting biomarker levels, including oral anticoagulants, non-selective beta blockers, and steroids. As the prevalence of daily aspirin use is about 30% in community-dwelling US adults aged 65 years or older [43], aspirin intake was not an exclusion criterion but treated as a control variable.
Participants underwent in home assessments every 12 months for a period of up to 3 years (i.e., for a maximum of 4 visits). Every 3 months research staff also made follow-up phone calls to check for changes in health status and in caregiver transitions (i.e., placement of the AD spouse in a long-term care facility or death of the AD spouse), and participants were additionally asked to call research staff when these transitions occurred. Post-transition assessments were set up at 3, 15, and 27 months after the transition. For all assessments, a research nurse gathered sociodemographic, medical, and psychosocial data using questionnaires. Participants kept their daily routine and were thus not required to fast for the collection of blood for the biomarker assessment. Blood was collected between 10:00 and 10:45 AM.
Out of the total enrolment of 186 study participants, 5 non-caregivers whose spouse had died during the study period and 12 participants with some missing baseline data were excluded from the present study. This yielded a final sample of 169 subjects (118 caregivers, 51 controls). All participants provided written informed consent to the study protocol that was approved by the UCSD Institutional Review Board.
Demographic and Health Assessment
Sociodemographic factors
We collected information on age, gender, ethnicity, years of education (reflecting socioeconomic status), years of caregiving, and hours of care per day.
Medical data
Participants were asked whether a doctor had informed them that they currently have or have ever had any CVD (comprising MI, congestive heart failure, angina, additional heart diseases, and stroke/transient ischemic attack) or diabetes. They were also provided a list of 21 health symptoms (e.g., sore throat, skin rash, toothache) to indicate how many of these they had experienced in the last month. For the assessment of subjective health, all participants were asked to rate their health in general, using a 5-point Likert scale ranging from 0 (“excellent”) to 4 (“poor”). BMI was calculated based on subjects’ self report of weight and height. Plasma low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) levels were determined by standard methodology at the clinical chemistry laboratories at the UCSD Medical Center. The LDL-C to HDL-C ratio was computed and used for statistical analysis. After a 15-minute resting period, the research nurse collected three systolic and diastolic BP measurements using a non-invasive Microlife Blood Pressure monitor. The average mean arterial pressure (MAP) was computed to be used in statistical analysis. Prescribed cholesterol- and BP-lowering medications, aspirin, and antidepressants were also noted.
Life style factors
Smoking status was defined as ever smoker (i.e., former or current smoking) vs. never smoker (only 3 participants smoked at baseline). The Rapid Assessment of Physical Activity (RAPA) scale was used to assess the amount of physical activity at light, moderate, and vigorous intensities in a typical week (score 0–6) [44]. Alcohol consumption in the last month was assessed by multiplying the number of days participants drank alcohol by the number of alcoholic drinks they usually drank on those days (score 0–36).
Sleep
We assessed subjective sleep quality with the interviewer-administered Pittsburg Sleep Quality Index comprising 19 items yielding a global score between 0 and 21 [45]. Higher scores indicate poorer sleep quality.
Affect
We used the Positive and Negative Affect Scale to assess the level of negative and positive mood in the last few weeks [46]. Participants rated 10 mood items for negative affect (e.g., irritable, nervous, hostile) and positive affect (e.g., excited, proud, active) each on a 5-point scale (1=very slightly or not at all, 5= extremely; score 10–50 for either scale).
Role overload
We used Pearlin’s Role Overload scale [47] to assess the extent to which caregivers and controls felt overwhelmed by life responsibilities. The scale consists of 4 items rated from 1 (not at all) to 4 (completely) (total score between 4 and 16); for example, “you work hard (as a caregiver) but never seem to make any progress.” The sections in parentheses specific to caregivers were excluded in the questionnaires given to controls.
Dementia severity
Caregivers were interviewed using the Clinical Dementia Rating (CDR) scale [48]; they indicated dementia symptoms of their spouses in 6 domains: 1) memory; 2) orientation; 3) judgment and problem solving; 4) community affairs; 5) home and hobbies; and 6) personal care. Based on item responses, an overall dementia severity score is given (0=no dementia, 1=mild dementia, 2=moderate dementia, 3=severe dementia).
Biomarkers
Circulating concentrations of biomarkers were determined in duplicates from EDTA plasma samples stored at −80°C. Concentrations of biomarkers were determined in duplicates using commercially available enzyme-linked immunosorbent assays per the manufacturers’ instructions (Meso Scale Discovery, Gaithersburg, MD: CRP, TNF-α, IL-6, IL-8, IL-10, IL-12p70, interferon-γ, sICAM-1, sVCAM-1; Quantikine, R&D Systems, Minneapolis, MN: endothelin-1; Asserachrom Stago, Asnières, France: VWF antigen, PAI-1, D-dimer. Intra- and inter-assay coefficients of variation were <10% for all biomarkers.
Data Analysis
Data were analyzed using PASW 18.0 statistical software package (SPSS Inc., Chicago, IL). Two-tailed level of significance was set at p<0.05. To approximate a normal distribution, all biomarker values were log10 transformed and values 3 SDs above the mean log10 transformed value were deleted as outliers (1 outlier for interferon-γ and sVCAM-1; 2 outliers for CRP and TNF-α; 3 outliers for endothelin-1; 4 outliers for IL-12p70 and IL-8; 6 outliers for IL-10; 7 outliers for IL-6). One VWF value <15% was deleted because of suspected VWF disease. Independent-samples t-test and chi-square test were used to compare caregivers and non-caregivers on baseline characteristics. Pearson’s correlation coefficient was computed to estimate the zero-order association between two variables.
We conducted a mixed (random-effects) regression analysis to examine the impact of caregiver status (i.e., caregivers vs. non-caregiving controls), years of caregiving, and caregiver transitions (i.e., placement and death of the AD spouse) on circulating levels of biomarkers over time. We made adjustments for sociodemographic, medical, life style and psychosocial factors, all of which might affect the concentrations of the various biomarkers. Mixed model regression is a powerful analysis that allows one to estimate an intercept and slope for each participant based on all available data for that individual (i.e., even when some data points are missing), augmented by the data from the entire sample. [49]. Our primary outcome was the change in CRP levels over time. Secondary analyses were performed with all other biomarkers as outcomes. Effect sizes are expressed as pseudo-R2 which indicates the amount of variance of the outcome that is explained by a model’s specific combination of independent variables [49].
To increase the interpretability of regression coefficients and to diminish problems associated with multicollinearity, we centered independent variables before conducting analysis [50] except for “time” (i.e., the number of assessments), which was linear in nature with the baseline assessment coded as “0.” Controlling for “time” may be important because with an increasing number of assessments, anticipatory arousal elicited by the unfamiliarity with the testing protocol and its probable effects on biomarker levels may decrease. Linear variables were centered around their grand means. Dummy coded categorical variables were centered at −0.5 (e.g. non-caregivers) and + 0.5 (e.g. caregivers). Because the dependent variables were log transformed values, several regression coefficients would be very small if using the original scaling of independent variables. Therefore, to provide estimates that can be interpreted, we express estimates for variables marked below with an “*” per a change in 3 units (i.e., we divided the values of these variables by 3 before being entered into the multivariate model).
The model included the following fixed effects: age*, gender, education*, CVD (yes/no), diabetes (yes/no), number of health symptoms*, subjective health, BMI*, LDL-C/HDL-C ratio, MAP*, cholesterol-lowering medication (yes/no), BP-lowering medication (yes/no), aspirin (yes/no), antidepressant medication (yes/no), smoking status, physical activity, alcohol consumption*, sleep quality*, negative affect*, positive affect*, role overload, caregiving status, years caregiving, placement status of the AD spouse (yes/no), and deceased status of the AD spouse (yes/no). Of these, age, CVD, diabetes, the number of health symptoms, subjective health, BMI, LDL-C/HDL-C ratio, MAP, medication categories, smoking status, physical activity, alcohol consumption, sleep quality, negative affect, positive affect, role overload, years caregiving, and caregiving transitions were all entered as time-varying. Random intercepts were modelled for participants. A significant effect of placement or death of the AD spouse would mean a change in the intercept of a biomarker (e.g., CRP levels) as a function of the transition (i.e., from pre- to post-transition). In case of a significant main effect for caregiver status, years caregiving, and caregiving transitions, we probed for interactions of these variables with covariates which significantly differentiated caregivers from controls at baseline. For significant interactions, we applied the Holmbeck method [51] to test whether high levels (+1 SD from the mean) vs. low levels (−1 SD from the mean) of a continuously scaled moderator variable would alter the association of caregiver status, years caregiving, and caregiving transitions with biomarker concentrations.
The 169 subjects contributed totally 483 assessments (mean of 2.9 assessments per participant). Data for all of the fixed effect variables were complete in 100% of assessments per the study design. Time-variant variables were complete in 100% of assessments for medication categories, physical activity, and transitions in the caregiving situation; in 98.3% for years caregiving; in 98.1% for sleep quality; in 97.9% for CVD, health symptoms, subjective health, BMI, smoking status, alcohol consumption, and role overload; in 97.7% for diabetes and positive and negative affect; in 93.8% for LDL-C/HDL-C ratio; and in 93.0% for MAP. After having deleted outliers, biomarker values were available in 87.4% (CRP) to 94.6% (TNF-α) of all assessments.
RESULTS
Characteristics of study participants at baseline
Of all the participants, the mean age±SD was 75±8 years (range 55–90), 68% were women and 92% were Caucasians. Caregivers had been providing care to their AD spouse for an average of 4.4±3.4 years (range 0.5–17.1). Caregivers spent 7.4±5.8 hours per day (range 1–24) caring for their spouse. The mean CDR total score of the care recipients was 1.64±0.59 (range 1–3) indicating mild to moderate dementia (only 7 AD spouses had severe dementia).
Table 1 shows the baseline characteristics of caregivers and controls. Compared to controls, caregivers had expectedly more physical symptomatology, worse subjective health and sleep, more negative affect and role overload, and less positive affect. Except from lower physical activity in caregivers, there were no significant group differences in other cardiovascular risk factors, as well as in medications and sociodemographic variables.
Table 1.
Baseline sociodemographic and health characteristics of 169 study participants
| Variables | Caregivers (n=118) |
Non-caregivers (n=51) |
P-value |
|---|---|---|---|
| Age (years) | 74.4 (8.1) | 74.4 (5.9) | 0.963 |
| Female gender (%) | 70.3 | 64.7 | 0.469 |
| Education (years) | 15.2 (3.1) | 15.7 (3.2) | 0.286 |
| Cardiovascular disease (%) | 16.9 | 9.8 | 0.345 |
| Diabetes (%) | 12.7 | 3.9 | 0.099 |
| Number of health symptoms | 1.96 (2.04) | 0.84 (1.38) | <0.001 |
| Subjective health (score) | 2.48 (0.90) | 3.10 (0.90) | <0.001 |
| Body mass index (kg/m2) | 26.6 (4.8) | 26.5 (6.2) | 0.929 |
| LDL cholesterol (mg/dl) | 106.1 (34.9) | 105.3 (26.9) | 0.888 |
| HDL cholesterol (mg/dl) | 52.2 (15.9) | 53.3 (16.7) | 0.686 |
| LDL cholesterol/HDL cholesterol ratio | 2.16 (0.82) | 2.17 (0.91) | 0.945 |
| Systolic blood pressure (mmHg) | 134.6 (15.3) | 133.9 (16.1) | 0.801 |
| Diastolic blood pressure (mmHg) | 75.9 (8.6) | 73.9 (10.5) | 0.206 |
| Mean arterial pressure (mmHg) | 95.4 (9.6) | 93.2 (11.2) | 0.370 |
| Cholesterol lowering medication (%) | 46.6 | 41.2 | 0.515 |
| Blood pressure lowering medication (%) | 60.2 | 54.9 | 0.523 |
| Aspirin (%) | 27.1 | 31.4 | 0.573 |
| Antidepressant medication (%) | 25.4 | 21.6 | 0.591 |
| Ever smoker (%) | 45.8 | 37.3 | 0.305 |
| Physical activity (score) | 3.41 (1.66) | 4.06 (1.58) | 0.018 |
| Alcohol consumption (score) | 5.53 (5.81) | 5.98 (6.30) | 0.649 |
| Pittsburgh Sleep Quality Index (score) | 6.62 (3.53) | 4.37 (2.47) | <0.001 |
| Negative affect (score) | 17.9 (6.1) | 13.7 (5.4) | <0.001 |
| Positive affect (score) | 31.9 (7.5) | 37.5 (5.8) | <0.001 |
| Role overload (score) | 5.14 (3.20) | 1.35 (2.04) | <0.001 |
Data are given as mean (SD) or percentages
HDL, high-density lipoprotein; LDL, low-density lipoprotein
Table 2 gives circulating levels of biomarkers at the baseline assessment as well as intercorrelations amongst the individual biomarkers. Elevated CRP levels were associated with greater levels of measures of endothelial dysfunction (i.e., endothelin-1, VWF:Ag, sICAM-1, and sVCAM-1), as well as impaired fibrinolysis (i.e., PAI-1), and IL-6 (i.e., a potent stimulant of liver CRP production).
Table 2.
Baseline concentrations and intercorrelations of biomarkers
| Biomarker | TNF-α | INF-γ | IL-12 | IL-6 | IL-8 | IL-10 | ET-1 | VWF:Ag | sICAM-1 | sVCAM-1 | D-dimer | PAI-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP (mg/l) 3.22 (5.70) |
0.04 | −0.10 | 0.11 | 0.29*** | −0.22** | 0.16 | 0.25** | 0.18* | 0.53*** | 0.61*** | −<0.01 | 0.21* |
| TNF-α (pg/ml) 5.96 (2.37) |
_ | 0.13 | 0.05 | 0.27*** | 0.58*** | 0.14 | −0.06 | −0.07 | 0.06 | 0.02 | 0.11 | 0.13 |
| INF-γ (pg/ml) 1.95 (1.67) |
_ | 0.14 | <0.01 | 0.29*** | 0.19* | −0.21* | −0.26** | −0.16* | −0.19* | <0.01 | 0.16 | |
| IL-12 (pg/ml) 5.56 (15.80) |
_ | 0.07 | −0.12 | 0.70*** | −0.03 | 0.06 | 0.10 | 0.06 | −0.10 | 0.06 | ||
| IL-6 (pg/ml) 1.56 (1.75) |
_ | 0.04 | 0.12 | 0.20* | 0.05 | 0.26** | 0.28*** | 0.09 | 0.16* | |||
| IL-8 (pg/ml) 7.09 (3.85) |
_ | −0.09 | −0.33*** | −0.27*** | −0.38*** | −0.48*** | −0.01 | 0.18* | ||||
| IL-10 (pg/ml) 3.16 (5.80) |
_ | −0.06 | 0.10 | 0.07 | 0.13 | −0.06 | 0.09 | |||||
| ET-1 (pg/ml) 1.22 (0.57) |
_ | 0.28*** | 0.37*** | 0.41*** | 0.10 | −0.03 | ||||||
| VWF:Ag (%) 176.1 (115.3) |
_ | 0.30*** | 0.34*** | 0.17* | 0.09 | |||||||
| sICAM-1 (ng/ml) 360.1 (209.3) |
_ | 0.89*** | 0.01 | 0.05 | ||||||||
| sVCAM-1 (ng/ml) 652.2 (346.5) |
_ | 0.04 | 0.02 | |||||||||
| D-dimer (ng/ml) 788.1 (473.9) |
_ | −0.10 | ||||||||||
| PAI-1 (ng/ml) 35.0 (30.5) |
_ |
Data are given as means (SD) for biomarker levels and Pearson correlation coefficients with significance level (*p<0.05, ** p<0.01; *** p<0.001) for intercorrelations between log transformed values of biomarkers.
CRP, C-reactive protein; ET, endothelin; IL, interleukin; INF, interferon; PAI, plasminogen activator inhibitor; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cellular adhesion molecule; TNF, tumor necrosis factor; VWF:Ag, von Willebrand factor antigen
Transitions in the caregiving situation
Over the course of the study, 30 (25.4%) caregivers placed their spouse in a long-term care facility and 20 (16.9%) experienced the death of their spouse. The initial post-transition assessments occurred at 3 months following placement or death of the AD spouse. The remaining assessments took place approximately 12 months (12 placements, 10 deaths) or 24 months (3 placements, 1 death) later.
Changes in C-reactive protein levels
There emerged significant zero-order associations between several key potential confounding variables and CRP levels over time. Higher CRP levels were associated with lower education (r=−0.10, p=0.037), more health symptoms (r=0.10, p=0.045), poorer subjective health (r=−0.22, p<0.001), fewer physical activity (r=−0.12, p=0.014), and greater negative affect (r=0.15, p=0.003). No significant associations were seen between CRP and age, gender, alcohol consumption, sleep quality, positive affect, and role overload.
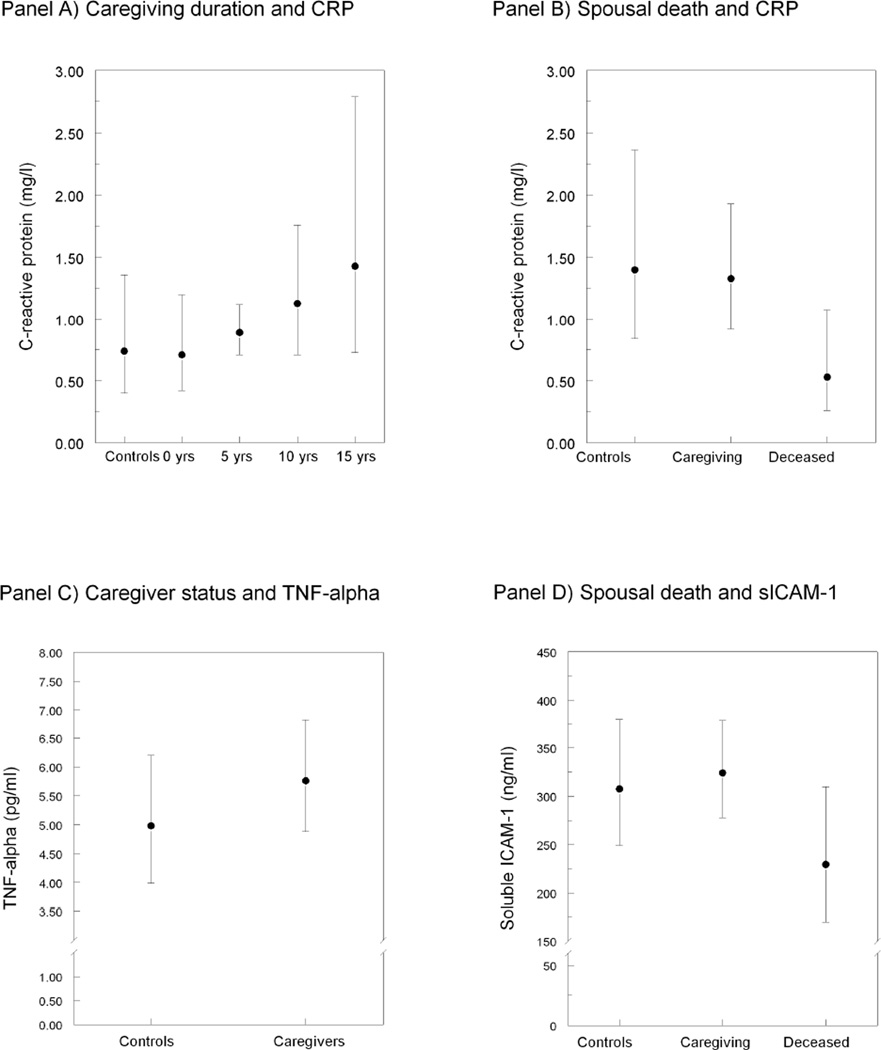
Table 3 shows the multivariate model for CRP levels. More years of caregiving (but not caregiver status per se) were associated with higher CRP levels over time (p=0.040; explained variance=1.21%). Figure 1A shows the increase in CRP levels across 5-year steps of caregiving duration. In caregivers who had been providing care to their AD spouse for 15 years, mean CRP levels were predicted to be two-fold higher [1.43 mg/l (95% CI 0.73–2.79)] than in caregivers who were at the beginning of their caregiver career [0.71 mg/l (95 % CI 0.42–1.20)] and in non-caregiving controls [0.74 mg/l (95% CI 0.40–1.36)], respectively, if all other covariates were held constant in the model.
Table 3.
Changes over time in circulating levels of C-reactive protein and cytokines
| Variables entered | CRP (mg/l) | TNF-α (pg/ml) | Interferon-γ (pg/ml) | IL-12p70 (pg/ml) | IL-6 (pg/ml) | IL-8 (pg/ml) | IL-10 (pg/ml) |
|---|---|---|---|---|---|---|---|
| Intercept | −0.064 (0.115) | 0.729 (0.040)*** | 0.061 (0.095) | 0.299 (0.141)* | 0.109 (0.076) | 0.766 (0.053)*** | 0.254 (0.112)* |
| Age | <0.001 (0.014) | 0.013 (0.005)** | 0.007 (0.011) | 0.024 (0.019) | 0.008 (0.010) | 0.010 (0.006) | 0.011 (0.015) |
| Gender, female | 0.108 (0.068) | 0.019 (0.024) | 0.115 (0.054)* | 0.170 (0.100) | 0.061 (0.049) | 0.055 (0.031) | 0.061 (0.077) |
| Education | −0.043 (0.030) | −0.003 (0.010) | −0.013 (0.024) | 0.004 (0.043) | 0.012 (0.022) | −0.001 (0.014) | −0.025 (0.033) |
| CVD | −0.016 (0.081) | 0.026 (0.028) | −0.067 (0.065) | 0.152 (0.107) | 0.009 (0.056) | 0.001 (0.038) | 0.070 (0.083) |
| Diabetes | −0.060 (0.107) | 0.007 (0.036) | 0.068 (0.084) | −0.152 (0.143) | 0.004 (0.073) | 0.060 (0.048) | 0.086 (0.110) |
| Health symptoms | 0.030 (0.052) | −0.018 (0.018) | 0.018 (0.044) | −0.048 (0.064) | −0.006 (0.035) | 0.023 (0.024) | −0.026 (0.051) |
| Subjective health | −0.111 (0.037)** | −0.007 (0.013) | 0.037 (0.031) | 0.030 (0.046) | −0.034 (0.025) | −0.014 (0.017) | 0.039 (0.037) |
| Body mass index | 0.013 (0.020) | 0.001 (0.007) | 0.003 (0.016) | 0.007 (0.027) | 0.027 (0.014) | −0.007 (0.009) | <0.001 (0.021) |
| LDL-C/HDL-C ratio | 0.093 (0.039)* | 0.008 (0.014) | 0.031 (0.032) | 0.006 (0.053) | 0.050 (0.027) | 0.006 (0.018) | 0.021 (0.041) |
| Mean arterial pressure | 0.009 (0.009) | <−0.001 (0.003) | −0.016 (0.007)* | −0.005 (0.011) | 0.008 (0.006) | −0.002 (0.004) | 0.004 (0.009) |
| Cholesterol meds | −0.041 (0.060) | 0.028 (0.021) | 0.030 (0.050) | 0.070 (0.080) | 0.074 (0.041) | 0.007 (0.028) | 0.068 (0.062) |
| Blood pressure meds | 0.073 (0.062) | 0.012 (0.022) | −0.059 (0.051) | −0.114 (0.083) | 0.009 (0.043) | −0.056 (0.029) | −0.050 (0.065) |
| Aspirin | −0.094 (0.067) | −0.010 (0.023) | −0.016 (0.055) | −0.113 (0.079) | −0.007 (0.044) | 0.040 (0.031) | −0.107 (0.063) |
| Antidepressants | 0.036 (0.066) | −0.022 (0.023) | 0.019 (0.055) | −0.115 (0.088) | −0.005 (0.046) | −0.021 (0.031) | −0.132 (0.068) |
| Ever smoker | 0.007 (0.061) | −0.001 (0.021) | −0.017 (0.049) | 0.060 (0.084) | 0.058 (0.043) | −0.028 (0.028) | 0.028 (0.066) |
| Physical activity | −0.014 (0.019) | −0.005 (0.007) | <−0.001 (0.016) | 0.016 (0.023) | 0.012 (0.012) | −0.009 (0.009) | 0.009 (0.018) |
| Alcohol consumption | 0.012 (0.016) | 0.010 (0.005) | 0.025 (0.012)* | −0.010 (0.020) | 0.009 (0.011) | 0.013 (0.007) | <−0.001 (0.016) |
| Sleep quality | −0.007 (0.030) | 0.016 (0.010) | 0.045 (0.024) | 0.125 (0.039)** | 0.019 (0.020) | 0.010 (0.014) | 0.083 (0.030)** |
| Negative affect | 0.029 (0.017) | −0.003 (0.006) | −0.010 (0.014) | −0.015 (0.021) | −0.003 (0.011) | −0.012 (0.007) | −0.024 (0.016) |
| Positive affect | 0.023 (0.014) | −0.003 (0.005) | <−0.001 (0.011) | 0.014 (0.017) | 0.004 (0.009) | −0.001 (0.006) | 0.004 (0.014) |
| Role overload | −0.017 (0.012) | −0.007 (0.004) | 0.004 (0.010) | −0.005 (0.014) | 0.003 (0.008) | −0.004 (0.005) | 0.008 (0.012) |
| Caregiver status | −0.022 (0.093) | 0.064 (0.032)* | 0.013 (0.074) | 0.039 (0.130) | −0.073 (0.066) | 0.023 (0.042) | 0.056 (0.101) |
| Years caregiving | 0.020 (0.010)* | −0.002 (0.003) | 0.006 (0.008) | 0.015 (0.014) | −0.001 (0.007) | 0.001 (0.004) | 0.005 (0.011) |
| Time | 0.066 (0.037) | −0.052 (0.013)*** | −0.179 (0.032)*** | −0.246 (0.042)*** | −0.012 (0.023) | −0.120 (0.017)*** | −0.014 (0.033) |
| Placed spouse | 0.058 (0.108) | 0.022 (0.037) | 0.034 (0.089) | 0.133 (0.131) | −0.007 (0.069) | −0.011 (0.050) | 0.122 (0.100) |
| Spouse deceased | −0.399 (0.133)** | −0.029 (0.046) | −0.069 (0.109) | 0.031 (0.152) | 0.092 (0.085) | −0.022 (0.062) | −0.010 (0.123) |
| Pseudo-R2 statistic | 16.97*** | 15.85*** | 16.32*** | 12.82*** | 10.76*** | 19.01*** | 7.02*** |
Data are given as log transformed slopes (s.e.),
p<0.05,
p<0.01;
p<0.001.
All independent variables were centered to the mean such that the intercepts show the mean biomarker concentrations in the entire sample. Categorical variables were contrast coded as female gender (+0.5) vs. male gender (−0.5), CVD (+0.5) vs. no CVD (−0.5), diabetes (+0.5) vs. no diabetes (−0.5), medications (+0.5) vs. no medications (−0.5), ever smoker (+0.5) vs. never smoker (−0.5), and caregiver (+0.5) vs. non-caregiver (−0.5). “Placed spouse” and “Spouse deceased” indicate the immediate change in the biomarker concentration assessed 3 months after the respective transition. “Time” indicates the change in the biomarker concentration per each assessment the participant was in the study.
CRP, C-reactive protein; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; TNF, tumor necrosis factor.
Figure 1. Parameters of dementia caregiving stress and changes in biomarker levels.
All values are given as multivariate-adjusted geometric means with 95%confidence intervals. CRP, C-reactive protein; TNF, tumor necrosis factor; sICAM-1, soluble intercellular adhesion molecule-1. Panel A illustrates the association between duration of caregiving across 5-year steps and change in CRP over time (0 years of caregiving in controls). Panel B and Panel D depict lowered CRP and sICAM-1 levels, respectively, in caregivers whose spouse had deceased relative to caregivers who are continuing to provide care for their spouse and controls. Panel C shows higher TNF-alpha levels over time in caregivers than in controls.
In addition, CRP levels had significantly dropped at three months after the death of the AD spouse (p=0.003, explained variance=2.15%). Figure 1B illustrates that caregivers whose spouse had died had 60% lower mean CRP levels than those who continued to provide care for their spouse [0.53 mg/l (95% CI 0.26–1.08) vs. 1.33 mg/l (95% CI 0.92–1.93)].
The association between longer duration of caregiving and CRP increase over time (0.019±0.008, p=0.014), as well as the drop in CRP levels after spousal death (−0.242±0.109, p=0.028) were also significant without making adjustments for covariates. The significance of both these associations further persisted when baseline characteristics that differentiated between caregivers and controls (i.e., number of health symptoms, subjective health, physical activity, sleep quality, negative affect, positive affect, and role overload) were added separately to the model, thereby suggesting that there were no mediational effects (data not shown). In the fully adjusted model, baseline characteristics differentiating caregivers from controls also did not turn out to be effect moderators, as they did not significantly interact with years of caregiving and spousal death in determining CRP levels (data not show).
Changes in Levels of Other Biomarkers
As can be seen in Tables 3 and 4, years of caregiving were not significantly associated with levels of any additional biomarker. However, caregivers showed greater TNF-α levels over time than non-caregiving controls (p=0.048, explained variance=0.94%); this association was also significant without covariate adjustment (0.054±0.021, p=0.010). Figure 1C shows that caregivers had 15.7% greater mean TNF-α levels over time than non-caregiving controls. This effect was not significantly mediated or moderated by baseline characteristics that differentiated between caregivers and controls (data not shown).
Table 4.
Changes over time in circulating biomarkers of endothelial function, cellular adhesion and hemostasis
| Variables entered | Endothelin-1 (pg/ml) | VWF:Ag (%) | sICAM-1 (ng/ml) | sVCAM-1 (ng/ml) | D-dimer (ng/ml) | PAI-1 (ng/ml) |
|---|---|---|---|---|---|---|
| Intercept | 0.044 (0.036) | 2.157 (0.062)*** | 2.424 (0.048)*** | 2.733 (0.043)*** | 2.863 (0.048)*** | 1.515 (0.080)*** |
| Age | 0.015 (0.005)** | 0.018 (0.007)* | −0.005 (0.005) | −0.005 (0.005) | 0.033 (0.007)*** | <−0.001 (0.011) |
| Gender, female | 0.015 (0.025) | −0.015 (0.037) | 0.014 (0.027) | 0.002 (0.025) | 0.033 (0.035) | 0.051 (0.059) |
| Education | 0.006 (0.011) | −0.015 (0.016) | 0.003 (0.012) | −0.005 (0.011) | −0.004 (0.015) | −0.014 (0.026) |
| CVD | 0.013 (0.027) | 0.097 (0.044)* | 0.033 (0.033) | 0.024 (0.030) | 0.033 (0.037) | 0.047 (0.061) |
| Diabetes | −0.074 (0.036)* | −0.011 (0.056) | 0.002 (0.043) | 0.003 (0.039) | −0.012 (0.049) | 0.094 (0.082) |
| Health symptoms | −0.003 (0.016) | −0.027 (0.027) | −0.002 (0.022) | −0.023 (0.019) | −0.003 (0.021) | 0.015 (0.035) |
| Subjective health | −0.001 (0.011) | −0.022 (0.020) | −0.017 (0.015) | −0.016 (0.013) | −0.028 (0.015) | −0.020 (0.026) |
| Body mass index | 0.012 (0.007) | 0.022 (0.011)* | −0.006 (0.010) | −0.002 (0.007) | −0.007 (0.009) | 0.054 (0.015)*** |
| LDL-C/HDL-C ratio | 0.002 (0.013) | 0.029 (0.021) | 0.055 (0.016)*** | 0.034 (0.014)* | −0.014 (0.018) | 0.107 (0.030)*** |
| Mean arterial pressure | 0.003 (0.003) | −0.001 (0.005) | 0.002 (0.004) | −0.001 (0.003) | 0.005 (0.004) | 0.021 (0.006)*** |
| Cholesterol meds | 0.004 (0.020) | 0.035 (0.033) | 0.035 (0.025) | 0.003 (0.022) | 0.022 (0.027) | 0.155 (0.045)*** |
| Blood pressure meds | 0.019 (0.021) | −0.021(0.033) | 0.021 (0.025) | 0.036 (0.023) | 0.001 (0.028) | 0.005 (0.047) |
| Aspirin | 0.005 (0.020) | −0.073 (0.035)* | −0.071 (0.028)* | −0.050 (0.025)* | 0.022 (0.027) | 0.005 (0.045) |
| Antidepressants | 0.010 (0.022) | 0.017 (0.036) | 0.010 (0.027) | 0.010 (0.025) | 0.068 (0.030)* | 0.059 (0.050) |
| Ever smoker | −0.036 (0.021) | 0.014 (0.033) | −0.002 (0.025) | 0.003 (0.022) | 0.030 (0.029) | −0.018 (0.049) |
| Physical activity | <−0.001 (0.006) | −0.004 (0.010) | 0.001 (0.008) | −0.002 (0.007) | −0.006 (0.008) | −0.025 (0.013) |
| Alcohol consumption | 0.006 (0.005) | <0.001 (0.008) | <0.001 (0.006) | 0.002 (0.006) | −0.009 (0.007) | 0.027 (0.012)* |
| Sleep quality | 0.014 (0.010) | −0.017 (0.016) | −0.002 (0.012) | −0.002 (0.011) | <−0.001 (0.013) | 0.007 (0.022) |
| Negative affect | −0.001 (0.005) | 0.009 (0.009) | 0.009 (0.007) | 0.011 (0.006) | −0.001 (0.007) | 0.002 (0.012) |
| Positive affect | −0.003 (0.004) | 0.010 (0.008) | 0.001 (0.006) | −0.003 (0.005) | −0.007 (0.006) | 0.016 (0.010) |
| Role overload | 0.001 (0.004) | 0.002 (0.006) | −0.014 (0.005)** | −0.012 (0.004)** | −0.005 (0.005) | 0.007 (0.008) |
| Caregiver status | −0.027 (0.033) | 0.056 (0.050) | 0.023 (0.037) | 0.018 (0.034) | 0.015 (0.046) | −0.065 (0.077) |
| Years caregiving | −0.002 (0.004) | −0.008 (0.005) | 0.001 (0.004) | −0.001 (0.004) | −0.008 (0.005) | 0.006 (0.008) |
| Time | 0.041 (0.010)*** | −0.010 (0.019) | 0.002 (0.016) | 0.010 (0.015) | −0.026 (0.013) | −0.073 (0.022)** |
| Placed spouse | 0.041 (0.031) | 0.021 (0.056) | 0.027 (0.042) | 0.035 (0.041) | 0.005 (0.041) | 0.058 (0.069) |
| Spouse deceased | −0.041 (0.040) | −0.066 (0.070) | −0.150 (0.056)** | −0.092 (0.050) | −0.009 (0.050) | 0.115 (0.083) |
| Pseudo-R2 statistic | 13.18*** | 10.89*** | 10.24*** | 8.12*** | 22.85*** | 30.36*** |
Data are given as log transformed slopes (s.e.),
p<0.05,
p<0.01;
p<0.001.
All independent variables were centered to the mean such that the intercepts show the mean biomarker concentrations in the entire sample. Categorical variables were contrast coded as female gender (+0.5) vs. male gender (−0.5), CVD (+0.5) vs. no CVD (−0.5), diabetes (+0.5) vs. no diabetes (−0.5), medications (+0.5) vs. no medications (−0.5), ever smoker (+0.5) vs. never smoker (−0.5), and caregiver (+0.5) vs. non-caregiver (−0.5). “Placed spouse” and “Spouse deceased” indicate the immediate change in the biomarker concentration assessed 3 months after the respective transition. “Time” indicates the change in the biomarker concentration per each assessment the participant was in the study.
CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAI, plasminogen activator inhibitor; sICAM; soluble intercellular adhesion molecule; sVCAM; soluble vascular cellular adhesion molecule; VWF:Ag, von Willebrand Factor Antigen
In terms of caregiving transitions, placement of the AD spouse in a long-term care facility was not significantly associated with changes in levels of any biomarker. However, sICAM-1 levels had significantly dropped at three months after the death of the AD spouse (p=0.008; explained variance=1.77%). Figure 1D illustrates that caregivers whose spouse had died had 29% lower mean sICAM-1 levels than those who continued to provide care for their spouse [230 ng/ml (95% CI 170–310) vs. 324 ng/ml (95% CI 278–378)]. The effect for spousal death on sICAM-1 levels remained significant without controlling for covariates (−0.107±0.044, p=0.015) and it was not mediated by baseline characteristics that differentiated between caregivers and controls (data not shown). Further exploratory analyses on sICAM-1 yielded significant interactions between death of the AD spouse and both physical activity (0.075±0.033, p=0.026; explained variance=1.25%) and the number of health symptoms (0.134±0.065, p=0.039; explained variance=1.05%). Post hoc probing of these moderating effects showed associations between spousal death and lowered sICAM-1 in caregivers with low physical activity level (−0.300±0.087, p<0.001), but not in those with high physical activity level (p=0.41), as well as in caregivers with a low number of health symptoms (−0.257±0.076, p<0.001), but not in those with a high number of health symptoms (p=0.17).
DISCUSSION
We found that the longer caregivers had been providing care to their AD spouse, the more their CRP levels increased over time. However, being a caregiver per se was not significantly associated with CRP levels suggesting that it might be the accumulation of the many factors making up caregiver burden that contributed to increased CRP. AD caregiver burden is understood as the sum of physical, psychological, social, and financial problems that can be experienced by family members caring for a demented relative [52]. Impaired sleep and poor self-care due to restricted time to engage in regular physical activity also contribute to caregiver burden [53]. In agreement with this conceptualization of AD caregiver burden, our caregivers had poorer physical and mental health, and they also slept more poorly and were less physically active than their non-caregiving counterparts at enrolment into the study. Poorer self-rated health, fewer physical activity, and greater negative affect also showed zero-order correlations with greater CRP levels. Even when taking into account differences in burden-eliciting factors, years of caregiving were associated with CRP levels suggesting that elevated CRP concentration could be an important physical marker of AD caregiver burden itself. Nevertheless, it remains possible that social and financial problems we could not consider for in our analysis accounted for at least part of the increased CRP levels with more years of caregiving. Caregiving strain is associated with increased SAM arousal [20] which could contribute to CRP levels with more years of exposure to caregiving strain, too. A recent study on family caregivers of patients with brain cancer found an increase in both daily output of salivary alpha-amylase (i.e., a marker of sympathetic nervous system activity) and circulating CRP levels sampled over a period of one year [54].
Caregiving for a disabled spouse was predictive of CHD [55] and all-cause mortality [56] and elevated CRP concentrations were associated with a greater risk of mortality from vascular and many non-vascular causes [42]. Moreover, even moderately elevated levels of CRP predicted incident frailty in individuals 65 years and older [57]. The relation of AD caregiving duration with CRP levels thus provides one possible pathway leading from chronic caregiving to poor cardiovascular health over time, but also to accelerated decline of physical health across a range of other biological systems. Years of caregiving explained a rather small amount of 1.2% of the variance in CRP levels over time. However, cutpoints of CRP for low risk (<1.0 mg/l), average risk (1.0–3.0 mg/l), and high risk (>3.0 mg/l) of CHD have been defined, whereby the high-risk category has an ≈2-fold increased relative risk compared to the low-risk group [28]. Mean CRP levels were in the low risk range in controls and in caregivers with shorter duration of caregiving, but in the average risk range in AD caregivers who had been providing care for 10 years or longer. This effect seems clinically meaningful as caregivers are expected to serve in this role for up to 15 years (2).
We found that death of the AD spouse was associated with a significant drop in caregivers’ CRP levels at three months post-transition. The same effect could be observed for sICAM-1 levels. The latter may seem an expected finding given that experimental studies found recombinant human CRP induces expression of ICAM-1 in human endothelial cells within 24 hours [58]. The soluble form of ICAM-1 reflects ICAM-1 expression on endothelial cells; ICAM-1 is involved in adherence and subsequent transmigration of circulating leukocytes across the vascular endothelium, thereby promoting inflammation of the coronary artery [59]. Underscoring the clinical value of sICAM-1 as a cardiovascular biomarker, elevated levels of sICAM-1 were prospectively associated with the risk of first-time MI in apparently healthy men [60]. Reduction in sICAM-1 was particularly seen in caregivers with fewer health symptoms and in those with lower physical activity levels at three months after spousal death. Although speculative, lowered sICAM-1 might mirror a reduction in health symptoms, as sICAM-1 is increased in many pathological conditions [61]. Moreover, as sICAM-1 increases with exercise [62], sICAM-1 might have been less detectable in the blood of poor exercisers. The decrease in CRP and sICAM-1 levels following the death of the AD spouse could reflect a lessening of cardiovascular burden with AD caregiving. This seems a clinically meaningful effect. For instance, on a population-based level, statin use is associated with a 12%lower CRP level than non-use [63], whereas caregivers whose spouse had died had a 60%lower mean CRP level compared to those who continued to provide care. Moreover, continued care was associated with CRP levels in the average risk range for CHD, whereas, after spousal death, caregivers’ mean CRP levels fell into the low risk range. Nevertheless, although these effects are intriguing, it should be noted that a large bereavement literature actually suggests that morbidity and mortality risk, including from cardiovascular causes, increases in the early months after partner loss [64].
Placement of the AD spouse in a long-term care facility did not significantly affect CRP levels, likely because the biobehavioral responses to spousal death are different from those elicited by placement of an AD spouse. For instance, in caregivers who were strained prior to the death of their spouse, the death itself did not increase their level of distress but instead, reduced health risk behaviors [65]. In contrast, while some burdens are lessened when a spouse is placed, others persist, or may even increase [66]. For instance, caregivers may stay involved in physical care during their visits and worry about the adequacy of treatment for the loved one and financial costs.
Relative to controls, caregivers showed higher levels of the proinflammatory cytokine TNF-α over time. This finding adds to the notion of an inflammatory state in AD caregivers [4]. However, except from TNF-α we did not find a significant association between caregiver status and circulating levels of any other biomarker, including CRP. Several explanations may apply to this observation. We controlled for possible confounders of biomarker levels, including medication use, life style, and sleep quality, all showing associations with some biomarkers (Tables 3 and 4). Assessment of biomarkers only three months after a major transition in the caregiving situation might have been too early to detect significant changes in some biomarkers. Most of the spouses suffered mild to moderate dementia. A greater proportion of spouses with severe dementia might have evoked greater caregiving stress with changes in biomarkers downstream. Healthier individuals are more likely to become caregivers and to remain in this role. Such a healthy caregiver effect might partially prevent biomarker changes in stressful situations. This notion is supported by the observation that covariates indicating compromised physical and mental health in caregivers did not turn out to moderate or mediate the caregiver-biomarker relationship.
The longitudinal design with on average three assessments per participant, relatively few missing follow-up data, and adjustment for important confounding variables, are all strengths of our study. However, the study also has its limitations. We are unable to make a statement about the trajectory in biomarker levels beyond the 3-month post-transition changes because we had too few data points available to reliably estimate post-transition slopes in biomarker concentrations. The ultimate health consequences of the changes in CRP levels in AD caregivers remain to be determined. Moreover, our findings might not generalize to populations of younger AD caregivers, those with a greater proportion of male caregivers, and to AD caregivers with greater impairments in physical health.
Taken together, we found that longer duration of caregiving and caregiver status were associated with increased CRP and TNF-α levels, respectively, with both these associations suggesting a proinflammatory state. In contrast, death of the AD spouse was associated with a decrease in inflammation-related biomarkers, namely in CRP and sICAM-1. Chronic caregiving stress is associated with SAM arousal [20] and CRP, TNF-α and sICAM-1 are responsive to chronic stress [67]. Therefore, cessation of caregiving stress and of the accompanying SAM arousal after spousal death could have favorably affected caregivers’ proinflammatory state. C-reactive protein might be a biomarker that integrates well several cardiovascular processes that are responsive to changes in caregiving burden. This would make CRP a promising candidate for the longitudinal investigation of atherothrombotic consequences of chronic AD caregiving.
ACKNOWLEDGMENTS
The authors wish to thank Susan Calleran and Christine Gonzaga for data collection. The study was supported by NIH/NIA award AG 15301 to Igor Grant, and additionally by awards AG 03090 to Brent Mausbach and AG 08415 to Sonia Ancoli-Israel.
REFERENCES
- 1.Schulz R, O'Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 2.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12:240–249. [PubMed] [Google Scholar]
- 4.Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15:251–259. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Känel R, Mausbach BT, Patterson TL, Dimsdale JE, Aschbacher K, Mills PJ, Ziegler MG, Ancoli-Israel S, Grant I. Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54:131–137. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- 6.Mausbach BT, Roepke SK, Ziegler MG, Milic M, von Känel R, Dimsdale JE, Mills PJ, Patterson TL, Allison MA, Ancoli-Israel S, Grant I. Association between chronic caregiving stress and impaired endothelial function in the elderly. J Am Coll Cardiol. 2010;55:2599–2606. doi: 10.1016/j.jacc.2009.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roepke SK, Allison M, von Känel R, Mausbach BT, Chattillion EA, Harmell AL, Patterson TL, Dimsdale JE, Mills PJ, Ziegler MG, Ancoli-Israel S, Grant I. Relationship between chronic stress and carotid intima-media thickness (IMT) in elderly Alzheimer's disease caregivers. Stress. doi: 10.3109/10253890.2011.596866. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschbacher K, von Känel R, Dimsdale JE, Patterson TL, Mills PJ, Mausbach BT, Allison MA, Ancoli-Israel S, Grant I. Dementia severity of the care receiver predicts procoagulant response in Alzheimer caregivers. Am J Geriatr Psychiatry. 2006;14:694–703. doi: 10.1097/01.JGP.0000227969.36850.eb. [DOI] [PubMed] [Google Scholar]
- 9.Adams KB. Specific effects of caring for a spouse with dementia: differences in depressive symptoms between caregiver and non-caregiver spouses. Int Psychogeriatr. 2008;20:508–520. doi: 10.1017/S1041610207006278. [DOI] [PubMed] [Google Scholar]
- 10.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Mausbach BT, Patterson TL, Rabinowitz YG, Grant I, Schulz R. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychol. 2007;26:539–544. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- 13.von Känel R, Ancoli-Israel S, Dimsdale JE, Mills PJ, Mausbach BT, Ziegler MG, Patterson TL, Grant I. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56:41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mausbach BT, von Känel R, Aschbacher K, Roepke SK, Dimsdale JE, Ziegler MG, Mills PJ, Patterson TL, Ancoli-Israel S, Grant I. Spousal caregivers of patients with Alzheimer's disease show longitudinal increases in plasma level of tissue-type plasminogen activator antigen. Psychosom Med. 2007;69:816–822. doi: 10.1097/PSY.0b013e318157d461. [DOI] [PubMed] [Google Scholar]
- 15.Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 16.von Känel R, Dimsdale JE, Mills PJ, Ancoli-Israel S, Patterson TL, Mausbach BT, Grant I. Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, C-reactive protein, and D-dimer. J Gerontol A Biol Sci Med Sci. 2006;61:963–969. doi: 10.1093/gerona/61.9.963. [DOI] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MD, Hong SC, Lee CI, Kim SY, Kang IO, Lee SY. Caregiver burden among caregivers of Koreans with dementia. Gerontology. 2009;55:106–113. doi: 10.1159/000176300. [DOI] [PubMed] [Google Scholar]
- 19.Mausbach BT, Aschbacher K, Patterson TL, von Känel R, Dimsdale JE, Mills PJ, Ancoli-Israel S, Grant I. Effects of placement and bereavement on psychological well being and cardiovascular risk in Alzheimer's caregivers: a longitudinal analysis. J Psychosom Res. 2007;62:439–445. doi: 10.1016/j.jpsychores.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Grant I. Caregiving may be hazardous to your health. Psychosom Med. 1999;61:420–423. doi: 10.1097/00006842-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Roepke SK, Chattillion EA, von Känel R, Allison M, Ziegler MG, Dimsdale JE, Mills PJ, Patterson TL, Ancoli-Israel S, Calleran S, Harmell AL, Grant I. Carotid plaque in Alzheimer caregivers and the role of sympathoadrenal arousal. Psychosom Med. 2011;73:206–213. doi: 10.1097/PSY.0b013e3182081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Känel R, Mausbach BT, Dimsdale JE, Mills PJ, Patterson TL, Ancoli-Israel S, Ziegler MG, Roepke SK, Harmell AL, Allison M, Grant I. Regular physical activity moderates cardiometabolic risk in Alzheimer’s caregivers. Med Sci Sports Exerc. 2011;43:181–189. doi: 10.1249/MSS.0b013e3181e6d478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitaliano PP, Persson R, Kiyak A, Saini H, Echeverria D. Caregiving and gingival symptom reports: psychophysiologic mediators. Psychosom Med. 2005;67:930–938. doi: 10.1097/01.psy.0000188485.65153.7b. [DOI] [PubMed] [Google Scholar]
- 24.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109(25) Suppl 1:IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 26.Koenig W. Cardiovascular biomarkers: added value with an integrated approach? Circulation. 2007;116:3–5. doi: 10.1161/CIRCULATIONAHA.107.707984. [DOI] [PubMed] [Google Scholar]
- 27.C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC) Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey-Smith G, Nordestgaard BG, Saleheen D, Samani NJ, Sandhu M, Anand S, Pepys MB, Smeeth L, Whittaker J, Casas JP, Thompson SG, Hingorani AD, Danesh J. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 29.Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly: results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoudi M, Curzen N, Gallagher PJ. Atherogenesis: the role of inflammation and infection. Histopathology. 2007;50:535–546. doi: 10.1111/j.1365-2559.2006.02503.x. [DOI] [PubMed] [Google Scholar]
- 31.Sarwar N, Thompson AJ, Di Angelantonio E. Markers of inflammation and risk of coronary heart disease. Dis Markers. 2009;26:217–225. doi: 10.3233/DMA-2009-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6:410–417. doi: 10.1038/nrcardio.2009.57. [DOI] [PubMed] [Google Scholar]
- 33.Schroecksnadel K, Frick B, Winkler C, Fuchs D. Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr Vasc Pharmacol. 2006;4:205–213. doi: 10.2174/157016106777698379. [DOI] [PubMed] [Google Scholar]
- 34.Zhou RH, Shi Q, Gao HQ, Shen BJ. Changes in serum interleukin-8 and interleukin-12 levels in patients with ischemic heart disease in a Chinese population. J Atheroscler Thromb. 2001;8:30–32. doi: 10.5551/jat1994.8.30. [DOI] [PubMed] [Google Scholar]
- 35.Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009;84:353–360. doi: 10.1093/cvr/cvp241. [DOI] [PubMed] [Google Scholar]
- 36.Lakoski SG, Liu Y, Brosnihan KB, Herrington DM. Interleukin-10 concentration and coronary heart disease (CHD) event risk in the estrogen replacement and atherosclerosis (ERA) study. Atherosclerosis. 2008;197:443–447. doi: 10.1016/j.atherosclerosis.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorin E, Webb DJ. Endothelium-derived endothelin-1. Pflugers Arch. 2010;459:951–958. doi: 10.1007/s00424-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulinska P, Spiel A, Jilma B. Role of von Willebrand factor in vascular disease. Hamostaseologie. 2009;29:32–38. [PubMed] [Google Scholar]
- 39.Hope SA, Meredith IT. Cellular adhesion molecules and cardiovascular disease. Part II. Their association with conventional and emerging risk factors, acute coronary events and cardiovascular risk prediction. Intern Med J. 2003;33:450–462. doi: 10.1046/j.1445-5994.2003.00379.x. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 41.Lowe GD. Fibrin D-dimer and cardiovascular risk. Semin Vasc Med. 2005;5:387–398. doi: 10.1055/s-2005-922485. [DOI] [PubMed] [Google Scholar]
- 42.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith NL, Psaty BM, Furberg CD, White R, Lima JA, Newman AB, Manolio TA. Temporal trends in the use of anticoagulants among older adults with atrial fibrillation. Arch Intern Med. 1999;159:1574–1578. doi: 10.1001/archinte.159.14.1574. [DOI] [PubMed] [Google Scholar]
- 44.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 46.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 47.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 48.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 49.Singer JD, Willett JB. Applied longitudinal data analysis: modelling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 50.Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- 52.George LK, Gwyther LP. Caregiver well-being: a multidimensional examination of family caregivers of demented adults. Gerontologist. 1986;26:253–259. doi: 10.1093/geront/26.3.253. [DOI] [PubMed] [Google Scholar]
- 53.Kaufer DI, Borson S, Kershaw P, Sadik K. Reduction of caregiver burden in Alzheimer's disease by treatment with galantamine. CNS Spectr. 2005;10:481–488. doi: 10.1017/s1092852900023178. [DOI] [PubMed] [Google Scholar]
- 54.Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J Clin Oncol. 2009;27:2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- 55.Lee S, Colditz GA, Berkman LF, Kawachi I. Caregiving and risk of coronary heart disease in U.S women: a prospective study. Am J Prev Med. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 56.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 57.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 58.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 59.Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, Ahern TJ, Buurman WA. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992;77:543–549. [PMC free article] [PubMed] [Google Scholar]
- 60.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 61.Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw. 2004;15:91–98. [PubMed] [Google Scholar]
- 62.Rehman J, Mills PJ, Carter SM, Chou J, Thomas J, Maisel AS. Dynamic exercise leads to an increase in circulating ICAM-1: further evidence for adrenergic modulation of cell adhesion. Brain Behav Immun. 1997;11:343–551. doi: 10.1006/brbi.1997.0498. [DOI] [PubMed] [Google Scholar]
- 63.Lyngdoh T, Vollenweider P, Waeber G, Marques-Vidal P. Association of statins with inflammatory cytokines: A population-based CoLaus study. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2011.07.117. (in press) [DOI] [PubMed] [Google Scholar]
- 64.Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- 65.Schulz R, Beach SR, Lind B, Martire LM, Zdaniuk B, Hirsch C, Jackson S, Burton L. Involvement in caregiving and adjustment to death of a spouse: findings from the caregiver health effects study. JAMA. 2001;285:3123–3129. doi: 10.1001/jama.285.24.3123. [DOI] [PubMed] [Google Scholar]
- 66.Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long-term care placement of dementia patients and caregiver health and well-being. JAMA. 2004;292:961–967. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 67.Hänsel A, Hong S, Cámara RJ, von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]