Abstract
We measured glutamine kinetics using L-[5-15N]glutamine and L-[ring-2H5]phenylalanine infusions in healthy subjects in the postabsorptive state and during ingestion of an amino acid mixture that included glutamine, alone or with additional glucose. Ingestion of the amino acid mixture increased arterial glutamine concentrations by ~20% (not by 30%; P < 0.05), irrespective of the presence or absence of glucose. Muscle free glutamine concentrations remained unchanged during ingestion of amino acids alone but decreased from 21.0 ± 1.0 to 16.4 ± 1.6 mmol/l (P < 0.05) during simultaneous ingestion of glucose due to a decrease in intramuscular release from protein breakdown and glutamine synthesis (0.82 ± 0.10 vs. 0.59 ± 0.06 μmol·100 ml leg−1 ·min−1; P < 0.05). In both protocols, muscle glutamine inward and outward transport and muscle glutamine utilization for protein synthesis increased during amino acid ingestion; leg glutamine net balance remained unchanged. In summary, ingestion of an amino acid mixture that includes glutamine increases glutamine availability and uptake by skeletal muscle in healthy subjects without causing an increase in the intramuscular free glutamine pool. Simultaneous ingestion of glucose diminishes the intramuscular glutamine concentration despite increased glutamine availability in the blood due to decreased glutamine production.
Keywords: transport, synthesis, stable isotopes, protein, amino acid, glucose
There is evidence from studies in vitro that intramuscular glutamine availability is involved in the regulation of muscle protein synthesis (28, 47) and breakdown (29, 47). The factors controlling muscle glutamine concentration (e.g., transmembrane transport and intracellular synthesis) have been extensively studied in vitro and in animals (36, 37). However, few quantitative studies have been performed in human subjects. In particular, the response of muscle glutamine metabolism to increased glutamine availability and the interaction of ingested glutamine with other nutrients are not known.
Muscle glutamine uptake is mediated primarily by transport system Nm (36, 37), which, in contrast to most of the amino acid transport systems, is insulin sensitive (14, 36). In perfused rat hindlimb models, it has been shown that glutamine availability to the muscle is an important regulator of muscle glutamine uptake and concentration (20). The addition of insulin to the perfusate increased the accumulation of intramuscular glutamine (20), and the uptake of glutamine into perfused hindlimbs of diabetic rats was diminished (21).
Some studies in trauma patients have shown improved plasma and muscle glutamine concentration after glutamine supplementation (e.g., Refs. 16 and 32), suggesting a stimulation of muscle glutamine uptake by increased glutamine availability in vivo. Others, however, have failed to demonstrate improvement or restoration of normal plasma (6, 8, 22, 26, 27, 33) and muscle (33) glutamine concentrations with glutamine supplementation. To our knowledge, no data on the effects of increased glutamine availability on muscle glutamine concentration are available in healthy people, and the direct effects of increased glutamine availability and insulin on muscle glutamine uptake/transport in human subjects have not been assessed.
The difference in the effects of glutamine supplementation on muscle glutamine concentration in patients may be due to different routes of glutamine administration (i.e., intravenous vs. enteral) and whether glutamine was given alone or in combination with other nutrients. Studies in vitro (43) and in vivo (17, 31) have shown that splanchnic tissues are able to extract a large percentage of the administered glutamine. Thus the availability of enterally administered glutamine to the muscle is likely to be less than if the same amount of glutamine is provided parenterally. Furthermore, muscle free glutamine concentration may be controlled by factors other than dietary glutamine availability (e.g., branched-chain amino acids and glucose) via effects on muscle glutamine metabolism.
Branched-chain amino acids are the predominant precursors for glutamine synthesis, and muscle glutamine release is stimulated during administration of branched-chain amino acids (1). However, it has been shown that, after protein (2) and mixed-meal ingestion (9), net glutamine release from the forearm is unchanged despite the transfer and uptake of large amounts of branched-chain amino acids by muscle (2). The underlying mechanisms for this are not known, but it may be related to increased intramuscular utilization of glutamine for protein synthesis and limited precursor (i.e., α-ketoglutarate/glutamate) availability for glutamine synthesis, which is likely related to the predominant fate of pyruvate in muscle during meal ingestion. An increased rate of glycolysis after glucose ingestion leads to an increase in pyruvate production (45). As a result, pyruvate is transaminated to alanine (45), which potentially limits the availability of the precursors glutamate and α-ketoglutarate.
To simultaneously determine the availability of dietary glutamine for skeletal muscle and its effects on muscle glutamine concentration in healthy subjects, we measured whole body and skeletal muscle glutamine kinetics in the postabsorptive state and during the ingestion of an amino acid mixture containing glutamine and glutamine plus glucose in healthy subjects. We hypothesized that increased dietary glutamine availability increases arterial glutamine concentration and stimulates glutamine uptake by skeletal muscle. Furthermore, we hypothesized that the addition of glucose to the amino acid mixture would increase the stimulation of muscle glutamine uptake to a greater extent than amino acids alone by increasing plasma insulin concentration. However, we also hypothesized that muscle glutamine synthesis would be decreased by the addition of glucose to the amino acid mixture due to accelerated pyruvate and alanine production, which limits glutamate availability for glutamine synthesis.
METHODS
Subjects
Eleven subjects [4 women, 7 men; age: 30 ± 2 yr; bodyweight: 69 ± 2 kg; body mass index (BMI): 25 ± 1 kg/m2] participated in this study. They were all healthy, as indicated by comprehensive history, physical examination, and standard blood and urine tests; all volunteers had normal oral glucose tolerance. The study was approved by the Institutional Review Board and the General Clinical Research Center of the University of Texas Medical Branch at Galveston. Informed consent was obtained from all subjects before enrollment in the study.
Experimental Design
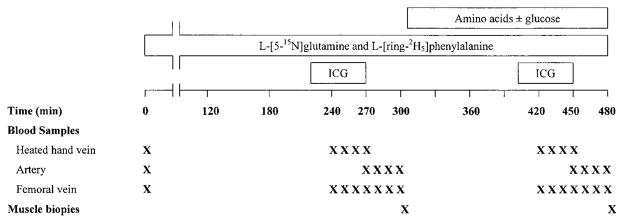
All experiments were performed in the morning, after the volunteers had fasted overnight (i.e., ~12 h). The time course of the experimental protocol is depicted in Fig. 1. Briefly, Teflon catheters were placed percutaneously into an antecubital vein for isotope infusion and into a contralateral dorsal hand vein, which was heated, for blood sampling. Two more catheters for blood sampling were placed in a femoral artery and vein. All catheters were kept patent by controlled infusion of 0.9% NaCl. The femoral arterial catheter was also used for the infusion of indocyanine green (ICG) dye for the determination of leg blood flow (23, 24). After the placement of all catheters and the collection of a background blood sample, primed continuous infusions of L-[5-15N]glutamine (0.25 μmol·kg−1 ·min−1; prime: 45 μmol/kg) and L-[ring-2 H5]phenylalanine (0.05 μmol·kg−1 ·min−1; prime: 2 μmol/kg) were started and maintained for 480 min. During the last 3 h (300–480 min), six subjects ingested a liquid amino acid mixture; the mixture was given in small aliquots (30 ml every 10 min) to maintain plasma amino acid concentrations at steady state. The total amount of amino acids in the mixture was 40 g (i.e., ~0.55 g/kg body wt); the proportional contribution of each amino acid in the mixture was similar to the composition of muscle protein (35). The glutamine content in the amino acid mixture was 5.8 g (i.e., ~2.8 μmol· kg−1·min−1). [1, 2-13C2]glutamine (6.8 mmol) and [ring-13C6]-phenylalanine (0.42 mmol) were added to the amino acid mixture for the determination of first-pass splanchnic extraction and the calculation of the endogenous rate of appearance (Ra) of glutamine during the administration of the amino acid mixture. The other five subjects ingested the same amino acid mixture plus 40 g of glucose. Calibrated syringe pumps (Harvard Apparatus, South Natick, MA) were used for infusions. For each infusion study, aliquots of the amino acid mixture and the isotope infusate were analyzed for the exact enrichment and concentration of tracers. All tracers were purchased from Cambridge Isotope Laboratories (Andover, MA).
Fig. 1.
Experimental design. ICG, indocyanine green dye. The amino acid mixture, which is described in the text, was dissolved in 540 ml Crystal Light and contained [ring-13C6]phenylalanine and [1,2-13C2]glutamine as described in the text.
Femoral arterial and venous blood samples (3 ml) were obtained at 120, 270, 280, 290, and 300 min (basal period) and 450, 460, 470, and 480 min (amino acid period) after the start of the tracer infusion for the determination of plasma amino acid concentration and plasma amino acid enrichment. Blood samples were collected in prechilled tubes containing EDTA as anticoagulant. Plasma was separated immediately after the completion of the study and was stored at −80°C until analysis. Muscle biopsies were taken from the lateral portion of the vastus lateralis of the catheterized leg, ~20 cm above the knee, using a 4-mm Bergström biopsy needle (Depuy, Warsaw, IN) at 300 min (basal period) and 480 min (amino acid period). The biopsies were taken from the same leg; the biopsy at 480 min was obtained through the same incision as the biopsy at 300 min. However, the biopsy needle was directed at the opposite side (i.e., at a 180° angle) of the biopsy at 300 min. With this technique, the biopsy sites are likely to be 3–5 in. apart. Therefore, changes in muscle amino acid kinetics are likely consistent. We have tested this procedure in a controlled way in our laboratory and found the same results as when separate legs were used (unpublished observations). The tissue was immediately frozen in liquid nitrogen and stored at −80°C until analysis.
To measure leg blood flow, a continuous infusion of ICG dye (0.5 mg/ml; 1 ml/min) was started in the femoral artery at 220 min and was maintained until 270 min. Between 240 and 270 min, four blood samples were taken every 10 min from the femoral vein and the heated hand vein to measure serum ICG concentration. For the determination of leg blood flow during the second study period (300–480 min), the ICG dye infusion and blood sampling protocol described above were repeated between 400 and 450 min.
Sample Analysis
Plasma and muscle samples were prepared as previously described (42, 44) to determine the enrichment and concentration of free glutamine and phenylalanine in plasma and muscle intracellular free water. Briefly, 50 μl of internal standard containing a known amount of [13C1]phenylalanine and [2H5]glutamine were added to 250 μl of the arterial and venous plasma samples. The plasma and internal standard mixture was acidified and passed through strong cation-exchange columns (Bio-Rad, Hercules, CA) and was eluted with NH4OH (2 N). The eluent was concentrated in a Speed-Vac (Savant Instruments, Farmingdale, NY) overnight, and 100 μl N-methyl-N-(tert-butyldimethylsilyl)trifluoro-acetamide and acetonitrile (1:1) were added to prepare the t-butyldimethylsilyl derivatives. After being heated for 1 h at 90°C, the samples were transferred to small sealed autosampler vials, and the enrichments of the samples and the internal standard solution were determined by gas chromatography-mass spectrometry (GC-MS; Hewlett Packard 5989).
Each muscle sample was weighed, internal standard solution was added (20 μl/mg tissue), and muscle protein was precipitated with 450 μl of 10% sulfosalicylic acid. The tissue was then homogenized and centrifuged, and the supernatant was collected. The remaining tissue was homogenized two more times with 450 μl of sulfosalicylic acid, and the supernatants were collected. The pooled supernatants (~1.2 ml) were processed further as described for plasma samples. The pellet was washed three times with absolute ethanol and dried at 50°C. Total water content in muscle was obtained from the difference between weights of wet and dried muscle samples. The intracellular water content was calculated on the basis of total muscle water content and previously published values for cellular hydration of skeletal muscle in healthy subjects (18).
Calculations
Amino acid kinetics
We used the average values of plasma glutamine and phenylalanine enrichments (tracer-to-tracee ratio) and concentrations obtained at 270–300 min and the glutamine and phenylalanine enrichments and concentrations in muscle intracellular free water obtained at 300 min for the calculation of glutamine and phenylalanine kinetics in the basal period. For the second study period (amino acid period), we used the average values of plasma glutamine and phenylalanine enrichments and concentrations obtained at 450–480 min and the glutamine and phenylalanine enrichments and concentrations in muscle intracellular free water obtained at 480 min.
Concentrations (C) of free amino acids in plasma and total muscle water were calculated as C = QIS/(V · EIS), where QIS is the amount of internal standard added to the sample, V is the volume of plasma or muscle water, and EIS is the internal standard tracer-to-tracee ratio in plasma or muscle water as measured by GC-MS. The intracellular water content was calculated based on total muscle water content and previously published values for cellular hydration of skeletal muscle in healthy subjects (18). The measured glutamine concentration and enrichment in total muscle water were corrected using these values to obtain the glutamine concentration and enrichment in intracellular free water.
The whole body Ra of glutamine and phenylalanine was calculated by dividing the tracer infusion rate (F) by the arterial enrichment (EA) of glutamine and phenylalanine, respectively. Hence
| (1) |
The endogenous Ra of glutamine and phenylalanine during the ingestion of the amino acid mixture was calculated by subtracting the amounts of glutamine and phenylalanine that are ingested and not taken up by splanchnic tissues (i.e., splanchnic escape; Eq. 2) from the total Ra calculated with the intravenous tracer (Eq. 1)
| (2) |
In this equation, splanchnic extraction is calculated as
| (3) |
where F is the rate of tracer delivery and E is the arterial enrichment of glutamine and phenylalanine in arterial plasma. Subscripts indicate the route of tracer administration (i.e., intravenous vs. oral) and the enrichments of these tracers in femoral arterial plasma.
Skeletal muscle glutamine kinetics were calculated using the three-pool model (Fig. 2), which has previously been developed in our laboratory (3). According to this model, the rate of delivery of glutamine to the leg via the femoral artery (Fin), the rate of glutamine inward transport from arterial plasma into the muscle intracellular free glutamine (FM,A), the rate of glutamine outward transport from the muscle intracellular free glutamine pool into venous plasma (FV,M), the rate of intracellular glutamine release (FM,O), which is the sum of glutamine release from muscle protein breakdown and intracellular glutamine synthesis, the rate of intracellular glutamine utilization (FO,M), and the leg net balance (NB) of glutamine were calculated as
Fig. 2.
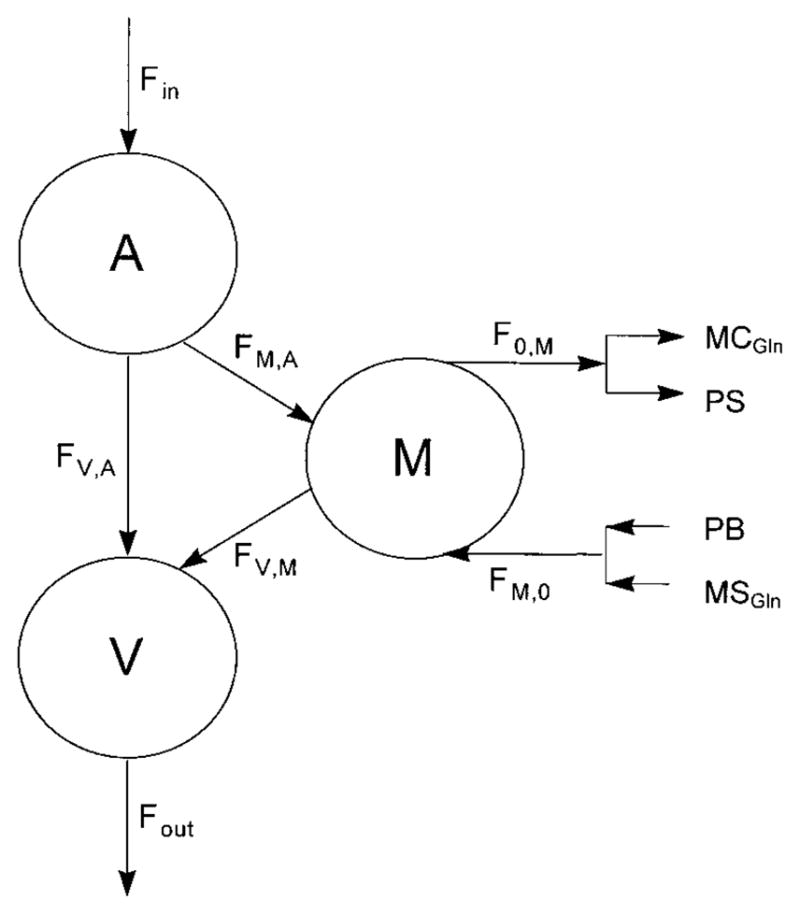
Three-pool model for the calculation of muscle glutamine kinetics. A, V, and M, glutamine pool in arterial and venous blood and muscle intracellular free water, respectively; Fin, rate of delivery of glutamine to the leg via the femoral artery; Fout, rate of delivery of glutamine from the leg via the femoral vein; FM,A, rate of glutamine inward transport from arterial plasma into the muscle intracellular free glutamine pool; FV,M, rate of glutamine outward transport from the muscle intracellular free glutamine pool into venous blood; FV,A, rate of arteriovenous shunting of glutamine; FM,O, rate of intracellular glutamine release, which is the sum of glutamine release from muscle protein breakdown (PB) and intracellular glutamine synthesis (MSGln); FO,M, rate of intracellular glutamine utilization, which is the sum of glutamine incorporation into muscle protein (PS) and intracellular glutamine catabolism (MCGln).
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
where E is the enrichment and C the concentration of glutamine; subscripts A, V, and M indicate enrichment and concentration in the artery, vein, and muscle intracellular free water, respectively; and BF is leg blood flow. Similarly, skeletal muscle phenylalanine kinetics were calculated using these equations.
Because glutamine is a nonessential amino acid, both glutamine release from protein breakdown and glutamine synthesis contribute to whole body glutamine Ra (Eq. 1) and intramuscular glutamine release (Eq. 7). Thus the whole body Ra of glutamine that comes from glutamine synthesis (SGln, Eq. 10) and the rate of muscle glutamine synthesis (MSGln, Eq. 11) were calculated by subtracting the rate of release of glutamine from protein breakdown from the whole body endogenous glutamine Ra and muscle glutamine production (FM,O), respectively. Whole body and muscle protein breakdown was estimated using whole body phenylalanine Ra (Ra Phe) and the rate of intramuscular phenylalanine release. Consequently, the rates of whole body and skeletal muscle glutamine synthesis were calculated as
| (10) |
| (11) |
where X indicates the proportional abundance of phenylalanine and glutamine in whole body/skeletal muscle protein, Ra Gln is whole body glutamine Ra, and FM,O Gln is the rate of intramuscular glutamine release. In the present studies, whole body glutamine release from protein breakdown was estimated as 0.83 × Ra of phenylalanine (31). For the calculation of intramuscular glutamine synthesis, we assumed a phenylalanine and glutamine content of 233 μmol and 460 μmol/g muscle protein, respectively, and a phenylalanine-to-glutamine ratio of 1:2 (4, 25). Consequently, the intramuscular glutamine rate of release from muscle protein breakdown was calculated as the intracellular rate of release (FM,O) of phenylalanine times two. This value was subtracted from the intracellular rate of release (FM,O) of glutamine to give an estimate of the rate of intracellular glutamine synthesis (MSGln).
The validity of the three-pool model for the calculation of glutamine kinetics has been questioned because it may take a long time for the intracellular glutamine pool to come to an isotopic equilibrium (40). This argument, however, was based on data from a study in which an inadequate glutamine priming dose was used such that the plasma glutamine enrichment was not at plateau (40). Therefore, extrapolations to our steady-state model are irrelevant. Furthermore, we have previously shown that, during continuous infusion of L-[5-15N]glutamine in healthy subjects, the muscle free glutamine enrichment is constant for the time period of our experiment (4).
To obtain measures for leg glutamine kinetics that are independent of the intracellular glutamine enrichment, we calculated leg glutamine uptake (Eq. 12) and release (Eq. 13) as
| (12) |
| (13) |
Both calculations rely only on plasma glutamine concentration and enrichment, for which an isotopic steady state can be achieved quickly (4, 7, 30, 31, 42).
Leg volume and blood flow
Leg volume was determined as described previously (23, 24) using a metric tape. Briefly, the leg was divided into six segments from the groin to the ankle. The volume of each segment was calculated on the basis of the height and the upper and lower circumference of the segment. The volume of the foot was calculated on the basis of its length and the height of the heel. Leg plasma flow was determined by dividing the infusion rate of ICG by the concentration difference of ICG in femoral venous and peripheral venous serum. The peripheral venous concentration of ICG was subtracted from the concentration in the femoral vein to account for recycling of the dye into the artery. The concentration of ICG in the infusate and in serum samples was determined using a spectrophotometer set at λ = 805 nm. With this method, we have found that the SE of the mean value for blood flow is generally within ±3% of the mean values.
Statistical Analysis
All data are expressed as means ± SE. ANOVA was used for testing the effects of different treatments. Significance was set at P < 0.05.
Choice of Tracer
The estimated values of glutamine first-pass splanchnic extraction and glutamine Ra could potentially be influenced by the choice of intravenous and oral tracers for the experiment. When L-[5-15N]glutamine is chosen as the oral tracer, it is possible, for instance, that labeled glutamine nitrogen could be lost during splanchnic passage via nitrogen exchange, thereby resulting in a loss of labeled glutamine that would not correspond to net uptake. This type of isotopic exchange would lead to an overestimation of glutamine splanchnic extraction. On the other hand, with carbon-labeled glutamine, such nitrogen exchange should not affect the calculated first-pass splanchnic extraction.
To validate the choice of tracer for the determination of first-pass splanchnic extraction and the endogenous Ra during ingestion of the amino acid mixture in our experiments, we performed two studies in a female volunteer (age 26 yr, body weight 78 kg, BMI 25 kg/m2) on two occasions, two days apart. The study design was similar to the second part (300–480 min) of the experimental design outlined in Fig. 1. On the first day, the L-[5-15N]glutamine tracer was infused, and the [1,2-13C2]glutamine tracer was given orally. On the second day, the tracers were alternated; the [13C]glutamine tracer was given intravenously, and the [15N]glutamine tracer was given orally.
The calculated first-pass splanchnic extraction of the glutamine tracer was not different (70 vs. 69%) when the 15N or the 13C tracer was added to the amino acid mixture and the 13C or the 15N tracer was administered intravenously. These results are confirmed by studies (15, 17) in which different glutamine tracers were extracted to the same extent by splanchnic tissues (15). Therefore, the glutamine tracers selected for our experiments are appropriate.
RESULTS
Plasma Glutamine and Phenylalanine Concentrations and Enrichments
During the last 30 min of the basal period (270–300 min) and during the ingestion of the amino acid mixture (450–480 min), plasma glutamine and phenylalanine concentrations and L-[5-15N]- and [13C2]glutamine and L-[ring-2H5]- and [ring-13C6]phenylalanine enrichments were at steady state. Ingestion of the amino acid mixture with glutamine alone and with additional glucose decreased the enrichment of both [15N]glutamine and [ring-2H5]phenylalanine in femoral arterial and venous plasma (Table 1). The arterial and venous plasma glutamine and phenylalanine concentrations increased during the ingestion of the amino acid alone and with additional glucose (Table 2).
Table 1.
Plasma and muscle intracellular free glutamine and phenylalanine enrichment during the last 30 min in the basal period and during ingestion of the amino acid mixture that included glutamine alone (amino acids) and with additional glucose (amino acids plus glucose)
| Basal | Amino Acids | Basal | Amino Acids Plus Glucose | |
|---|---|---|---|---|
| [15N1]glutamine | ||||
| Femoral artery | 0.066±0.005 | 0.047±0.003* | 0.072±0.006 | 0.056±0.004* |
| Femoral vein | 0.052±0.003 | 0.037±0.003* | 0.054±0.005 | 0.045±0.004* |
| Muscle | 0.016±0.002 | 0.021±0.002* | 0.020±0.005 | 0.033±0.003* |
| Muscle-to-artery ratio | 0.24±0.04 | 0.45±0.06* | 0.28±0.07 | 0.59±0.04* |
| [13C2]glutamine | ||||
| Femoral artery | 0.032±0.003 | 0.026±0.003 | ||
| [ring-2H5]phenylalanine | ||||
| Femoral artery | 0.069±0.006 | 0.039±0.003* | 0.078±0.003 | 0.047±0.003* |
| Femoral vein | 0.054±0.003 | 0.034±0.003* | 0.059±0.004 | 0.043±0.003* |
| Muscle | 0.039±0.006 | 0.030±0.003* | 0.037±0.005 | 0.037±0.004 |
| [ring-13C6]phenylalanine | ||||
| Femoral artery | 0.047±0.005 | 0.058±0.002 | ||
Data are expressed as means ± SE.
Significantly different from basal (P < 0.05).
Table 2.
Plasma and muscle intracellular free glutamine and phenylalanine concentrations in the basal period and during ingestion of the amino acid mixture that included glutamine alone (amino acids) and with additional glucose (amino acids plus glucose)
| Basal | Amino Acids | Basal | Amino Acids Plus Glucose | |
|---|---|---|---|---|
| Glutamine | ||||
| Artery, μmol/l | 595±59 | 729±61* | 596±60 | 684±65* |
| Femoral vein, μmol/l | 670±50 | 772±50* | 720±60 | 813±82* |
| Muscle, mmol/l | 21.5±2.8 | 22.8±1.5 | 21.0±1.0 | 16.4±1.6* |
| Phenylalanine | ||||
| Artery, μmol/l | 57±3 | 117±11* | 60±2 | 133±18* |
| Femoral vein, μmol/l | 63±4 | 112±11* | 69±2 | 118±9* |
| Muscle, μmol/l | 124±18 | 199±25* | 130±12 | 185±36* |
Data are expressed as means ± SE.
Significantly different from basal (P < 0.05).
Plasma Glucose and Insulin Concentrations
Plasma glucose and insulin concentrations during the ingestion of the amino acid mixture with glutamine alone were similar to their basal concentrations (Table 3); both increased significantly when glucose was added to the amino acid mixture (Table 3).
Table 3.
Plasma glucose and insulin concentrations in the basal period and during ingestion of the amino acid mixture that included glutamine alone (amino acids) and with additional glucose (amino acids plus glucose)
| Basal | Amino Acids | Basal | Amino Acids Plus Glucose | |
|---|---|---|---|---|
| Glucose, mmol/l | 5.4±0.5 | 5.8±0.6 | 5.5±0.6 | 6.9±0.5* |
| Insulin, μU/ml | 5.7±1.0 | 9.4±2.1 | 6.3±0.7 | 33.7±4.2* |
Data are expressed as means ± SE.
Significantly different from basal (P < 0.05).
Muscle Glutamine and Phenylalanine Concentrations and Enrichments
The muscle intracellular free glutamine concentration remained unchanged from basal during ingestion of the amino acid mixture alone but decreased during ingestion of amino acids plus glucose (Table 2). In contrast, the muscle intracellular free phenylalanine concentration increased during ingestion of both amino acid mixtures (Table 2). The [15N]glutamine enrichment in intracellular free water increased in all subjects by ~30% during ingestion of amino acids alone to 60% during ingestion of amino acids plus glucose (Table 1). The [ring-2H5]phenylalanine enrichment decreased during ingestion of amino acids alone but remained unchanged during ingestion of amino acids plus glucose (Table 1).
Glutamine First-Pass Splanchnic Extraction and Whole Body Glutamine Kinetics
Most of the ingested glutamine was extracted in the first pass by splanchnic tissues (Table 4); the extraction was higher during ingestion of amino acids plus glucose than during ingestion of amino acids alone (Table 4). The endogenous Ra of glutamine was unaffected by the ingestion of the amino acid mixture, either with or without glucose (Table 4). Similarly, whole body protein breakdown and thus the Ra of glutamine from protein breakdown during ingestion of the amino acid mixture with and without glucose were not different from the basal values (Table 4). The Ra of glutamine coming from glutamine synthesis and its contribution to whole body endogenous glutamine Ra were also not affected by the ingestion of amino acids alone and amino acids plus glucose (Table 4).
Table 4.
Glutamine first-pass splanchnic extraction and whole body glutamine kinetics in the basal period and during ingestion of the amino acid mixture that included glutamine alone (amino acids) and with additional glucose (amino acids plus glucose)
| Basal | Amino Acids | Basal | Amino Acids Plus Glucose | |
|---|---|---|---|---|
| First-pass splanchnic extraction, % | 66±4 | 76±2* | ||
| Endogenous Ra, μmol·kg−1 ·min−1 | 4.1±0.3 | 4.6±0.3 | 3.9±0.3 | 4.3±0.3 |
| Protein breakdown, μmol·kg−1 ·min−1 | 0.7±0.1 | 0.5±0.1 | 0.7±0.2 | 0.7±0.3 |
| Synthesis, μmol·kg−1 ·min−1 | 3.4±0.3 | 4.1±0.6 | 3.2±0.3 | 3.6±0.4 |
| Synthesis/endogenous Ra, % | 82±2.0 | 84±1.0 | 83±1.0 | 83±2.0 |
Data are expressed as means ± SE. Ra, rate of appearance. Protein breakdown, Ra of glutamine from whole body protein breakdown; Synthesis, Ra of glutamine from glutamine synthesis.
Significantly different from protocol 1 (P < 0.05).
Leg Blood Flow
Leg blood flow during the ingestion of the amino acid mixture alone (3.2 ± 0.4 ml·100 ml leg−1 ·min−1) was similar to basal leg blood flow (3.2 ± 0.5 ml·100 ml leg−1 ·min−1). Ingestion of amino acids plus glucose increased leg blood flow significantly (4.3 ± 0.3 vs. 3.4 ± 0.2 ml·100 ml leg−1 ·min−1, basal; P < 0.05).
Glutamine Delivery To and Glutamine and Phenylalanine Net Balance Across the Leg
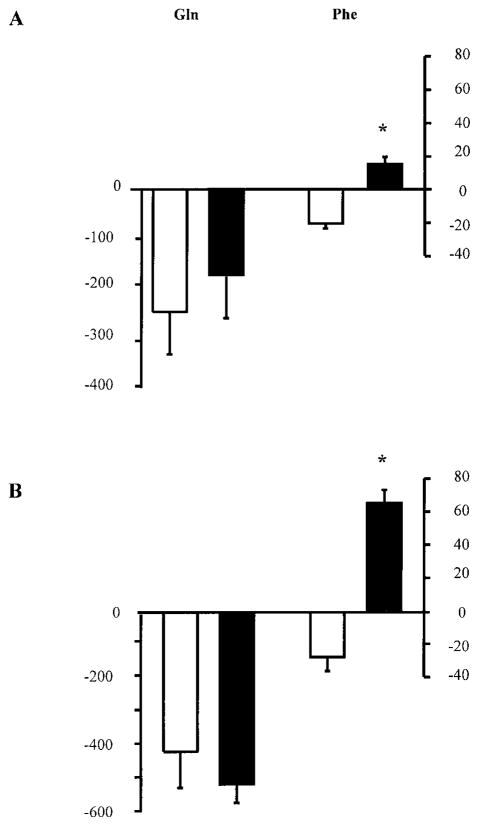
In all subjects, the rate of glutamine delivery to the leg (Fin), increased both during the ingestion of amino acids alone (2.6 ± 0.3 vs. 1.8 ± 0.3 μmol·100 ml leg−1 ·min−1, basal; P < 0.05) and during ingestion of amino acids plus glucose (2.9 ± 0.3 vs. 1.9 ± 0.1 μmol·100 ml leg−1 ·min−1, basal; P < 0.05). Leg glutamine net balance across the leg was not affected by the ingestion of amino acids alone or amino acids plus glucose (Fig. 3). In contrast, phenylalanine net balance changed from net release in the basal state to net uptake during ingestion of the amino acids, both with and without glucose (Fig. 3).
Fig. 3.
Glutamine and phenylalanine net balance across the leg (nmol·100 ml−1 ·min−1) in the basal state (open bars) and during ingestion of the amino acid mixture, which contains glutamine, alone (filled bars, A) and with additional glucose (filled bars, B). Data are expressed as means ± SE. *Significantly different from basal (P < 0.05).
Muscle Glutamine Kinetics
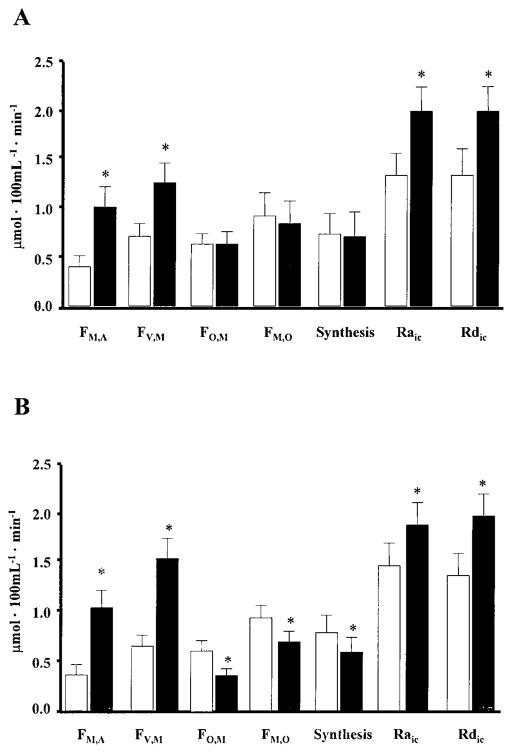
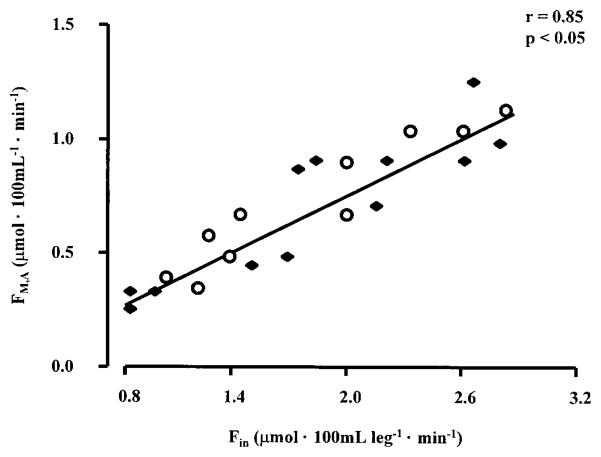
The rate of muscle glutamine inward (FM,A) and outward (FV,M) transport approximately doubled during ingestion of amino acids alone and with glucose (Fig. 4). Similarly, leg glutamine uptake and release increased by ~120 and 60% during ingestion of amino acids alone and amino acids plus glucose, respectively (P < 0.05 vs. basal). The inward transport rate (FM,A) was positively correlated with the rate of delivery (Fin) of glutamine (Fig. 5).
Fig. 4.
Effect of ingestion of an amino acid mixture that contains glutamine, alone (A) and with additional glucose (B), on muscle glutamine kinetics. Synthesis, intracellular glutamine synthesis; Raic, total rate of appearance of glutamine in the intracellular free glutamine pool (i.e., the sum of inward transport, release from muscle protein via proteolysis, and glutamine synthesis); Rdic, total loss of intramuscular glutamine (i.e., outward transport, incorporation into muscle protein, and glutamine catabolism). Data are expressed as means ± SE. *Significantly different from basal (P < 0.05).
Fig. 5.
Relationship between the rate of delivery of glutamine to the leg via the femoral artery (Fin) and inward glutamine transport from the femoral artery into the muscle intracellular free glutamine pool (FM,A). ●, Data from subjects that were studied in the basal state and during ingestion of amino acids alone; ○, data from subjects that were studied in the basal state and during ingestion of amino acids plus glucose.
Total muscle glutamine utilization (FO,M) remained unchanged during ingestion of amino acids alone but decreased during ingestion of amino acids plus glucose (Fig. 4). However, muscle protein synthesis, and thus the utilization of glutamine for protein synthesis, increased both during ingestion of amino acids alone (154 ± 11 vs. 94 ± 8 nmol·100 ml leg−1 ·min−1, basal) and during ingestion of amino acids plus glucose (278 ± 70 vs. 121 ± 34 nmol·100 ml leg−1 ·min−1, basal).
Intramuscular glutamine release (FM,O) and muscle glutamine synthesis were not affected by the ingestion of amino acids alone (Fig. 4). However, both decreased during ingestion of amino acids plus glucose (Fig. 4). The release of glutamine from muscle protein breakdown remained unchanged during ingestion of amino acids alone (108 ± 22 vs. 122 ± 38 nmol·100 ml leg−1 ·min−1, basal) and during ingestion of amino acids plus glucose (174 ± 48 vs. 186 ± 34 nmol·100 ml leg−1 ·min−1, basal).
Both the total Ra of glutamine in the intracellular free glutamine pool (i.e., the sum of inward transport, release from muscle protein and glutamine synthesis) and the total loss of intramuscular glutamine (i.e., outward transport, incorporation into muscle protein and glutamine catabolism) increased significantly during ingestion of the amino acid mixture with and without glucose (Fig. 4). The contribution of inward transport to total glutamine appearance was 25 ± 3% in the basal period and 43 ± 5% during ingestion of the amino acids alone. During ingestion of amino acids plus glucose, the contribution of inward transport increased to a similar extent from 28 ± 7% in the basal period to 59 ± 4%.
DISCUSSION
We found that, during ingestion of an amino acid mixture that included glutamine, a large fraction of the ingested glutamine (66 ± 4%, amino acids alone; 76% ± 2 amino acids plus glucose) from the amino acid mixture was extracted by splanchnic tissues. The arterial glutamine concentration, however, increased during ingestion of the amino acid mixture with and without additional glucose despite the high extraction of glutamine by splanchnic tissues. Nonetheless, the muscle intracellular free glutamine concentration did not increase during ingestion of amino acids alone and decreased during ingestion of amino acids plus glucose. The decrease in muscle intracellular free glutamine concentration when glucose was added to the amino acid mixture was a consequence of a suppression of glutamine synthesis in the muscle.
The capability of splanchnic tissues to extract large doses of glutamine is well known from studies in vitro (43) and in vivo (17, 31). In people, it has been found that the first-pass splanchnic glutamine extraction was higher (~75%) when small amounts of glutamine (~1.5 g/h) were given compared with the first-pass splanchnic extraction (~55%) when large doses of glutamine (~9 g/h) were given (17). The splanchnic extraction of glutamine from a mixed amino acid solution has not been quantified previously. The observed high splanchnic glutamine extraction from a mixed meal lends support to findings from previous studies that failed to observe an increase in plasma glutamine concentrations in patients after supplementation of enteral diets with glutamine (8, 22, 27, 33). However, in the present studies, we observed a significant increase in arterial glutamine concentration in healthy volunteers despite the high first-pass extraction of glutamine by splanchnic tissues. Hence, enterally administered glutamine, when given in sufficient amounts, increases glutamine availability for skeletal muscle in healthy subjects.
When glutamine alone (i.e., without glucose) was given in the form of an amino acid mixture, glutamine uptake by skeletal muscle increased significantly. The significant and positive correlation of the rate of glutamine delivery to the leg via the femoral artery (Fin) and the rate of inward transport of glutamine from arterial blood into the intramuscular free glutamine pool (FM,A) indicate a strong relationship between glutamine transport into the muscle cells and arterial glutamine availability. This stimulation of transport across muscle cells during abundant substrate supply is similar to the effect seen with other amino acids during enhanced availability (5, 41). Furthermore, studies with perfused rat hindlimbs have shown that the availability of glutamine is essential for glutamine transport to operate at its maximal capacity (37, 36, 39). However, unlike the situation with other amino acids (5, 41), we did not observe an increase in intramuscular glutamine concentration during the ingestion of the amino acid mixture. This lack of increase in the muscle free glutamine pool can be attributed to the fact that the utilization of glutamine for protein synthesis and outward transport (FV,M) increased simultaneously. Hence, there may be an upper limit for intramuscular glutamine accumulation in humans. This notion is supported by findings from rat hindlimb perfusion studies in which intramuscular glutamine concentration increased with increasing perfusate glutamine concentration from 0 to 2.5 mM (28). However, no further increase was observed with a perfusate glutamine concentration of 5 mM (28). Furthermore, it has been shown that the intracellular glutamine concentration rises in a curvilinear fashion with time of incubation in a medium containing glutamine, reaching a plateau; prolongation of incubation did not result in a further increase in intracellular glutamine concentrations (10). Also, glutamine infusion had no effect on the muscle free glutamine concentration in healthy rats (19), whereas it increased when glutamine was given to rats in which the muscle free glutamine pool was depleted (46); this was probably due to a “glutamine depletion-stimulated” increase in glutamine synthetase activity and thus glutamine synthesis, rather than increased inward transport (39). We conclude that elevated plasma glutamine concentrations and thus increased glutamine availability to the muscle stimulate glutamine uptake by skeletal muscle in healthy volunteers. However, the simultaneous increase in glutamine release from the muscle and accelerated intracellular glutamine utilization prevent changes in the intramuscular free glutamine pool.
Skeletal muscle glutamine uptake was also stimulated by the amino acid-glucose mixture, but the observed increase in uptake (~2-fold) was no greater than the response observed during ingestion of amino acids alone. This is in contrast to findings in vitro in which addition of insulin to rat hindlimb perfusate augmented, although not markedly, the effect of increased glutamine availability on skeletal muscle glutamine uptake and concentration (20), whereas glutamine uptake in muscles from diabetic rats was inhibited, presumably via insulin resistance (21). In the hindlimb studies (20), glutamine was the only amino acid in the muscle perfusate. Therefore, it is possible that in our studies, competitive inhibition of glutamine transporters by other amino acids prevented a similar stimulation of glutamine uptake by insulin. Furthermore, the increase in plasma insulin in response to glucose ingestion may have been insufficient to stimulate muscle glutamine uptake in our studies.
On the basis of our finding that muscle glutamine turnover was stimulated during ingestion of the amino acid mixture, one might expect whole body glutamine Ra to increase if muscle is the major source of whole body glutamine Ra. However, given a leg muscle mass of ~5 liters and the assumption that it represents ~20% of total skeletal muscle mass, we found that the rate of release of glutamine from muscle represents only ~50% of whole body glutamine Ra into plasma in the basal state. This is similar to what has previously been observed in rats. In contrast, during the ingestion of amino acids, muscle glutamine release accounts for most of the endogenous whole body glutamine Ra. Thus tissues other than skeletal muscle, most likely splanchnic tissues, contribute significantly to basal whole body glutamine Ra. It is therefore possible that whole body endogenous Ra remained unchanged even if leg glutamine release increased during the ingestion of amino acids, if the increased release of glutamine from skeletal muscle is counteracted by a reduction in glutamine release by other tissues.
The muscle free glutamine concentration decreased during the ingestion of the amino acid-glucose mixture despite an increase in glutamine uptake by muscle cells due to a reduction in muscle glutamine synthesis. The increase in muscle glutamine utilization for muscle protein synthesis during ingestion of the amino acid-glucose mixture made only a minor contribution to the observed drop in the muscle free glutamine concentration. The reduction in glutamine synthesis during ingestion of amino acids and glucose could have been the result of both an inhibitory effect of insulin on the activity of glutamine synthetase and the availability of α-ketoglutarate/glutamate for glutamine synthesis. Although no data are available on the direct effects of insulin on glutamine synthetase activity, studies in rats have shown that streptozotocin-induced diabetes resulted in a significant increase in glutamine synthetase activity (11), the effect of which was reversed by the administration of protamine zinc insulin (11), suggesting an inhibitory effect of insulin on glutamine synthetase. The availability of α-ketoglutarate/glutamate is likely related to the predominant fate of pyruvate in muscle. An increased rate of glycolysis after glucose ingestion leads to an increase in pyruvate production (45). As a result, pyruvate is transaminated to alanine at an accelerated rate (45), which potentially limits the availability of glutamate, the immediate precursor of glutamine. Although we did not directly test this hypothesis in our study, it has previously been shown that decreasing pyruvate availability by infusion of dichloroacetate increases muscle free glutamine concentration in burn patients (12).
In the present studies, muscle glutamine kinetics were assessed using a three-pool model that requires isotopic steady state in the sampled pools (i.e., arterial and venous blood and muscle intracellular free water). Several investigators have shown that plasma (4, 7, 30, 31, 42) and muscle (4) glutamine enrichment is at steady state during the time period of our experiment. However, others have shown that it may take a long time for the intracellular glutamine pool to come to an isotopic equilibrium (40), possibly due to the relatively slow rate of entry of glutamine from blood compared with the large size of the intramuscular free glutamine pool. In that study (40), however, an insufficient priming dose was given, as reflected by the failure of plasma glutamine enrichment to reach an isotopic equilibrium. Recycling of the nitrogen label into the amino nitrogen of glutamine could also contribute to the increase in glutamine enrichment with time. This pathway, however, has been shown to be of only minor importance during the time period of our experiment (34); the contribution of nitrogen recycling into glutamine could, however, increase during prolonged tracer infusion. If indeed, in our experiment, an isotopic steady state in the muscle free glutamine pool had not been achieved, we would have underestimated muscle glutamine inward transport and overestimated muscle glutamine synthesis, particularly during the basal period. However, despite potential quantitative errors, the conclusions from our studies would still be valid, as the plasma glutamine enrichment decreased by ~30% during ingestion of the amino acid mixture with and without additional glucose, whereas the muscle free glutamine enrichment increased by ~30% during ingestion of the amino acid mixture alone and by ~60% during ingestion of the amino acid mixture plus glucose (Table 1). It is unlikely that the observed increase in muscle free glutamine enrichment under these circumstances is solely due to a failure of achieving an isotopic plateau in the muscle intracellular free glutamine pool. In fact, to obtain an estimate of the possible impact of changes in the intracellular free glutamine enrichment with time, we also obtained a muscle biopsy at 120 min after the start of the tracer infusion in some of the subjects in the present study and found that the enrichment in the muscle intracellular free glutamine enrichment at 120 min was already ~80% of the enrichment measured at 300 min (end of the basal period). During that time (from 120 to 300 min), the plasma glutamine enrichment was unchanged. On the basis of these data, we would expect the muscle intracellular glutamine enrichment to rise by ~7%/h from the end of the basal study period to the end of the amino acid ingestion if the plasma glutamine enrichment was unchanged and if the intracellular glutamine enrichment at 300 min did not represent the isotopic plateau but rose in a linear fashion during the 8-h study period (highest possible increase). However, the plasma glutamine enrichment decreased by ~30% during the ingestion of the amino acid mixture. Therefore, the muscle intracellular glutamine enrichment should have been unchanged if the ingestion of amino acids had no effect. A similar conclusion could be reached if we used the results from the study by van Acker et al. (40), according to which we would expect the muscle intracellular glutamine enrichment in our study to rise 4%/h from the end of the basal study period to the end of the amino acid ingestion. This probably represents the upper limit of the expected increase simply due to tracer infusion, as the glutamine enrichment in plasma in their study increased as well. The muscle intracellular glutamine enrichment in the present study, however, increased by ~30% during the ingestion of amino acids alone and by ~60% during ingestion of amino acids plus glucose without taking into account the simultaneous decrease in plasma glutamine enrichment. Therefore, we feel confident in saying that the rise in muscle glutamine enrichment as a result of the ingestion of the amino acid mixture was due at least in part, if not mainly, to changes in the muscle glutamine kinetics.
Furthermore, we found that conclusions based on leg glutamine net balance and intracellular glutamine concentration, both of which are tracer independent, are consistent with the tracer data. Specifically, an increase in net muscle protein synthesis and no change in glutamine net balance across the leg and intracellular free glutamine concentration during ingestion of amino acids alone suggest that the net Ra of glutamine from other sources (i.e., net transport and net intracellular utilization) in the muscle cell must have increased. Similarly, an increase in net protein synthesis but no change in leg glutamine net balance and decreased intracellular free glutamine concentration suggest that the net Ra of glutamine from these other sources in the muscle cell must have decreased. Based on the available data from the literature, the likely candidate responsible for the increased net Ra during increased amino acid supply is accelerated inward transport. Thus a decreased net Ra under these circumstances is likely due to decreased net production.
The observed increase in skeletal muscle protein synthesis during ingestion of the amino acid-glucose mixture, which resulted in a decrease in the muscle free glutamine concentration, is against the general belief that a positive association exists between intramuscular glutamine concentration and net muscle protein anabolism (19, 28, 29, 47). It could be that the intramuscular free glutamine content in our study did not drop below the threshold level required to limit protein synthesis or stimulate breakdown, as the depletion of muscle glutamine in catabolic patients is generally much greater than that induced by glucose in this study. Also, it is possible that the stimulatory effect of insulin on muscle protein synthesis would have been even greater if the intramuscular glutamine concentration had been maintained. On the other hand, there is little evidence to support a direct relationship between muscle free glutamine concentration and protein synthesis in vivo. The most compelling association is that muscle wasting occurs when intramuscular free glutamine stores are depleted (i.e., during critical illness). However, this relationship may be coincidental; evidence directly linking muscle free glutamine concentration and protein synthesis is lacking. In severely burned patients, muscle free glutamine stores are depleted, yet muscle protein synthesis is elevated significantly (13, 38). Although this could reflect the response to increased amino acid availability stemming from increased muscle protein breakdown, it does not support the notion that a low muscle glutamine concentration limits the rate of muscle protein synthesis. The only direct evidence that protein synthesis is stimulated (28) and protein breakdown is inhibited (29, 47) by increased glutamine availability is from in vitro experiments. The present study fails to support the notion that maintenance of intramuscular free glutamine availability is required for a stimulation of net muscle protein anabolism to occur.
We conclude that, in healthy volunteers, enteral administration of glutamine in the form of a mixed amino acid mixture increases plasma glutamine concentration and thus glutamine availability for peripheral tissues despite a high first-pass splanchnic extraction. Glutamine uptake by skeletal muscle is stimulated by increased glutamine availability. However, a simultaneous increase in glutamine release from the muscle and accelerated intracellular glutamine utilization for protein synthesis prevent an increase in the intramuscular free glutamine pool. Furthermore, intramuscular glutamine synthesis is attenuated during ingestion of glucose, and increased peripheral glutamine availability cannot overcome the lack of intracellular glutamine synthesis. Thus intramuscular glutamine concentration decreases. However, skeletal muscle protein synthesis can be stimulated despite a significant reduction in intramuscular glutamine concentration.
Acknowledgments
We appreciate the assistance of Dr. D. C. Gore and the nursing staff at the General Clinical Research Center at the University of Texas Medical Branch at Galveston.
This work was supported by National Institutes of Health Grants DK-33952, AG-15780, and MO1-RR00073 and by Shriners’ Burns Hospital Grant 8490.
References
- 1.Aoki TT, Brennan MF, Fitzpatrick GF, Knight DC. Leucine meal increases glutamine and total nitrogen release from forearm muscle. J Clin Invest. 1981;68:1522–1528. doi: 10.1172/JCI110406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki TT, Brennan MF, Muller WA, Soeldner JS, Alpert JS, Kaufmann RL, Tan MH, Cahill GF., Jr Amino acid levels across normal forearm muscle and splanchnic bed after a protein meal. Am J Clin Nutr. 1976;29:340–350. doi: 10.1093/ajcn/29.4.340. [DOI] [PubMed] [Google Scholar]
- 3.Biolo G, Chinkes D, Zhang X-J, Wolfe RR. A new model to determine in vivo the relationship between amino acid trans-membrane transport and protein kinetics in muscle. J Parenter Enteral Nutr. 1992;16:305–315. doi: 10.1177/0148607192016004305. [DOI] [PubMed] [Google Scholar]
- 4.Biolo G, Declan-Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab. 1995;268:E75–E84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol Endocrinol Metab. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 6.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. J Parenter Enteral Nutr. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 7.Darmaun D, Matthews DE, Bier DM. Glutamine and glutamate kinetics in humans. Am J Physiol Endocrinol Metab. 1986;251:E117–E126. doi: 10.1152/ajpendo.1986.251.1.E117. [DOI] [PubMed] [Google Scholar]
- 8.Darmaun D, Roig JD, Auestad N, Sager BK, Neu J. Glutamine metabolism in very low birth weight infants. Pediatr Res. 1997;41:391–396. doi: 10.1203/00006450-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Elia M, Folmer C, Schlatmann A, Goren A, Austin S. Amino acid metabolism in muscle and in the whole body of man before and after ingestion of a single mixed meal. Am J Clin Nutr. 1989;49:1203–1210. doi: 10.1093/ajcn/49.6.1203. [DOI] [PubMed] [Google Scholar]
- 10.Fang CH, James JH, Fischer JE, Hasselgreen PO. Is muscle protein turnover regulated by intracellular glutamine during sepsis? J Parenter Enteral Nutr. 1995;19:279–285. doi: 10.1177/0148607195019004279. [DOI] [PubMed] [Google Scholar]
- 11.Feng B, Banner C, Max SR. Effect of diabetes on glutamine synthetase expression in rat skeletal muscles. Am J Physiol Endocrinol Metab. 1990;258:E762–E766. doi: 10.1152/ajpendo.1990.258.5.E762. [DOI] [PubMed] [Google Scholar]
- 12.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. Acute dichloroacetate administration increases skeletal muscle free glutamine concentrations after burn injury. Ann Surg. 1998;228:249–256. doi: 10.1097/00000658-199808000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrando AA, Chinkes DL, Wolf SE, Matin S, Herndon DN, Wolfe RR. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidotto GG, Borghetti AF, Gazzola GC. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978;515:329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- 15.Haisch M, Fukagawa NK, Matthews DE. Splanchnic bed oxidation of enteral glutamine in humans (Abstract) FASEB J. 1998;12:A986. [Google Scholar]
- 16.Hammarqvist F, Wernerman J, Ali R, van der Decken A, Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989;209:455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hankard RG, Darmaun D, Sager BK, d’Amore D, Reed Parsons W, Haymond M. Response of glutamine metabolism to exogenous glutamine in humans. Am J Physiol Endocrinol Metab. 1995;269:E663–E670. doi: 10.1152/ajpendo.1995.269.4.E663. [DOI] [PubMed] [Google Scholar]
- 18.Häussinger D, Roth E, Lang E, Gerok W. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet. 1993;341:1330–1333. doi: 10.1016/0140-6736(93)90828-5. [DOI] [PubMed] [Google Scholar]
- 19.Hickson RC, Czerwinski SM, Wegrzyn LE. Glutamine prevents downregulation of myosin heavy chain synthesis and muscle atrophy from glucocorticoids. Am J Physiol Endocrinol Metab. 1995;268:E730–E734. doi: 10.1152/ajpendo.1995.268.4.E730. [DOI] [PubMed] [Google Scholar]
- 20.Hundal HS, Rennie MJ, Watt PW. Characteristics of L-glutamine transport in perfused rat skeletal muscle. J Physiol (Lond) 1987;393:283–305. doi: 10.1113/jphysiol.1987.sp016824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hundal HS, Taylor PM, Willhoft NM, Mackenzie B, Low SY, Ward MR, Rennie MJ. A role for membrane transport in modulation of intramuscular free glutamine turnover in streptozotocin diabetic rats. Biochim Biophys Acta. 1992;1180:137–146. doi: 10.1016/0925-4439(92)90062-r. [DOI] [PubMed] [Google Scholar]
- 22.Jensen GL, Miller RH, Talabiska DG, Fish J, Gianferante L. A double-blind, prospective, randomized study of glutamine-enriched compared with standard peptide-based feeding in critically ill patients. Am J Clin Nutr. 1996;64:615–621. doi: 10.1093/ajcn/64.4.615. [DOI] [PubMed] [Google Scholar]
- 23.Jorfeldt L, Juhlin-Daunfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 24.Jorfeldt L, Waren J. Leg blood flow during exercise in man. Clin Sci (Colch) 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn KS, Schuhmann K, Stehle P, Darmaun D, Fürst P. Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis-derived endogenous glutamine production. Am J Clin Nutr. 1999;70:484–489. doi: 10.1093/ajcn/70.4.484. [DOI] [PubMed] [Google Scholar]
- 26.Long CL, Borghesi L, Stahl R, Clark JA, Geiger JW, Di-Rienzo DB, Weis JK, Laws HL, Blakemore WS. Impact of enteral feeding of a glutamine-supplemented formula on the hypoaminoacidemic response in trauma patients. J Trauma. 1996;40:97–102. doi: 10.1097/00005373-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Long CL, Nelson KM, DiRenzo DB, Weis JK, Stahl RD, Broussard TD, Theus WL, Clark JA, Pinson TW, Geiger JW, Laws HL, Blakemore WS, Carraway RP. Glutamine supplementation of enteral nutrition: impact on whole body protein kinetics and glucose metabolism in critically ill patients. J Parenter Enteral Nutr. 1995;19:470–476. doi: 10.1177/0148607195019006470. [DOI] [PubMed] [Google Scholar]
- 28.MacLennan PA, Brown RA, Rennie MJ. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett. 1987;215:187–191. doi: 10.1016/0014-5793(87)80139-4. [DOI] [PubMed] [Google Scholar]
- 29.MacLennan PA, Smith K, Weryk B, Watt PW, Rennie MJ. Inhibition of protein breakdown by glutamine in perfused rat skeletal muscle. FEBS Lett. 1988;237:133–136. doi: 10.1016/0014-5793(88)80186-8. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DE, Campbell RJ. The effect of dietary protein intake on glutamine and glutamate nitrogen metabolism. Am J Clin Nutr. 1992;55:963–970. doi: 10.1093/ajcn/55.5.963. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of glutamine and glutamic acid in humans. Am J Physiol Endocrinol Metab. 1993;264:E848–E854. doi: 10.1152/ajpendo.1993.264.6.E848. [DOI] [PubMed] [Google Scholar]
- 32.Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Koller M, König W, Fürst P, Puchstein C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double-blind, controlled study. Ann Surg. 1998;227:302–308. doi: 10.1097/00000658-199802000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer TE, Griffiths RD, Jones C. Effect of parenteral L-glutamine on muscle in the very severly ill. Nutrition. 1996;12:316–320. doi: 10.1016/s0899-9007(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 34.Patterson BW, Carraro F, Klein S, Wolfe RR. Quantification of incorporation of [15N]ammonia into plasma amino acids and urea. Am J Physiol Endocrinol Metab. 1995;269:E508–E515. doi: 10.1152/ajpendo.1995.269.3.E508. [DOI] [PubMed] [Google Scholar]
- 35.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–910. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 36.Rennie MJ, MacLennan DA, Hundal HS, Weryk B, Smith K, Taylor PM, Egan C, Watt PW. Skeletal muscle glutamine transport, intramuscular glutamine concentration, and muscle protein turnover. Metabolism. 1989;38:47–51. doi: 10.1016/0026-0495(89)90140-6. [DOI] [PubMed] [Google Scholar]
- 37.Rennie MJ, Tadros L, Khogali S, Ahmed A, Taylor PM. Glutamine transport and its metabolic effects. J Nutr. 1994;124:1503S–1508S. doi: 10.1093/jn/124.suppl_8.1503S. [DOI] [PubMed] [Google Scholar]
- 38.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, Patterson BW, Wolfe RR. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned children. Ann Surg. 1995;222:283–297. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadros LB, Willhoft NM, Taylor PM, Rennie MJ. Effects of glutamine deprivation on glutamine transport and synthesis in primary culture of rat skeletal muscle. Am J Physiol Endocrinol Metab. 1993;265:E935–E942. doi: 10.1152/ajpendo.1993.265.6.E935. [DOI] [PubMed] [Google Scholar]
- 40.Van Acker BA, Hulsewe KW, Wagenmakers AJ, Deutz NE, vanKreel BK, Halliday D, Matthews DE, Soeters PB, von Meyenfeldt MF. Absence of glutamine isotopic steady state: implications for the assessment of whole-body glutamine production rate. Clin Sci (Colch) 1998;95:339–346. [PubMed] [Google Scholar]
- 41.Volpi E, Ferrando AA, Tipton KD, Wolfe RR. Exogenous amino acids stimulate muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams BD, Wolfe RR. Determination of amino- and amide-15N glutamine enrichment with tertiary butyldimethylsilyl derivatives. Biol Mass Spectrom. 1994;23:682–688. doi: 10.1002/bms.1200231106. [DOI] [PubMed] [Google Scholar]
- 43.Windmüller HG. Glutamine utilization by the small intestine. Adv Enzymol Relat Areas Mol Biol. 1982;53:201–237. doi: 10.1002/9780470122983.ch6. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 45.Wolfe RR, Jahoor F, Herndon DN, Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substances in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991;110:54–67. [PubMed] [Google Scholar]
- 46.Wusteman M, Elia M. Effect of glutamine infusions on glutamine concentration and protein synthetic rate in rat muscle. J Parenter Enteral Nutr. 1991;15:521–525. doi: 10.1177/0148607191015005521. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, Thompson JR. Regulation of protein turnover by glutamine in heat-shocked skeletal myotubes. Biochim Biophys Acta. 1997;1357:234–242. doi: 10.1016/s0167-4889(97)00035-9. [DOI] [PubMed] [Google Scholar]