Abstract
Cell invasion plays a central role in a wide variety of biological phenomena, and is the cause of tumor growth and metastasis. Understanding the biochemical mechanisms that control cell invasion is one of the major goals of our laboratory. Podosomes and invadopodia are specialized cellular structures present in cells with physiological or pathological invasive behaviors. These transient structures are localized at the ventral cell surface, contain an array of different proteins, and facilitate cell-substrate adhesion as well as the local proteolytic activity necessary for extracellular matrix remodeling and subsequent cellular invasion. We have previously shown that the adaptor proteins and Src substrates Tks4 and Tks5 are required for podosome and invadopodia formation, for cancer cell invasion in vitro, and for tumor growth in vivo. We have also defined a role for the Tks-mediated generation of reactive oxygen species (ROS) in both podosome and invadopodia formation, and invasive behavior. Tks4 and Tks5 are also required for proper embryonic development, likely because of their roles in cell migration. Finally, we recently implicated podosome formation as part of the synthetic phenotype of vascular smooth muscle cells. Inhibitors of podosome and invadopodia formation might have utility in the treatment of vascular diseases and cancer. We have therefore developed a high content, cell-based high throughput screening assay that allows us to identify inhibitors and activators of podosome/invadopodia formation. We have used this assay to screen for small molecule inhibitors, and defined novel regulators of invadopodia formation. Here we will review these recent findings.
Keywords: Src, cancer, atherosclerosis, embryonic development, reactive oxygen species
Podosomes and invadopodia
Podosomes and invadopodia can be defined as dynamic, actin-rich protrusions of the ventral membrane of certain cell types [1]. They are the sites of attachment to, and degradation of, the extracellular matrix (ECM). Their presence correlates with migratory and invasive ability of cells, and they represent an increasingly important area of research. The term podosome is used to define the structures found in normal cell types, such as osteoclasts, macrophages, endothelial cells and vascular smooth muscle cells. The term invadopodia is used to describe the structures found in invasive cancer cells. Despite these different names, there are far more similarities than differences between the two structures. And where differences have been noted, for example in turnover time and length of protrusion, it is not clear whether these are intrinsic, or related to the different culture conditions used for normal and cancer cells. Certainly, the key components of podosomes are shared with invadopodia. For a more detailed description of podosome and invadopodia components, see our recent review [1].
The Tks adaptor proteins
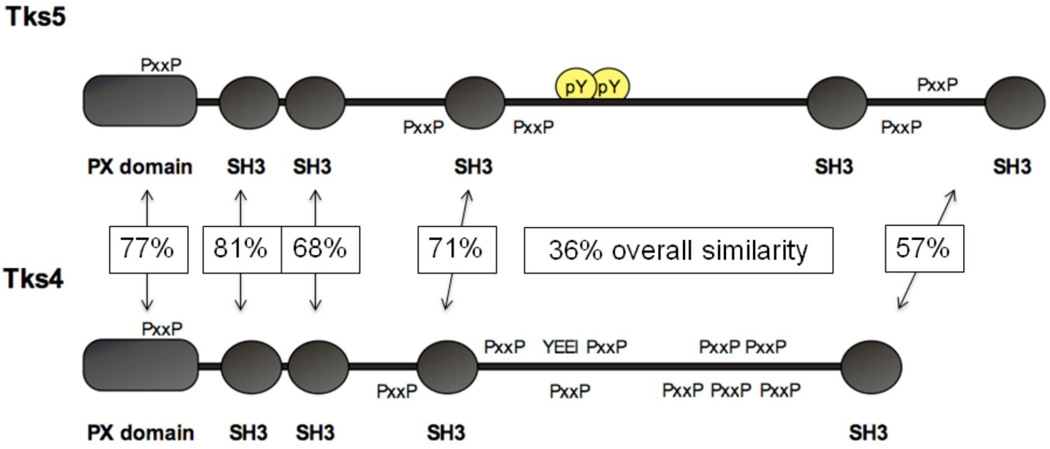
Our interest in podosomes and invadopodia began when we realized that a novel Src substrate and adaptor protein we had discovered, known as Tks5 (Figure 1), localized to invadopodia [2, 3]. We went on to show that Tks5 is required for both invadopodia formation and invasive behavior in a number of human cancer cell lines, as well as in the Src-transformed mouse fibroblasts (Src-3T3 cells) we use to study all aspects of Src transformation [4]. Tks5 thus joined a growing number of proteins shown to be necessary for invadopodia formation. Whereas most invadopodia components are broadly expressed in all cell types, we noticed that Tks5 is expressed in invasive cancer cells, but not in non-invasive cells. This suggested that Tks5 might play a central role in the initiation of invadopodia formation. To investigate this, we introduced Tks5 (along with Src to phosphorylate it) into a non-invasive breast cancer cell line, and detected the robust formation of invadopodia [4]. In this assay, invadopodia formation was dependent on the PX domain of Tks5, which suggests that lipid binding to the PX domain of Tks5 initiates invadopodia formation. In keeping with this, Oikawa et al subsequently showed that invadopodia are initiated at membrane sites rich in PI-3,4P2, a lipid known to bind to the PX domain of Tks5 [2, 5]. Other studies have shown that recruitment of Tks5 and cortactin are the first events in invadopodia formation [6].
Figure 1. Schematic of the Tks adaptor proteins.
The architecture of Tks5 (top) and Tks4 (bottom) is shown, illustrating the PX and SH3 domains, Src phosphorylation sites (pY and YEEI) and proline-rich motifs (PxxP). The boxed figures represent the percent similarity of the arrowed domains.
More recently, we have characterized the Tks5-related protein Tks4, which is also a Src substrate and adaptor protein, with a PX domain followed by 4 SH3 domains (Figure 1) [7]. An examination of Tks4 null fibroblasts revealed that Tks4 is also required for Src-driven invadopodia formation. In the absence of Tks4, several invadopodia proteins, including Tks5, accumulate together at the membrane, but actin polymerization and ECM degradation do not take place. Over time in culture, Tks5 levels rise in Tks4 null cells, and actin polymerization is now visualized at these pre-invadopodia sites. But high levels of Tks5 cannot rescue ECM degradation. This is likely because Tks4 has a non-redundant role in the localization of MT1-MMP (a transmembrane metalloprotease) to invadopodia.
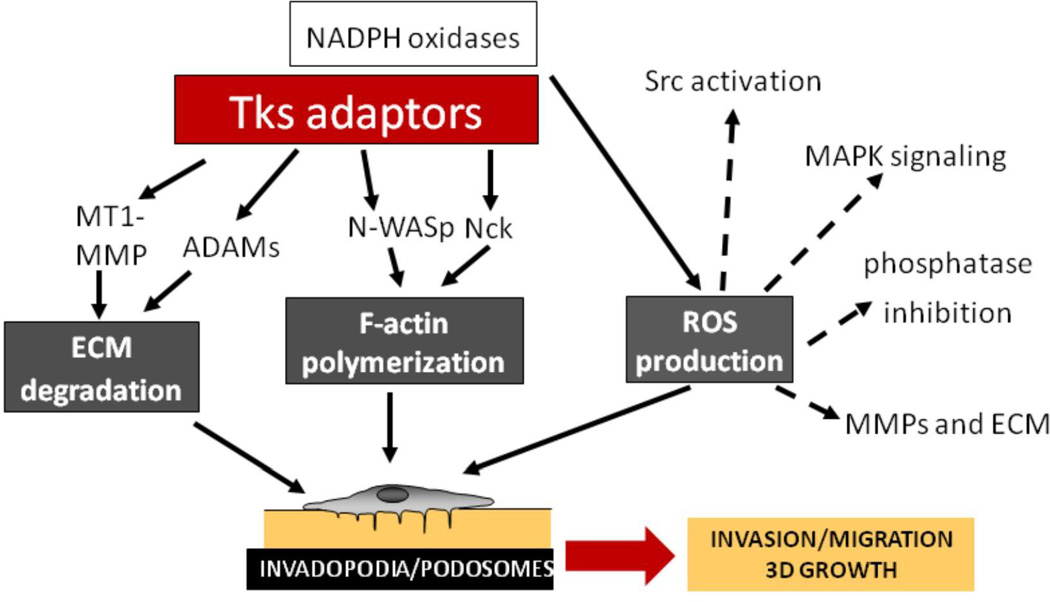
The defining properties of invadopodia and podosomes are the polymerization of F-actin and the degradation of the ECM. We are beginning to have a better understanding of how the Tks adaptor proteins participate in these processes. For example, Tks5 can associate with the actin regulatory proteins N-WASP [5] and Nck [8]. Both Tks4 and Tks5 associate with ADAMs family metalloproteases [2, 7], and we have already mentioned the key role of Tks4 in recruiting MT1-MMP to invadopodia. Recently, we determined that both Tks proteins can act as organizers for the production of reactive oxygen species (ROS) by the NADPH oxidase family of enzymes [9, 10]. ROS can be detected in invadopodia, and are necessary for both podosome and invadopodia formation. While the mechanisms by which ROS function in podosome and invadopodia assembly are not yet fully established, their known roles in promoting signal transduction, in transiently inhibiting the activity of some phosphatases, and in increasing matrix metalloprotease synthesis, are likely to prove important. Our current understanding of how the Tks adaptors might integrate diverse aspects of podosome and invadopodia formation and function are shown in Figure 2. Clearly it will be important to complete our understanding of the roles of Tks4 and Tks5 with more biochemical studies, to define the role of each SH3 domain, phosphorylation site, and proline-rich motif.
Figure 2. An overview of the functions of the Tks adaptor proteins.
The interactions of the Tks proteins with proteases, actin regulators and NADPH oxidases are shown, suggesting a way in which key features of invadopodia and podosomes might be under the control of the Tks proteins. The dotted lines highlight ways in which ROS production might contribute to invadopodia and podosome formation, and to invasion, migration and growth.
Tks proteins and embryonic development
Cell migration and invasion is a property of specialized cell types in the adult organism, and can also occur during pathological processes such as atherosclerosis. But during embryonic development, many cell types are migratory, and cell migration is required to pattern the developing embryo. We have been interested to determine whether the Tks adaptors are also involved in cell migration during embryonic development. To address this question for Tks5, we used zebrafish, since embryonic development is complete within 5 days, and the embryos of zebrafish are transparent, making the analysis of cell movements relatively facile. We used morpholino technology to reduce Tks5 expression in zebrafish embryos and observed a number of severe defects [11]. These include craniofacial and pigmentation abnormalities, heart malformations, lack of movement and edema. Many of the defects observed are in cell types that arise from the differentiation of neural crest stem cells. We further evaluated the role of Tks5 using zebrafish expressing red fluorescent protein tagged neural crest cells. We found that loss of Tks5 has little effect on neural crest cell number, but does impair their ventral migration, coincident with a loss of protrusive structures in the migrating cells. In vitro studies using a mouse neural crest stem cell line showed the formation of Src and Tks5-dependent podosome-like structures in response to TGFβ. Tks5 is also required for neural crest cell migration in vitro. Our data suggests that neural crest cell migration requires the formation of podosomes, implying that similar mechanisms are involved in cell migration during embryogenesis and cancer metastasis.
To evaluate embryonic roles for Tks4, we have analyzed mice bearing a gene-trap cassette in the gene encoding Tks4, which fail to express any Tks4 protein [12]. Tks4 null mice are born at mendelian ratios, but approximately 20% die in the first three weeks of life of undetermined causes. At birth, Tks4 null mice are on average the same size as their wild type and heterozygous littermates, but by weaning the surviving nulls are 40% smaller than normal littermates. They have severe craniofacial developmental defects, as well as other skeletal defects, heart abnormalities, glaucoma, and a striking size reduction of white adipose tissue (WAT) depots. As we were characterizing the Tks4 null mice, Dr. van Bokhoven and colleagues used homozygosity mapping and copy number analysis to determine that mutations of the gene encoding Tks4 are associated with some cases of Frank-Ter Haar syndrome [FTHS (MIM 249420)], a rare, fatal, autosomal recessive disorder characterized by skeletal, cardiovascular and eye abnormalities. Analysis of seven FTHS families revealed five different homozygous mutations in the Tks4 gene [12]. No Tks4 mutations were detected in six other FTHS families. However, we found that dermal fibroblasts from one of these individuals nevertheless express reduced levels of the Tks4 protein, suggesting a common mechanism underlying disease causation. These findings underscore the importance of Tks4 in embryonic development. While it is not yet clear whether failure to produce podosomes underlies each of the developmental abnormalities observed in FTHS, the fact that several other podosome-associated proteins are mutated in disorders involving craniofacial and other skeletal abnormalities suggests that this is an idea worth pursuing in the future [13].
Tks proteins and cancer progression
Most experiments on invadopodia are conducted in vitro. In the future it will be important to visualize these structures in vivo, and to characterize their roles in tumor progression in detail. To date, the roles for just a handful of invadopodia proteins have been probed in animal models. Expression of a form of AMAP1 that cannot mediate association between cortactin and paxillin has little effect on breast cancer growth in mice, but a more pronounced effect on metastasis of these cells [14]. In contrast, knockdown of either MT1-MMP or cortactin affects the growth of implanted tumors [15, 16]. The same is also true for Tks5: Src-3T3 cells with reduced Tks5 expression grow poorly when implanted subcutaneously, likely as a result of increased apoptosis and reduced tumor vascularization [17]. These results are not in keeping with a role for invadopodia solely in crossing basement membrane barriers in the process of metastasis, and suggest instead a broader role for invadopodia in tumor invasion and growth. Perhaps the pericellular proteolytic activity controlled by invadopodia is required not only for ECM degradation, but also for the local production and release of growth factors and other cytokines necessary to establish a productive tumor microenvironment.
Isolating invadopodia regulators
The growing evidence for a role for invadopodia in tumor progression, as well as the role of vascular smooth muscle cell podosomes in atherosclerosis [18], suggests that targeting these structures might represent a valuable therapeutic approach. We set out to develop an assay to screen for invadopodia regulators. We opted for a high content screening assay, using Src-3T3 cells stained with DAPI to visualize nuclei and rhodamine-labeled phalloidin to stain F-actin. We screened the LOPAC collection; 1280 small molecules annotated to inhibit a variety of targets. We chose 7 compounds for further analysis; 2 activators and 5 inhibitors [19]. The activators were cantharidin (a serine/threonine phosphatase inhibitor) and paclitaxel, a chemotherapeutic agent used in the treatment of a variety of solid tumors. We verified that paclitaxel promoted invadopodia formation and invasive behavior in human cancer cell lines, including those resistant to the cytotoxic effects of the drug. These findings raise concerns about the use of paclitaxel in the neo-adjuvant setting (that is, prior to the removal of the primary tumor) as well as once resistance has emerged. The most interesting inhibitors to emerge from our screen were annotated as cyclin-dependent kinase inhibitors. We ruled out a role for cell cycle progression in the formation of invadopodia, which suggested that Cdks 1 and 2 were unlikely to be the targets of the inhibitors. We therefore focused on Cdk5, a related kinase known to be involved in neuronal cell migration [20]. We validated Cdk5 as a positive regulator of invadopodia formation, and determined that Cdk5 and its associated protein, p35, are widely over-expressed in cancer cells. We determined that the mechanism by which Cdk5 promotes invadopodia formation is by phosphorylating and targeting for destruction the actin-binding protein caldesmon, which was previously described as a negative regulator of podosomes and invadopodia. Thus, Cdk5 represents a validated invadopodia target.
Future directions
Even while much more is now known about podosomes and invadopodia than just five years ago, there remain many important unanswered questions which will be the focus of our research in the coming years. What do invadopodia look like in vivo? What stimuli promote their formation? How do they regulate pericellular proteolysis? How common is it for cancers to use invadopodia for invasion and metastasis? Will podosome and invadopodia inhibitors be of therapeutic use?
ACKNOWLEDGEMENTS
I am grateful to all the members of my laboratory, past and present, for their commitment to and passion for research. Important contributions to our understanding of podosome and invadopodia biology were made by Peter Lock, Clare Abram, Darren Seals, Eduardo Azucena, Ian Pass, Paul Bromann, Barbara Blouw, Danielle Murphy, Manuela Quintavalle, Begoña Diaz, Matt Buschman and Pilar Cejudo-Martin. This research would not have been possible without our fine collaborators, including James Resau, Gary Bokoch, Hans van Bokhoven, Pilar Ruiz-Lozano, José Luis Millán, James Lindsay, Gianluigi Condorelli, Rodney Stewart, Juan Carlos Izpisua-Belmonte, Susanne Heynen-Genel, Jeffrey Price, Jeff Tsai and Leonardo Elia. Our research is supported by the National Cancer Institute of the National Institutes of Health, and by the Mathers Foundation.
Abbreviations
- ROS
reactive oxygen species
- Tks
tyrosine kinase substrate
- ECM
extracellular matrix
- PX
phox homology
- PI
phosphatidylinositol
- MMP
matrix metalloprotease
- SH
Src homology
- TGF
transforming growth factor
- FTHS
Frank-ter Haar syndrome
- Cdk
cyclin-dependent kinase
REFERENCES
- 1.Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J Biol Chem. 2003;278:16844–16851. doi: 10.1074/jbc.M300267200. [DOI] [PubMed] [Google Scholar]
- 3.Lock P, Abram CL, Gibson T, Courtneidge SA. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. Embo J. 1998;17:4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–165. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Oikawa T, Itoh T, Takenawa T. Sequential signals toward podosome formation in NIH-src cells. J Cell Biol. 2008;182:157–169. doi: 10.1083/jcb.200801042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Courtneidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol Biol Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stylli SS, Stacey TT, Verhagen AM, Xu SS, Pass I, Courtneidge SA, Lock P. Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J Cell Sci. 2009;122:2727–2740. doi: 10.1242/jcs.046680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianni D, Diaz B, Taulet N, Fowler B, Courtneidge SA, Bokoch GM. Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci Signal. 2009;2:ra54. doi: 10.1126/scisignal.2000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz B, Shani G, Pass I, Anderson D, Quintavalle M, Courtneidge SA. Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy DA, Diaz B, Bromann PA, Tsai JH, Kawakami Y, Maurer J, Stewart RA, Izpisua-Belmonte JC, Courtneidge SA. A Src-Tks5 Pathway Is Required for Neural Crest Cell Migration during Embryonic Development. PLoS One. 2011;6:e22499. doi: 10.1371/journal.pone.0022499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal Z, Cejudo-Martin P, de Brouwer A, van der Zwaag B, Ruiz-Lozano P, Scimia MC, Lindsey JD, Weinreb R, Albrecht B, Megarbane A, Alanay Y, Ben-Neriah Z, Amenduni M, Artuso R, Veltman JA, van Beusekom E, Oudakker A, Millan JL, Hennekam R, Hamel B, Courtneidge SA, van Bokhoven H. Disruption of the Podosome Adaptor Protein TKS4 (SH3PXD2B) Causes the Skeletal Dysplasia, Eye, and Cardiac Abnormalities of Frank-Ter Haar Syndrome. American Journal of Human Genetics. 2010;86:254–261. doi: 10.1016/j.ajhg.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cejudo-Martin P, Courtneidge SA. Podosomal proteins as causes of human syndromes: a role in craniofacial development? Genesis. 2011;49:209–221. doi: 10.1002/dvg.20732. [DOI] [PubMed] [Google Scholar]
- 14.Nam JM, Onodera Y, Mazaki Y, Miyoshi H, Hashimoto S, Sabe H. CIN85, a Cbl-interacting protein, is a component of AMAP1-mediated breast cancer invasion machinery. EMBO J. 2007;26:647–656. doi: 10.1038/sj.emboj.7601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus - independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark ES, Brown B, Whigham AS, Kochaishvili A, Yarbrough WG, Weaver AM. Aggressiveness of HNSCC tumors depends on expression levels of cortactin, a gene in the 11q13 amplicon. Oncogene. 2009;28:431–444. doi: 10.1038/onc.2008.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol. 2008;87:555–567. doi: 10.1016/j.ejcb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal. 2011;4:ra49. doi: 10.1126/scisignal.2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A, Tsai LH. Cyclin-dependent kinase 5 and neuronal migration in the neocortex. Neurosignals. 2003;12:173–179. doi: 10.1159/000074618. [DOI] [PubMed] [Google Scholar]