Abstract
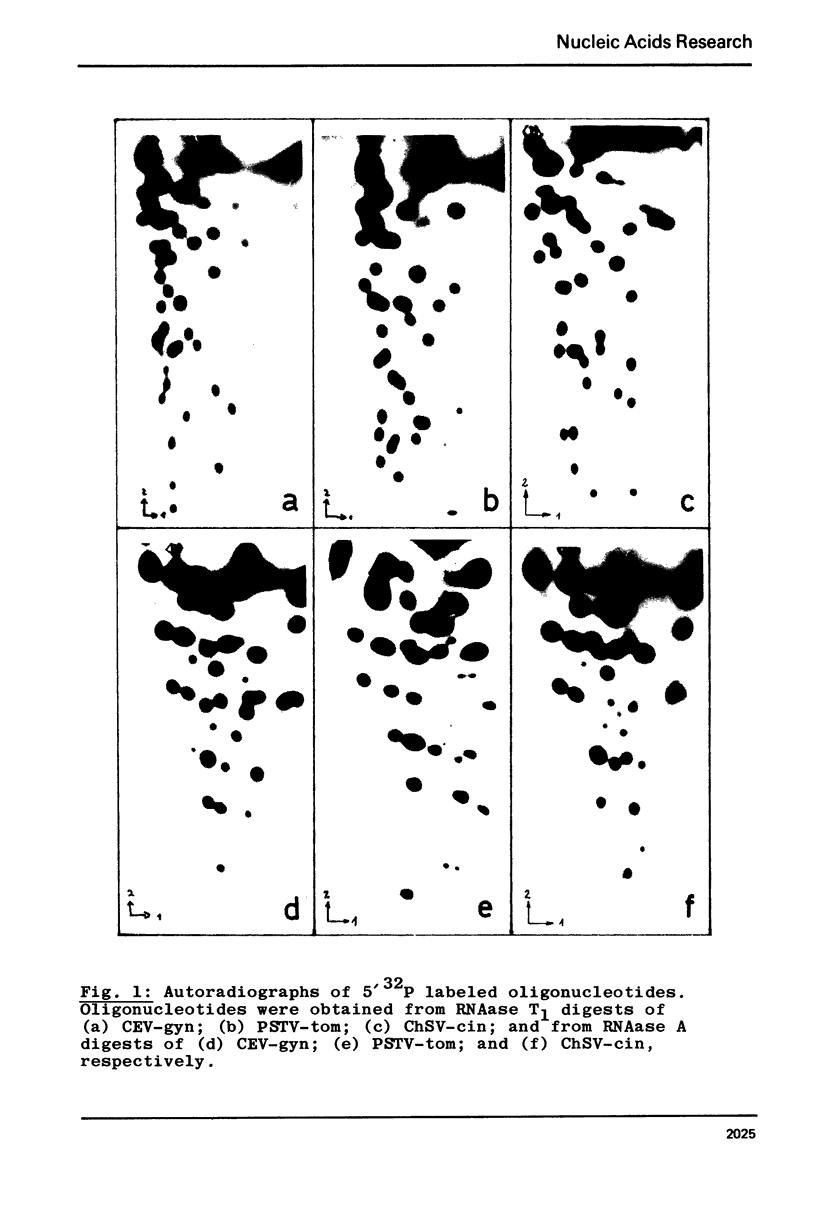
5' Phosphorylation in vitro with gamma-32P-ATP and T4 phage induced polynucleotide kinase was used to obtain RNAase A and RNAase T1 fingerprints of three plant viroids: Potato spindle tuber viroid from tomato (PSTV-tom), chrysanthemum stunt viroid from cineraria (ChSV-cin) and citrus exocortis viroid from Gynura aurantiaca (CEV-gyn). These three viroids differ significantly from each other as judged from their oligonucleotide patterns. This supports the concept of individual viroid species.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dickson E., Prensky W., Robertson H. D. Comparative studies of two viroids: analysis of potato spindle tuber and citrus exocortis viroids by RNA fingerprinting and polyacrylamide-gel electrophoresis. Virology. 1975 Dec;68(2):309–316. doi: 10.1016/0042-6822(75)90274-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Lawson R. H. Chrysanthemum stunt: a viroid disease. Virology. 1973 Jan;51(1):94–101. doi: 10.1016/0042-6822(73)90369-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Viroids. Adv Virus Res. 1972;17:295–313. doi: 10.1016/s0065-3527(08)60754-x. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Ghosh H. P., Simsek M., Raj Bhandary U. L. Initiator methionine transfer ribonucleic acid from wheat embryo. Purification, properties, and partial nucleotide sequences. J Biol Chem. 1974 Aug 10;249(15):4720–4729. [PubMed] [Google Scholar]
- Gillum A. M., Roe B. A., Anandaraj M. P., RajBhandary U. L. Nucleotide sequence of human placenta cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):407–413. doi: 10.1016/0092-8674(75)90190-7. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henco K., Riesner D., Sanger H. L. Conformation of viroids. Nucleic Acids Res. 1977 Jan;4(1):177–194. doi: 10.1093/nar/4.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Randles J. W., Rillo E. P., Diener T. O. The viroidlike structure and cellular location of anomalous RNA associated with the cadang-cadang disease. Virology. 1976 Oct 1;74(1):128–139. doi: 10.1016/0042-6822(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaine C. P., Horst R. K. Suggested viroid etiology for chrysanthemum chlorotic mottle disease. Virology. 1975 Mar;64(1):86–95. doi: 10.1016/0042-6822(75)90081-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Magnuson D. S., Weathers L. G. Potato spindle tuber disease produced by pathogenic RNA from citrus exocortis disease: evidence for the identity of the causal agents. Virology. 1973 Mar;52(1):292–294. doi: 10.1016/0042-6822(73)90419-4. [DOI] [PubMed] [Google Scholar]
- Simsek M., Petrissant G., Rajbhandary U. L. Replacement of the sequence G-T-phi-C-G(A)- by G-A-U-C-G- in initiator transfer RNA of rabbit-liver cytoplasm. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2600–2604. doi: 10.1073/pnas.70.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Michniewicz J. J., Narang S. A. Separation of potato spindle tuber viroid ribonucleic acid from Scopolia sinensis into three infectious forms and the purification and oligonucleotide pattern of fraction II RNA. Part IX. Can J Biochem. 1976 Jul;54(7):600–608. doi: 10.1139/o76-089. [DOI] [PubMed] [Google Scholar]
- Southern E. M. An improved method for transferring nucleotides from electrophoresis strips to thin layers of ion-exchange cellulose. Anal Biochem. 1974 Nov;62(1):317–318. doi: 10.1016/0003-2697(74)90395-9. [DOI] [PubMed] [Google Scholar]
- Székely M., Sanger F. Use of polynucleotide kinase in fingerprinting non-radioactive nucleic acids. J Mol Biol. 1969 Aug 14;43(3):607–617. doi: 10.1016/0022-2836(69)90362-3. [DOI] [PubMed] [Google Scholar]