Abstract
Presenilin-1 (PS1) is a transmembrane protein that is in many cases responsible for the development of early-onset familial Alzheimer’s disease. PS1 is essential for neurogenesis, somitogenesis, angiogenesis, and cardiac morphogenesis. We report here that PS1 is also required for maturation and/or maintenance of the pituitary gland. We generated PS1-conditional knockout (PS1-cKO) mice by crossing floxed PS1 and Wnt1-cre mice, in which PS1 was lacking in the neural crest-derived cell lineage. Although the PS1-cKO mice exhibited no obvious phenotypic abnormalities for several days after birth, reduced body weight in the mutant was evident by the age of 3 to 5 weeks. Pituitary weight and serum insulin-like growth factor (IGF)-1 level were also reduced in the mutant. Histologic analysis revealed severe atrophy of the cytosol in the anterior and intermediate pituitary lobes of the mutant. Immunohistochemistry did not reveal clear differences in the expression levels of thyroid-stimulating hormone, adrenocorticotropic hormone, or prolactin in the mutant pituitary. In contrast, growth hormone expression levels were reduced in the anterior lobe of the mutant. PS1 was defective in the posterior lobe, but not the anterior or intermediate lobes, in the mutant pituitary. These findings suggest that PS1 indirectly mediates the development and/or maintenance of the anterior and intermediate lobes in the pituitary gland via actions in other regions, such as the posterior lobe.
Introduction
Presenilin-1 (PS1) is a transmembrane protein that is in many cases responsible for the development of early-onset familial Alzheimer’s disease (Cruts & Van Broeckhoven 1998a, 1998b). Full-length PS1 undergoes endoproteolytic cleavage, generating stable N-terminal and C-terminal fragments that interact with other proteins to form a macromolecular complex with γ-secretase activity. This enzyme is required for regulated intramembrane proteolysis of several membrane proteins, such as amyloid precursor protein, Notch, and cadherins (De Strooper et al. 1999; Marambaud et al. 2002; Marambaud et al. 2003; Koo & Kopan 2004). In addition to the proteolytic γ-secretase function, PS1 has an important role in the turnover of β-catenin, a molecule essential for Wnt signaling and cell adhesion (Kang et al. 2002; Gottardi & Gumbiner 2004).
The biochemical functions as well as the biologic significance of PS1 in vivo have been revealed. Earlier studies of PS1-knockout null mice have contributed to our understanding of the in vivo functions of PS1 in neurogenesis, somitogenesis, angiogenesis, and cardiac morphogenesis (Shen et al. 1997; Wong et al. 1997; Handler et al. 2000; Koizumi et al. 2001; Yuasa et al. 2002; Nakajima et al. 2003; Nakajima et al. 2004). The role of PS1 in the postnatal stages, however, has not been examined because PS1 null mice die during late gestation. A relatively new approach, however, using conditional knockout (cKO) mice, which are born and grow up, has allowed for the examination of PS1 function during the postnatal period (Yu et al. 2001; Saura et al. 2004).
Neural crest cells are multipotent progenitors that possess the unique property of contributing to the development of a variety of adult tissues, such as the peripheral and enteric nervous system, bone and connective tissue of the head and neck, and the outflow tract of the developing heart (Chai et al. 2000; Jiang et al. 2000). During early embryogenesis, neural crest cells migrate from the dorsal neural tube to each final differentiation site. Many cellular mechanisms crucial to embryonic development depend on this cell lineage.
In the present study, we examined the role of PS1 in the neural crest cell lineage using mice conditionally lacking PS1 in this cell lineage. Our findings indicate that PS1 is required for the development and/or maintenance of the anterior and intermediate lobes of the pituitary gland.
Experimental procedures
Animals
All mice were maintained under a controlled temperature and photoperiod (23°C, 12 h light and 12 h dark) with food and water provided ad libitum. All experimental procedures followed the Guideline for Animals Experimentation prepared by the Animal Care and Use Committee of Matsuyama University.
PS1 floxed mice were described previously (Yu et al. 2001). Wnt1-cre transgenic mice (Danielian et al. 1998) were obtained from The Jackson Laboratory (Bar Harbor, ME; #003829). The Wnt1-cre transgene has been used extensively to inactivate floxed genes in almost all neural crest cell lineages (Chai et al., 2000; Jiang et al., 2000; Brewer et al., 2004). ROSA26-cre-reporter mice (Mao et al. 1999) were also from The Jackson Laboratory (#003504). Mice with a C57BL/6J and 129 hybrid background were used. Embryonic age was determined according to the vaginal plug, with noon of the day of plug observation defined as E0.5.
Skeletal analysis
Skeletal preparations were made using an alcian blue-alizarin red procedure, staining cartilage in blue and ossified, calcium-containing tissue in red (Kessel & Gruss 1991). One-day old mice were eviscerated, fixed in 100% ethanol for 2 days, kept in acetone for 2 days, and rinsed with water. They were stained for 3 days in staining solution consisting of 1 volume (vol.) of 0.3% alcian blue 8GX (Sigma, St. Louis, MO; #A3157) in 70% ethanol, 1 vol. of 0.1% alizarin red S (WAKO, Osaka, Japan; #1–119) in 95% ethanol, 1 vol. of 100% acetic acid, and 17 vol. of ethanol. After rinsing, the specimens were kept in 1% potassium hydroxide for 11 days with replacement every 2 to 3 days, and then kept in 50% ethanol and 20% glycerol solution for 3 days for clarification. Photographs were taken under a stereomicroscope.
Serum and tissue collection
Blood was collected by cardiac puncture in anesthetized mice. Serum was frozen and stored at −80°C. Insulin-like growth factor (IGF)-1 level in the sera was determined by Quantikine Mouse IGF-1 Immunoassay (R&D Systems, Minneapolis, MN). Pituitaries were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C overnight and weighed.
Histology, immunohistochemistry, and detection of β-galactosidase (lacZ) activities
For hematoxylin and eosin staining, pituitaries were fixed in 4% paraformaldehyde in PBS at 4°C, embedded in paraffin, and sectioned at a thickness of 4 µm.
For immunohistochemistry, pituitaries were fixed in 4% paraformaldehyde in PBS at 4°C, and embedded in paraffin or frozen in OCT compound (Sakura Finetechnical, Tokyo, Japan) following serial penetrations with 10%, 20%, and 30% sucrose in PBS. In the immunohistochemistry using paraffin sections, antigen retrieval was performed after deparaffinization and rehydration by treatment of slides with boiling Target Retrieval Solution (DAKO no. S1700, Carpinteria, CA) for 40 min. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in distilled water for 10 min. The sections were incubated with anti-PS1-C-terminal fragment antibody (R007, 1:100 dilution, Koizumi et al. 2001) or anti-mouse growth hormone (GH) (1:2000 dilution, A. Parlow, National Hormone and Peptide Program, Torrance, CA) overnight. After washing in PBS, the immunoreactivities were detected with ENVISION+ system HRP Rabbit (DAKO, #K4002) and diaminobenzidine. The sections were counterstained with methylgreen.
In the case of the immunohistochemistry using frozen sections (16 µm thick), specimens were postfixed in 4% paraformaldehyde in PBS for 15 min. Endogenous peroxidase activity was blocked by incubation with 0.5% hydrogen peroxide in methanol for 30 min. The sections were incubated with anti-mouse GH (1:2000 dilution), anti-rat thyroid-stimulating hormone (TSH) beta (1:2000 dilution, A. Parlow), anti-mouse prolactin (PRL) (1:2000 dilution, A. Parlow), or anti-rat adrenocorticotropic hormone (ACTH) (1:5000 dilution, A. Parlow) overnight. After washing in PBS, immunoreactivity was detected with ENVISION+ system HRP Rabbit and diaminobenzidine. The sections were counterstained with methylgreen.
The pituitaries of the 5-week-old mice were stained for β-galactosidase activity according to the standard procedures. The pituitaries were fixed for 60 min at room temperature in fixation buffer containing 0.2% glutaraldehyde, 5 mM EGTA (pH8.0), 2 mM MgCl2, 1.5% formaldehyde, and 100 mM sodium phosphate (pH8.0). The fixed tissues were then washed three times with a washing buffer containing 0.01% sodium deoxycholate, 0.02% NP-40, 2 mM MgCl2, and 100 mM sodium phosphate (pH8.0), and subjected to X-gal staining. The reactions were performed for 1 day at room temperature with a staining solution including 0.1% X-gal, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide in the washing buffer. After staining, the tissues were rinsed twice in PBS and postfixed in 3.7% formaldehyde in PBS. The pituitaries were sectioned to observe lacZ expression at the cellular level; the pituitaries stained by X-gal were embedded with OCT compound. Sections were cut at a 16-µm thickness and counterstained with Nuclear Fast Red (Sigma, #N3020).
Statistics
Statistical significance was determined by a two-tailed paired Student’s t-test or Z-test. A p value of less than 0.05 was considered statistically significant.
Results
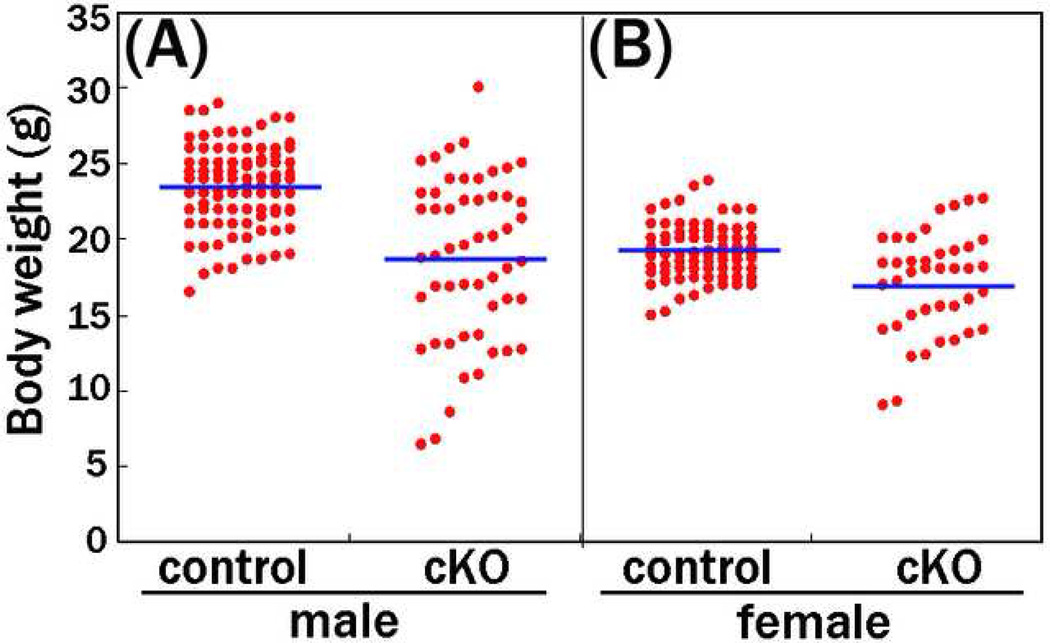
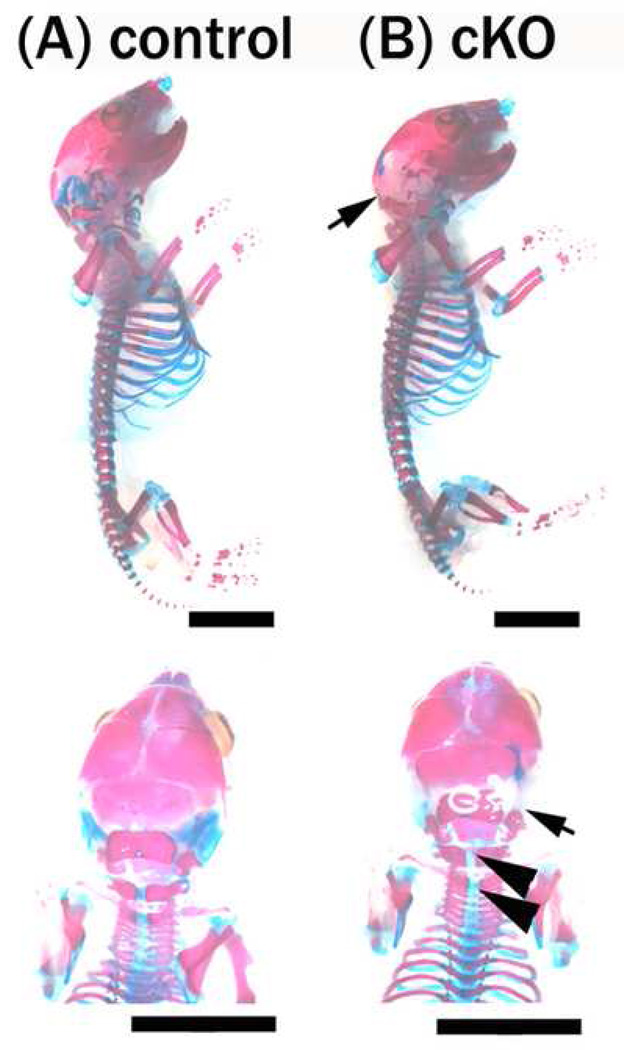
To generate mice lacking PS1 in the neural crest cell lineage, we crossed floxed PS1 and Wnt1-cre mice, which were previously described (Danielian et al. 1998; Yu et al. 2001; Saura et al. 2004). In contrast to PS1 null mice, which die perinatally (Shen et al. 1997; Wong et al. 1997; Handler et al. 2000; Koizumi et al. 2001; Yuasa et al. 2002; Nakajima et al. 2003; Nakajima et al. 2004), the PS1-cKO mice were viable with no obvious phenotypic abnormalities for several days after birth. Both male and female mutant mice, however, showed a significantly reduced weight by the time of weaning (Fig. 1). Although extremely low-weight mutant mice died at around 3 to 7 weeks of age, the remaining mutant mice matured with a reduced weight and were fertile. In this report, we refer to mice under 17.9 g for males and 15.6 g for females (mean minus two standard deviations) at 5 weeks of age as “lightweight mice”. Because most of the lightweight mutant mice had a shortened as well as lean trunk (Fig. 2), we first examined whether abnormal axial bone formation caused the reduced weight in the mutant. Although analysis of the skeletal preparation of 1-day-old mutants revealed that most of the cartilage was missing in the occipital region, bone malformations were restricted to only this region (Fig. 3), indicating that bone malformation was not a major cause of the reduced weight.
Figure 1. Comparison between body weight of PS1-cKO and control mice.
Body weight of PS1-cKO and control mice was measured at the age of 5 weeks. Mean +/− SEM body weight in PS1-cKO and control male mice (A) was 18.78 +/− 0.74 g (n=53) and 23.35 +/− 0.29 g (n=91), respectively. Mean +/− SEM body weight in PS1-cKO and control female mice (B) was 17.06 +/− 0.53 g (n=40) and 19.15 +/− 0.20 g (n=77), respectively. The difference in the bodyweight means between the PS1-cKO and control mice was significant in (A) males (P < 1×10−8) and (B) females (P < 0.0005), respectively. Blue bar = mean body weight.
Figure 2. External appearance of PS1-cKO mouse.
Lightweight PS1-cKO mice had a shortened trunk in addition to decreased body-weight compared to control mice (4 weeks old, male)
Figure 3. Bone formation in PS1-cKO mice.
The skeleton of PS1-cKO and control mice was examined by alizarin red and alcian blue staining on postnatal day 1. Ossified, calcium-containing tissues stained in red in (B) PS1-cKO mice were comparable to those in (A) control mice. A slight defect in cartilage (stained in blue) was detected in the occipital region of the PS1-cKO mice, as indicated by the arrows (otic capsule) and arrowheads (the 1st and 2nd vertebrae) in (B). Bar = 5 mm.
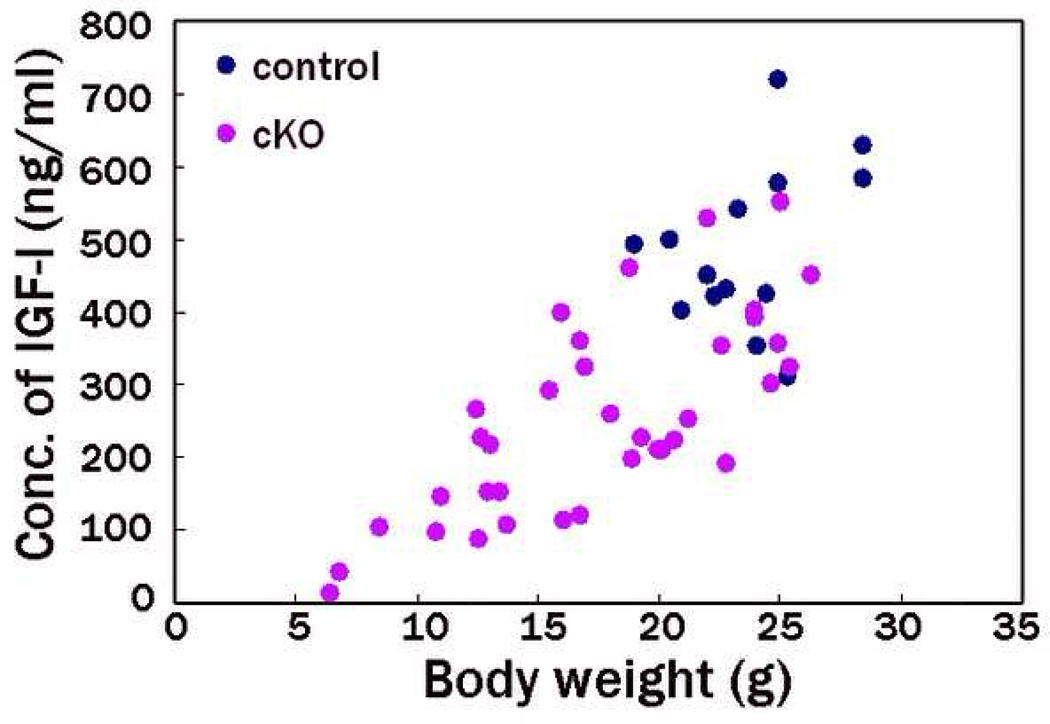
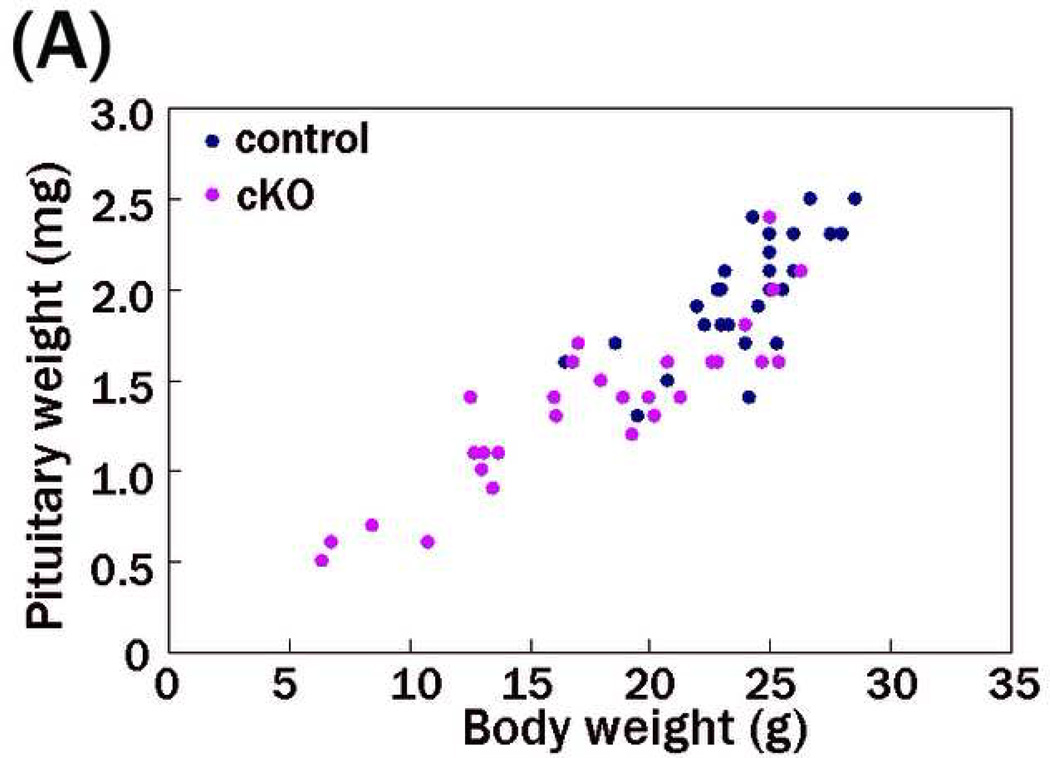
We then focused on hormonal regulation abnormalities as a cause of the reduced weight. As the phenotype of reduced weight in the cKO male mice was more severe than that in females, we used male mice for subsequent analyses. Because we failed to detect GH in the sera from the cKO and control mice by radioimmunoassay methods (< 3.2 ng/ml in 5-week-old mice), we measured serum IGF-1 levels by enzyme-linked immunosorbent assay instead. IGF-1 expression is regulated by GH and mediates many of the postnatal effects of GH (Daughaday & Rotwein 1989). The mean serum IGF-1 levels in PS1-cKO were significantly reduced (P < 1×10−6; Fig. 4). The lightweight mutant mice exhibited low serum IGF-1 levels, while IGF-1 levels of the non-lightweight mutants were comparable to those of control mice. We then measured the pituitary gland weight in the mutants. Mean pituitary weight in PS1-cKO was significantly reduced (P < 1×10−6). The lightweight mutant mice showed a reduced pituitary weight, whereas the pituitary weight of the non-lightweight mutants was comparable to that of control mice (Fig. 5), indicating that the lightweight PS1-cKO mice had a small pituitary with reduced IGF-1 expression.
Figure 4. Serum IGF-I deficiency in PS1-cKO mice.
Serum IGF-I levels in PS1-cKO and control male mice were measured at the age of 5 weeks by enzyme-linked immunosorbent assay. The IGF-1 concentration values are plotted on the ordinate corresponding to each body weight on the abscissa. Mean +/− SEM serum IGF-1 level in PS1-cKO and control mice was 253 +/− 134 ng/ml (n=36) and 482 +/− 111 ng/ml (n=15), respectively. The difference of the means is significant (P < 1×10−6).
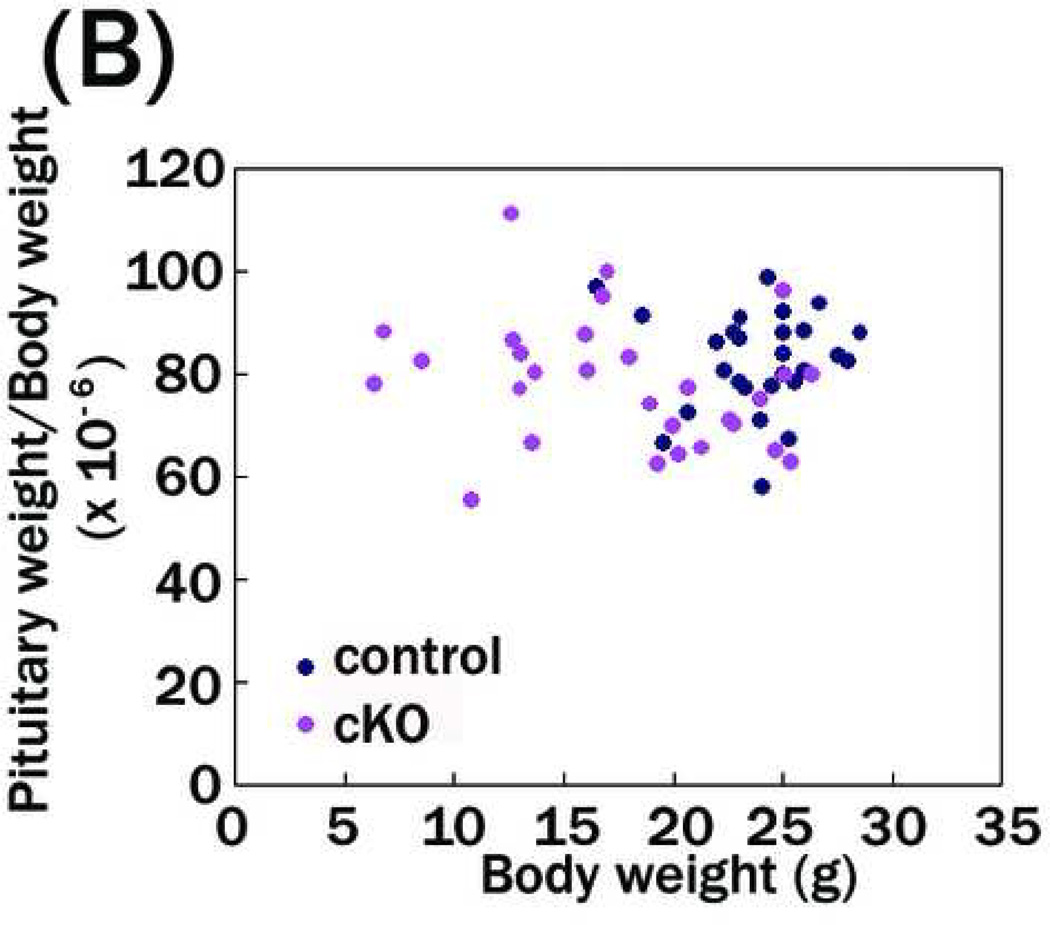
Figure 5. Reduced pituitary weight in PS1-cKO mice.
Pituitary weight in PS1-cKO and control male mice was measured at 5 weeks of age. The values of pituitary weights or pituitary weight/body weight are plotted on the ordinate corresponding to each body weight on the abscissa. (A) Mean +/− SEM pituitary weight in PS1-cKO mice (1.36 +/− 0.45 mg, n=29) was significantly lower than that in control mice (1.99 +/− 0.33 mg, n=28; P < 1×10−6). (B) Mean pituitary weight/body weight in PS1-cKO mice ((78.26 +/− 2.32)×10−6, n=29) was not different from that in control mice ((82.67 +/− 1.80)×10−6, n=28; P = 0.14).
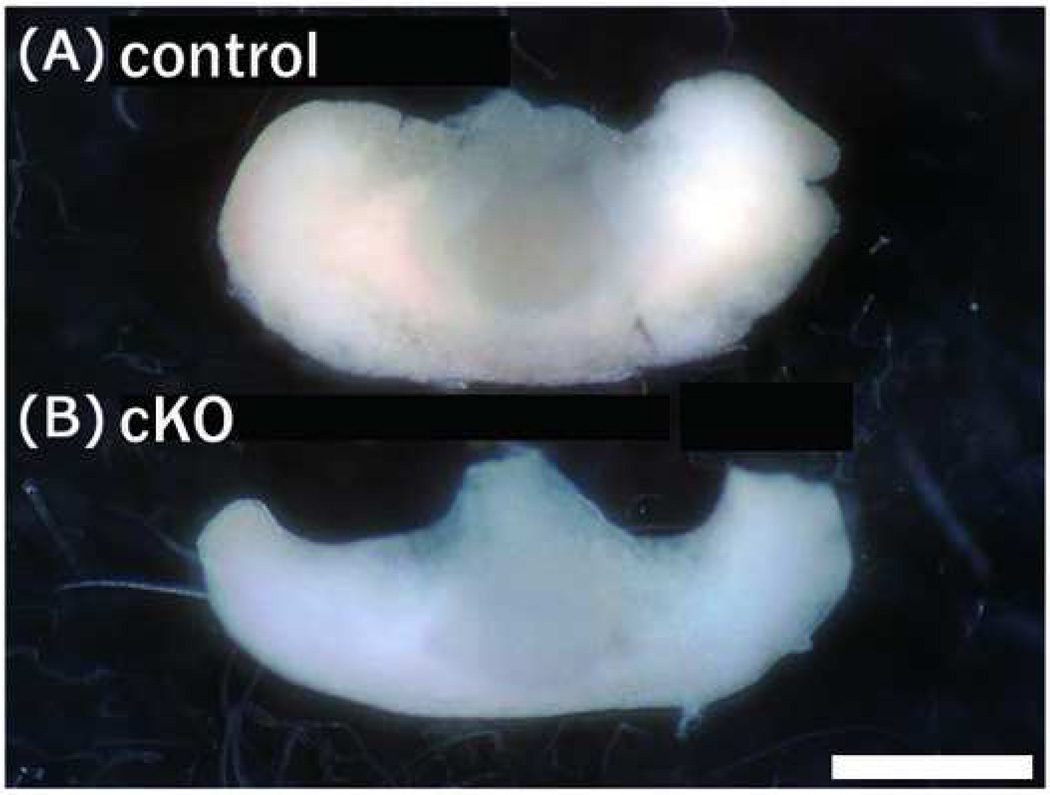
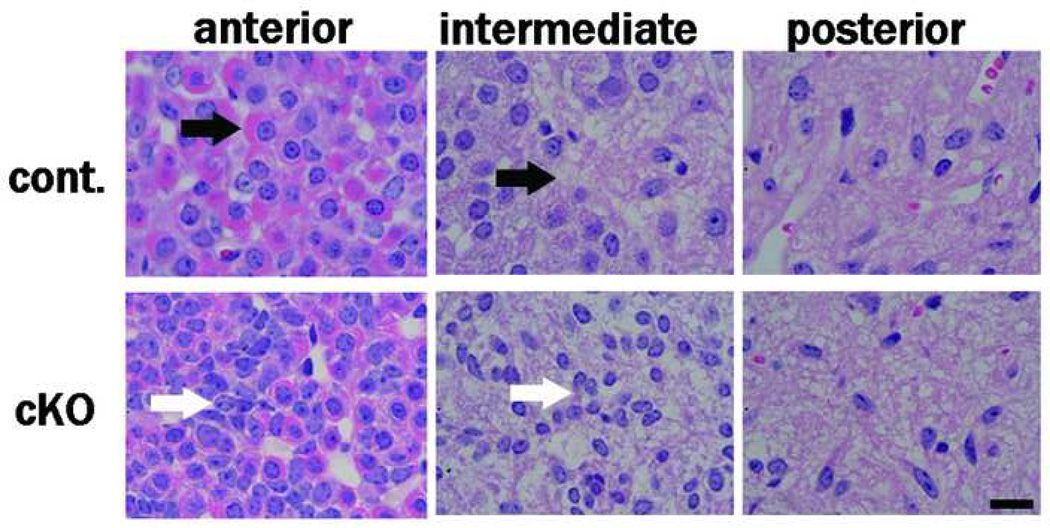
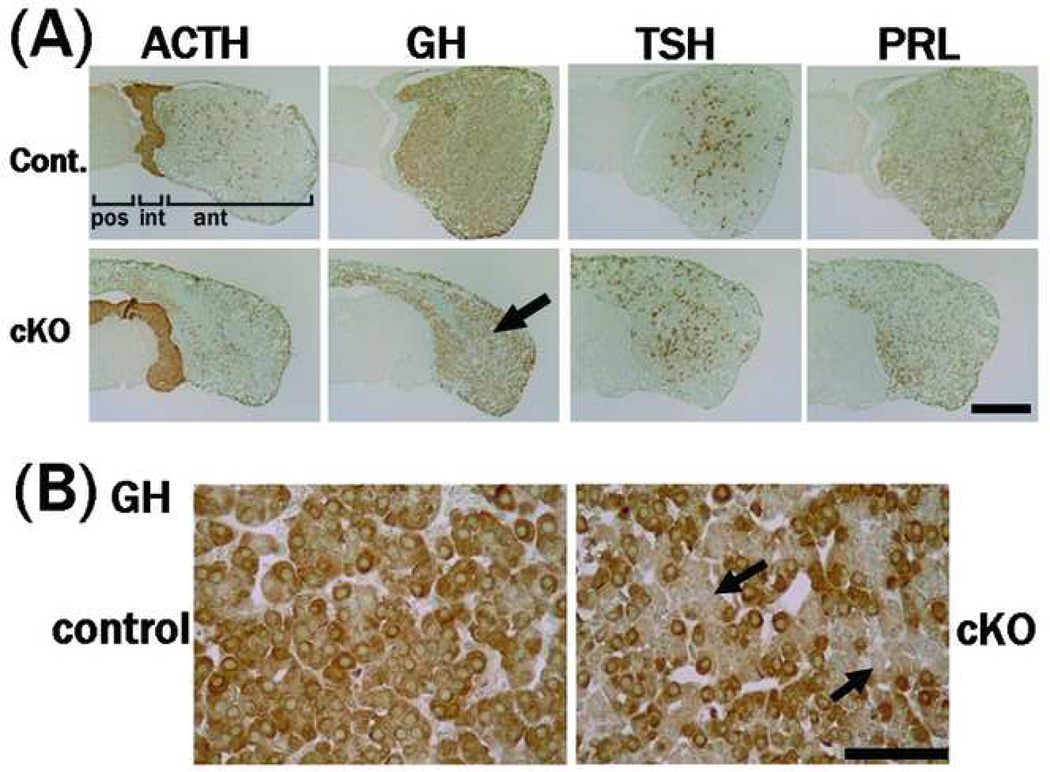
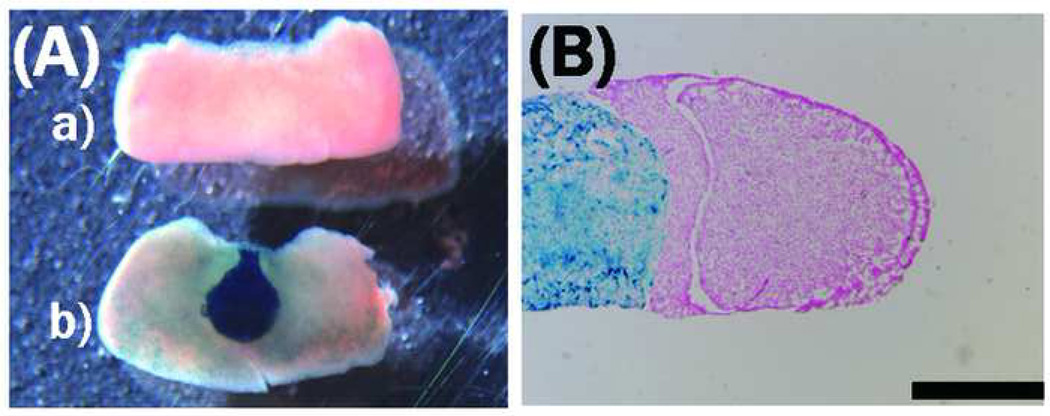
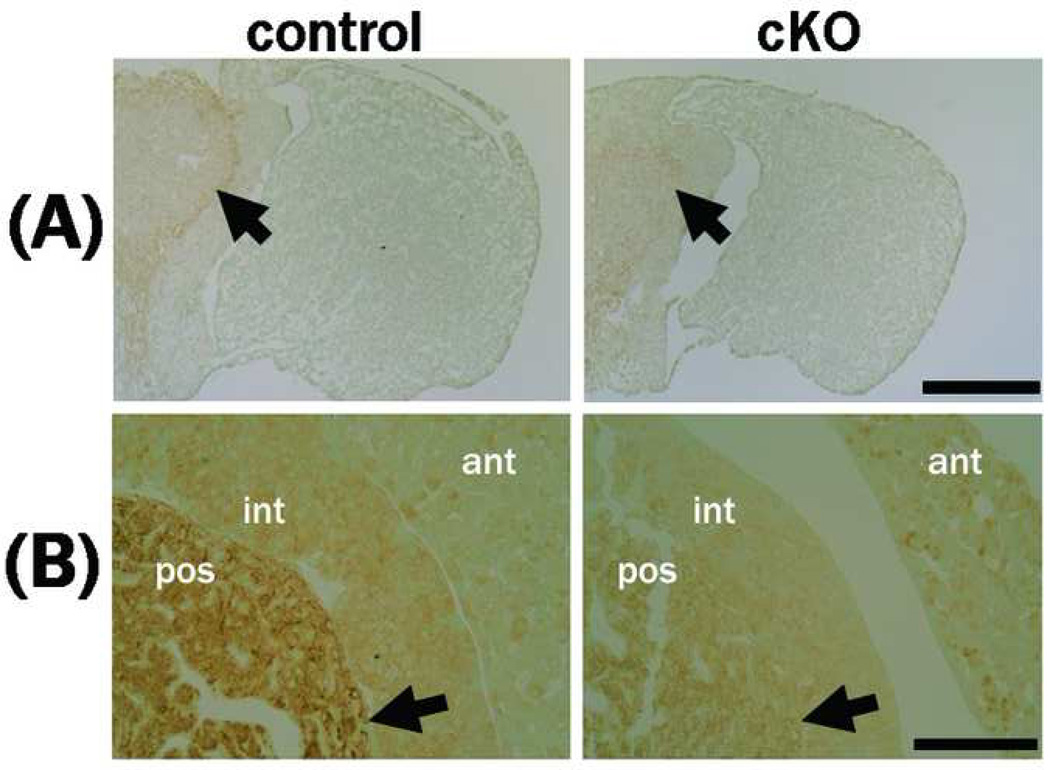
The morphologic defect of the pituitary in the mutant mice in which the anterior lobe is contracted is shown in Fig. 6. Histologic analysis using hematoxylin eosin staining revealed severe atrophy of the cytosol in the anterior and intermediate lobes of the mutant (Fig. 7). Immunohistochemistry did not reveal clear differences in the expression levels of TSH, ACTH, or PRL in the mutant pituitary (Fig. 8). The GH expression levels, however, were reduced in the anterior lobe of the mutant; cells with no GH immunoreactivity were increased in the anterior lobe of the mutant compared to the control (Fig. 8), and the anterior region containing GH-producing cells was particularly small in the mutant (Fig. 6).
Figure 6. Atrophy in the anterior pituitary of PS1-cKO mice.
Appearance of the pituitary in (B) PS1-cKO and (A) control male mice was compared at the age of 5 weeks. Atrophy in the anterior region of the mutant is evident. Body weight; control 21 g, cKO 11 g. Bar = 1 mm.
Figure 7. Atrophy of the cytosol in the anterior and intermediate lobes of PS1-cKO mice.
HE staining of paraffin sections revealed the histologic abnormality in the pituitary of PS1-cKO male mice (5 weeks old). The interval between the nuclei is reduced in the anterior and intermediate lobes, but not in the posterior lobe, of the PS1-cKO mice. The contraction of the cytosol is obvious in the anterior and intermediate lobes of the mutant (white arrows). The black arrows indicate mature cytosol in the anterior and intermediate lobes of the control. Bodyweight; control 21 g, cKO 11 g. Bar = 10 mm.
Figure 8. Immunohistochemical analysis of pituitary hormones.
(A) Cryostat sections of the pituitary were stained with anti-ACTH, -GH, -TSH, or –PRL antibodies. The immunoreactivities for ACTH, TSH, or PRL in PS1-cKO pituitary were comparable with that in control. Reduction of the GH-immunoreactivities was detected in the anterior lobe of PS1-cKO mice (male, 5 weeks old). (B) Immunohistochemistry using paraffin sections revealed sporadic GH-immunoreactivities in the mutant anterior lobe (male, 5 weeks old). The arrows in (A) and (B) indicate the decrease in GH immunoreactivity. Body weight; control 25 g, cKO 13 g (A), control 23 g, cKO 13 g (B). ant, anterior lobe; int, intermediate lobe; pos, posterior lobe. Bar = 0.4 mm in (A) or 50 mm in (B).
To identify the areas lacking the Ps1 gene in the PS1-cKO mice, X-gal staining of the pituitary was performed using ROSA26 reporter mice crossed with the Wnt1-cre mice. X-gal staining suggested a defect of the floxed Ps1 gene in the posterior lobe, but not in the anterior or intermediate lobes (Fig. 9). Reduced PS1 protein levels in the posterior lobe in the mutant were confirmed by immunohistochemistry using anti-PS1 antibody, whereas residual PS1-protein expression was present in the anterior lobe (Fig.10).
Figure 9. Distribution of cells in the pituitary in which loxP sites were recombined by Wnt1-cre allele.
Presence of a conditional Rosa26-LacZ allele allowed X-gal staining of cells in which loxP sites were recombined by Wnt1-cre allele. Most cells were stained with X-gal reagent in the posterior lobe of mice carrying the deleter Wnt1-cre allele (Wnt1-cre +/Tg;Rosa26-LacZ +/floxed) (A-b), but not in control mice (Wnt1-cre +/+;Rosa26-LacZ +/floxed) (A-a). The pituitary of (A-b) was sectioned and counterstained with nuclear fast red reagent (B). Bar = 0.4 mm in (B).
Figure 10. Immunohistochemistry for PS1 in the pituitary.
(A) Cryostat sections or (B) paraffin sections from the pituitary were stained with anti-PS1-CTF antibody. Reduction of the PS1-immunoreactivities is obvious in the anterior lobe of PS1-cKO male mice (5 weeks old). The arrows indicate the boundary between the anterior and intermediate lobes. ant, anterior lobe; int, intermediate lobe; pos, posterior lobe. Bar = 0.4 mm in (A) or 0.1 mm in (B).
Discussion
To elucidate the possible role of PS1 in the neural crest cell lineage, we developed Wnt1-cre-induced PS1-cKO mice in which PS1 is lacking in the tissues comprising neural crest-derived cells. We failed to detect any obvious abnormalities in the embryos of PS1-cKO mice, in which deletion of the floxed PS1 gene with cre recombinase expressed under the control of the Wnt1 promoter was confirmed by whole mount X-gal staining in the peripheral nervous system, such as the dorsal root ganglia, craniofacial regions, cerebellum, mesencephalon, and diencephalon (data not shown). Several weeks after birth, however, the mutant mice exhibited an unexpected phenotype of reduced weight. Several lines of evidence, including the reduction of IGF-I, pituitary weight, and GH immunoreactivity in the anterior lobe of the pituitary, indicate that PS1-cKO mice might be an animal model of dwarfism with hypopituitarism.
PS1-cKO mice showed great variations in body weight, IGF-I level, and pituitary weight. Uneven expression of the Wnt1 promoter or inconsistent efficiency of the Cre recombinase would not account for the variations because lacZ expression by the Wnt1-cre allele was detected almost all over the posterior lobe of ROSA26 reporter mice (Figure 9) and PS1 protein was completely defective in the mutant posterior lobe (Figure 10). One possible reason for this discrepancy is the usage of C57BL/6J and 129 hybrid-background mice. Presenilin-2 (PS2), a highly homologous protein to PS1, is known to have functional redundancy with PS1 (Donoviel et al., 1999). Differential PS2 expression due to differences in the mouse strain background might lead to great variations in the phenotype.
GH regulates the expression of IGF-1 from the liver and peripheral tissues. IGF-1 acts on target tissues by autocrine, paracrine, and endocrine mechanisms. Consequently, the effects of GH on somatic growth are achieved by the direct actions of GH and indirect actions of IGF-1 (Daughaday & Rotwein 1989). The actions of the GH/IGF-1 axis involve postnatal growth, including bone development (Donahue & Beamer 1992; Baker et al. 1993; Mohan et al. 2003). Thus, the decreased IGF-1 levels in the sera from PS1-cKO mice may be due to the reduced production of GH in the pituitary and the defect of the GH/IGF-1 axis results in the reduced body weight and shortened trunk in the mutant.
PS1 is a major component of γ-secretase, which processes the intramembrane or cytoplasmic domain of several membrane proteins, such as amyloid precursor protein, Notch, and cadherins (De Strooper et al. 1999; Marambaud et al. 2002; Marambaud et al. 2003; Koo & Kopan 2004). The subsequent localization to the nucleus of the processed cytoplasmic domains of these proteins is suggested to mediate the PS1 signal transduction. Recently, the GH receptor was reported to be a new member of these γ-secretase substrates (Cowan et al., 2005). Accordingly, GH receptor processing and its potential downstream event, such as IGF-1 expression, might be affected by the conditional inactivation of floxed PS1 alleles in the neural crest cell lineage of the PS1-cKO mice. The crucial reduction in serum IGF-1 levels in the mutant mice demonstrated in the present study would be, however, not due to the abrogation of GH receptor processing because the liver, the major IGF-1-releasing organ, is not derived from neural crest and PS1 is not inactivated in the liver.
Morphologic and histologic analyses of the pituitary revealed why the pituitary weight correlated well with body weight in the PS1-cKO mice. The lightweight PS1-cKO mice had lightweight pituitaries, not just because they are small, but because the pituitaries were atrophied in specific regions such as the anterior and intermediate lobes, and the defect in the anterior lobe might have led to the reduction in GH immunoreactivity, IGF-1 levels, and body weight.
The oral ectoderm forming Rathke’s pouch arises as a midline structure from the anterior neural ridge immediately anterior to the adjacent region of the neural plate from which the neural ectoderm of the diencephalon later develops (Couly & Le Dourain 1988; Eagelson & Harris 1990). The close interaction between the oral ectoderm and the neural ectoderm is crucial for the initial development of the pituitary gland. The initial phase of pituitary development is suggested to require signals from the oral ectoderm and neural ectoderm of the ventral diencephalon. The ventral diencephalic signals include members of the bone morphogenetic protein, fibroblast growth factor, and Wnt gene families. The onset of expression of these factors coincides with the initial development of Rathke’s pouch from the oral ectoderm (Treier et al. 1998). In the present study, we found an isolated GH deficiency in the PS1-cKO mice. The sites of pathology were restricted to the GH-producing somatotropes in the anterior lobe of the mutant pituitary, whereas the PS1 deficiency was detected in the posterior lobe, but not the anterior or intermediate lobes. The hint to solving this paradox may be in the reciprocal extrinsic-signaling system between the primordia of the anterior and posterior lobes in the developing stages, as mentioned above. The simplest scenario is that the defects of extrinsic signaling molecules such as bone morphogenetic proteins, fibroblast growth factors, or others are triggered by a PS1 deficiency in the posterior lobe, which affects the development and/or maintenance of the anterior and intermediate lobes in the pituitary gland.
It remains a question as to how isolation in the pituitary hormone deficiency is established in the PS1-cKO mice. One possible explanation is that hypothalamic modulators such as GH-releasing hormone or somatostatin are affected by a PS1 deficiency. If GH release was disturbed by changes in these hypothalamic modulators, GH immunoreactivity would increase in the pituitary anterior lobe, but GH immunoreactivity was rather reduced in the mutant pituitary. Another possible explanation is that extrinsic signaling molecules from the posterior lobe are defective in the developing mutant mice, which could affect anterior lobe development. Alterations in the extrinsic signals from the mutant posterior lobe might affect the expression of critical molecules in the anterior lobe, such as HESX1, which is a causative gene for isolated GH deficiency (Brickman et al. 2001) or Math3, which is a gene specifically required for GH and GHRHR expression (Zhu et al., 2006).
Notch signaling, which has PS1-dependency, is the major stream of underlying molecular mechanisms in pituitary morphogenesis. Mammals have four Notch receptors and five ligands. Following ligand-receptor binding, Notch receptors undergo successive proteolytic cleavages that read to the release of the Notch intracellular domain and subsequent nuclear translocation and gene expression. It was recently demonstrated that Notch signaling is involved in the development of the anterior lobe of the pituitary gland (Zhu et al., 2006). Notch signaling is required for sustained expression of the tissue-specific paired-like homeodomain transcription factor, Prop1, which is required for the generation of the Pit1-positive cell lineage (Pit1 is a tissue-specific POU-class homeodomain transcription factor). Attenuation of Notch signaling is necessary for terminal differentiation in post-mitotic Pit1-positive cells. At this time point, the expression of Math3 gene which is repressed by Notch signaling and activated by Pit1, is promoted. The protein product of Math3 is suggested to be specifically required in somatotropes for the expression of GH and GHRHR genes, as described above. Math3−/− mice express postnatal dwarfism (Tomita et al., 2000). These Notch-mediated developmental regulations in the anterior lobe might be affected indirectly by the PS1 defect in the posterior lobe of the pituitary gland.
Congenital GH deficiency encompasses a group of different etiologic disorders. It occurs in isolation (isolated GH deficiency) or in association with other pituitary hormone deficiencies (combined pituitary hormone deficiency). Mutations within GH-1, GHRHR, and HESX1 genes are suggested to be causal for isolated GH deficiency, whereas POU1F1, PROP1, and HESX1 are causative genes for combined pituitary hormone deficiency (Dattani 2005). The findings of the present study suggest that PS1 is a possible candidate gene for isolated GH deficiency with hypoplasia in humans.
Acknowledgments
We thank Dr. Haruhiko Koseki for technical assistance with the skeletal preparation. This work was supported in part by Grant-in-Aid for Scientific Research (C) to Mitsunari Nakajima.
List of abbreviations
- PS-1
presenilin-1
- cKO
conditional knockout
- IGF-1
insulin-like growth factor-1
- GH
growth hormone
- TSH
thyroid-stimulating hormone
- ACTH
adrenocorticotropic hormone
- PRL
prolactin
References
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cremediated deletion of AP-2a causes multiple neural crest-related defects. Dev Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Brickman JM, Clements M, Tyrell R, McNay D, Woods K, Warner J, Stewart A, Beddington RS, Dattani M. Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders. Development. 2001;128:5189–5199. doi: 10.1242/dev.128.24.5189. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Couly G, Le Douarin NM. The fate map of the cephalic neural primordium at the presomitic to the 3-somite stage in the avian embryo. Development. 1988;103(Suppl):101–113. doi: 10.1242/dev.103.Supplement.101. [DOI] [PubMed] [Google Scholar]
- Cowan JW, Wang X, Guan R, He K, Jiang J, Baumann G, Black RA, Wolfe MS, Frank SJ. Growth hormone receptor is a target for presenilin-dependent gamma-secretase cleavage. J Biol Chem. 2005;280:19331–19342. doi: 10.1074/jbc.M500621200. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Molecular genetics of Alzheimer's disease. Ann Med. 1998a;30:560–565. doi: 10.3109/07853899809002605. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Presenilin mutations in Alzheimer's disease. Hum Mutat. 1998b;11:183–190. doi: 10.1002/(SICI)1098-1004(1998)11:3<183::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Dattani MT. Growth hormone deficiency and combined pituitary hormone deficiency: does the genotype matter? Clin Endocrinol (Oxf) 2005;63:121–130. doi: 10.1111/j.1365-2265.2005.02289.x. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Donahue LR, Beamer WG. Growth hormone deficiency in 'little' mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2,-1 or -4. J Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol. 1990;21:427–440. doi: 10.1002/neu.480210305. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Nakajima M, Yuasa S, Saga Y, Sakai T, Kuriyama T, Shirasawa T, Koseki H. The role of presenilin 1 during somite segmentation. Development. 2001;128:1391–1402. doi: 10.1242/dev.128.8.1391. [DOI] [PubMed] [Google Scholar]
- Koo EH, Kopan R. Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nat Med. 2004;10:S26–S33. doi: 10.1038/nm1065. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Mohan S, Richman C, Guo R, Amaar Y, Donahue LR, Wergedal J, Baylink DJ. Insulin-like growth factor regulates peak bone mineral density in mice by both growth hormone-dependent and -independent mechanisms. Endocrinology. 2003;144:929–936. doi: 10.1210/en.2002-220948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Moriizumi E, Koseki H, Shirasawa T. Presenilin 1 is essential for cardiac morphogenesis. Dev Dynamics. 2004;230:795–799. doi: 10.1002/dvdy.20098. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yuasa S, Ueno M, Takakura N, Koseki H, Shirasawa T. Abnormal blood vessel development in mice lacking presenilin-1. Mech Dev. 2003;120:657–667. doi: 10.1016/s0925-4773(03)00064-9. [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RH, 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg Lh, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, Younkin S, Kandel ER, Kirkwood A, Shen J. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Nakajima M, Aizawa H, Sahara N, Koizumi K, Sakai T, Usami M, Kobayashi S, Kuroyanagi H, Mori H, Koseki H, Shirasawa T. Impaired cell cycle control of neuronal precursor cells in the neocortical primordium of presenilin-1-deficient mice. J Neurosci Res. 2002;70:501–513. doi: 10.1002/jnr.10430. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]