For many years, the prevailing view has been that the initiating event in type 2 diabetes is development of insulin resistance in peripheral tissues that triggers overproduction of insulin by pancreatic β-cells. Eventually, β-cells reach a stage at which they are unable to compensate for the insulin resistance, resulting in hyperglycemia and overt diabetes. The underlying cause of tissue insulin resistance is not well understood, but several putative mechanisms including ectopic lipid accumulation and low-grade activation of inflammatory pathways have been proposed (1). Recent evidence suggests that β-cell failure may occur in parallel with the development of insulin resistance (2), and β-cell failure may in itself be a cause of insulin resistance (3).
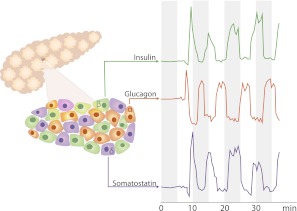
Insulin is secreted into the portal vein in a pulsatile fashion with approximately 5-min cycles (Fig. 1) (4–6). Insulin pulses may account for as much as 70% of the total insulin secretion in the basal state (4). This pulsatile β-cell secretion pattern is controlled by an intrinsic rhythm of intracellular Ca2+ oscillations (7). Adjacent β-cells in the islets adapt to each other via autocrine interaction, resulting in a coordinated secretion pattern. In addition, synchronization of all islets so that insulin release by the entire pancreas occurs in distinct peaks results from autonomic nervous system involvement (8). Interestingly, a pulsatile secretion of somatostatin takes place in phase with the insulin release, whereas pulsatile glucagon secretion is antisynchronous to that of both insulin and somatostatin (Fig. 1) (5). The resulting >20-fold variations in portal vein insulin-to-glucagon ratio may be of importance for short-term regulation of hepatic glucose metabolism.
FIG. 1.
Schematic representation of the pulsatile nature and temporal relationships of insulin, glucagon, and somatostatin secretion by glucose-stimulated human β-cells. Hormone concentration tracings from Ref. 5 with permission.
A substantial decline in β-cell function is now believed to occur in the early stages of type 2 diabetes development. β-Cell mass is reduced by approximately 40–65% when patients present with type 2 diabetes (9). To compensate for decreased β-cell mass, the remaining β-cells produce high levels of insulin, but the pulsatile secretion pattern is modified in that the insulin pulses are markedly attenuated (4). This altered insulin secretion has several consequences, including effects on the autocrine regulation of insulin secretion (10), higher plasma levels of uncleaved proinsulin (11), and development of hepatic insulin resistance in both animals and humans. In addition, more insulin seems to escape hepatic retention, leading to elevated peripheral insulin levels. Insulin resistance has partly been ascribed to decreased number and downregulation of insulin receptors with subsequent effects on the intracellular signaling system, including insulin receptor substrate 1 (IRS-1). Normally, IRS-1 binds to the cytoplasmatic portion of the activated, autophosphorylated insulin receptor and becomes phosphorylated at several tyrosine residues, thereby initiating the intracellular signaling cascade of insulin (12).
In this issue of Diabetes, Matveyenko et al. (13) present new evidence regarding the effect of different modes of intraportal insulin delivery on hepatic intracellular insulin signaling and whole-body glucose utilization in animals. Insulin administration was either pulsatile (representing the healthy state), constant rate, or with reduced magnitude of the pulses (mimicking the defective insulin secretion of type 2 diabetes). Activation of intracellular insulin signaling via IRS-1 and IRS-2, Akt, and Foxo1 was evaluated. The investigators provide evidence that phosphorylation of these signaling proteins, as well as the mRNA expression of glucokinase, was delayed and impaired following constant rate and type 2 diabetes–like insulin infusions as compared with the pulsatile infusion. These findings provide the first direct evidence that the loss of pulsatile insulin secretion leads to intrahepatic molecular changes and altered gene expression consistent with the development of hepatic insulin resistance. Moreover, pulsatile insulin administration was accompanied by a modest decrease in plasma glucose levels, whereas both the constant infusion and the type 2 diabetes–mimicking infusion resulted in increased plasma glucose levels in the fasting state. The insulin secretion pattern appears to influence both blood glucose regulation and tissue insulin resistance. Finally, to extend these short-term results, the investigators used the human islet amyloid polypeptide (HIP) transgenic rat model of type 2 diabetes in studies covering up to 12 months. Compared with age-matched Sprague-Dawley control rats, the HIP rats exhibited a progressive loss of β-cell mass, decreased mass of insulin pulses, elevated fasting levels of glucose, and decreased levels of glucokinase mRNA.
The pulsatile secretion of insulin by the β-cells is not a piece of evolutionary whimsy, yet its possible functional importance and the consequences of the loss of this function in type 2 diabetes have received only limited attention to date. Therefore, the present results by Matveyenko et al. (13) represent a welcome tour de force of new information in this area and bring to the foreground the distinct possibility that loss of insulin pulsatility and β-cell dysfunction may not only be initiating events, but also drivers in the development of hepatic insulin resistance and progression of type 2 diabetes. The extent to which the present findings for rats and dogs translate to people with type 2 diabetes needs to be evaluated. A primary role for β-cell dysfunction in the pathogenesis of type 2 diabetes may not apply to all forms of this disorder, but support for the hypothesis is provided by the fact that among the several common genetic variations associated with type 2 diabetes, β-cell α2A-adrenergic receptor is overexpressed, potentially mediating suppression of insulin secretion (14). Moreover, epidemiological data show that β-cell dysfunction, loss of pulsatile insulin secretion, and insulin resistance are frequently observed in relatives of patients with type 2 diabetes (15). Finally, the findings by Matveyenko et al. may have therapeutic implications. If confirmed, the findings imply that increasing circulating insulin levels by secretagogues or administration of long-acting insulin preparations may contribute to the development of tissue insulin resistance, and the possibility of providing insulin in a pulsatile manner should be explored (16).
ACKNOWLEDGMENTS
Å.K. is employed by Cebix. No other potential conflicts of interest relevant to this article were reported.
The excellent work with the illustration by Gunilla Elam Design, Stockholm, is gratefully acknowledged.
Footnotes
See accompanying original article, p. 2269.
REFERENCES
- 1.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting beta-cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95:4206–4216 [DOI] [PubMed] [Google Scholar]
- 3.Schofield CJ, Sutherland C. Disordered insulin secretion in the development of insulin resistance and type 2 diabetes. Diabet Med. 24 March 2012 [Epub ahead of print] [DOI] [PubMed]
- 4.Pørksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol 1995;269:E478–E488 [DOI] [PubMed] [Google Scholar]
- 5.Hellman B, Salehi A, Gylfe E, Dansk H, Grapengiesser E. Glucose generates coincident insulin and somatostatin pulses and antisynchronous glucagon pulses from human pancreatic islets. Endocrinology 2009;150:5334–5340 [DOI] [PubMed] [Google Scholar]
- 6.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab 2000;85:4491–4499 [DOI] [PubMed] [Google Scholar]
- 7.Gylfe E, Grapengiesser E, Hellman B. Propagation of cytoplasmic Ca2+ oscillations in clusters of pancreatic beta-cells exposed to glucose. Cell Calcium 1991;12:229–240 [DOI] [PubMed] [Google Scholar]
- 8.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 2000;43:393–410 [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 10.Stapelfeldt W, Bender H, Schusdziarra V, Pfeiffer EF. Effect of continuous and oscillatory portal vein insulin infusion upon glucose-induced insulin release in rats. Res Exp Med (Berl) 1984;184:67–71 [DOI] [PubMed] [Google Scholar]
- 11.Temple RC, Carrington CA, Luzio SD, et al. Insulin deficiency in non-insulin-dependent diabetes. Lancet 1989;1:293–295 [DOI] [PubMed] [Google Scholar]
- 12.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005;87:99–109 [DOI] [PubMed] [Google Scholar]
- 13.Matveyenko AV, Liuwantara D, Gurlo T, et al. Pulsatile portal vein insulin delivery enhances hepatic insulin action and signaling. Diabetes 2012;61:2269–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosengren AH, Jokubka R, Tojjar D, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 2010;327:217–220 [DOI] [PubMed] [Google Scholar]
- 15.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med 1988;318:1225–1230 [DOI] [PubMed] [Google Scholar]
- 16.Mirbolooki MR, Taylor GE, Knutzen VK, Scharp DW, Willcourt R, Lakey JR. Pulsatile intravenous insulin therapy: the best practice to reverse diabetes complications? Med Hypotheses 2009;73:363–369 [DOI] [PubMed] [Google Scholar]