Abstract
Rapamycin/interleukin-2 (IL-2) combination treatment of NOD mice effectively treats autoimmune diabetes. We performed a phase 1 clinical trial to test the safety and immunologic effects of rapamycin/IL-2 combination therapy in type 1 diabetic (T1D) patients. Nine T1D subjects were treated with 2–4 mg/day rapamycin orally for 3 months and 4.5 × 106 IU IL-2 s.c. three times per week for 1 month. β-Cell function was monitored by measuring C-peptide. Immunologic changes were monitored using flow cytometry and serum analyses. Regulatory T cells (Tregs) increased within the first month of therapy, yet clinical and metabolic data demonstrated a transient worsening in all subjects. The increase in Tregs was transient, paralleling IL-2 treatment, whereas the response of Tregs to IL-2, as measured by STAT5 phosphorylation, increased and persisted after treatment. No differences were observed in effector T-cell subset frequencies, but an increase in natural killer cells and eosinophils occurred with IL-2 therapy. Rapamycin/IL-2 therapy, as given in this phase 1 study, resulted in transient β-cell dysfunction despite an increase in Tregs. Such results highlight the difficulties in translating therapies to the clinic and emphasize the importance of broadly interrogating the immune system to evaluate the effects of therapy.
Type 1 diabetes (TID) results from immune-mediated destruction of pancreatic β-cells, leading to hyperglycemia and life-long dependence on exogenous insulin. Although targeting T cells, B cells, or costimulation have all induced transient maintenance of β-cell function in patients with recently diagnosed T1D, treatment resulting in lasting remission of disease remains elusive (1–4). Thus, alternative approaches, such as combination therapy, may be required to treat and prevent T1D.
An imbalance of autoreactive regulatory and effector cells is important in T1D pathogenesis. One predominant family of CD4+ regulatory T cells (Tregs) express high levels of CD25 (the high-affinity interleukin-2 [IL-2] receptor), and the forkhead box P3 (FOXP3) transcription factor. Impaired Treg function and decreased Treg persistence in response to IL-2 are present in T1D patients (5) and occur along with apparent increased activity of pathogenic T-effector cells (Teffs) (6).
Rapamycin is used in transplantation due to its ability to block activation of the mammalian target of rapamycin complex 1 (mTORC1), which results in growth factor–driven cell-cycle progression (7). Rapamycin preferentially inhibits proliferation of T helper (Th)1 and Th17 Teffs, with a weaker effect on Tregs, which do not require mTORC1 signaling for survival and growth (8–10). In a small study of long-standing T1D subjects, low doses of rapamycin enhanced FOXP3+ Treg function (11).
IL-2 acts on multiple cell types expressing the IL-2 receptor (IL-2R) (12), including CD25hi Tregs, which are highly IL-2 dependent (13). In NOD mice and T1D patients, defects in IL-2 production and IL-2R signaling appear to play a central role in T1D (14). In the NOD mouse, IL-2 both prevents and reverses hyperglycemia through selective activation and expansion of FOXP3+ Tregs (15–17). Moreover, in recently reported clinical trials of low-dose IL-2 in graft-versus-host disease (GVHD) (18) and autoimmune vasculitis (19), IL-2 therapy resulted in increased Treg numbers and beneficial clinical outcomes in some patients.
Treatment of NOD mice with rapamycin and IL-2 resulted in prevention of both spontaneous diabetes and recurrent diabetes after islet transplant (20). We performed an open-label phase 1 clinical trial in adult T1D subjects with rapamycin (Rapamune) plus IL-2 (Proleukin). β-Cell function, based on C-peptide values, transiently declined, and significant changes were observed in Tregs, Teffs, and natural killer (NK) cells. Thus, rapamycin/IL-2 therapy boosted Tregs, but also promoted a proinflammatory environment that may have resulted in transient β-cell dysfunction. Clinical and mechanistic data from this study will help guide future uses of IL-2 and combination therapy.
RESEARCH DESIGN AND METHODS
Trial design and patient characteristics.
After institutional review board approval and informed consent at Benaroya Research Institute, Oregon Health Sciences University, or Columbia University, subjects were screened for trial entry with the aim to enroll 10 subjects (21). Otherwise-healthy, autoantibody-positive subjects between 4 and 48 months from T1D diagnosis with peak C-peptide of ≥0.4 pmol/mL after mixed-meal tolerance test (MMTT) were eligible. IL-2 (Proleukin) was administered at 4.5 × 106 IU s.c., three times per week for 4 weeks for a total of 12 doses. Rapamycin (Rapamune or Sirolimus) was administered without a loading dose at 2 mg/day, with adjustments to maintain trough blood levels of 5–10 ng/mL for 3 months (Supplementary Fig. 1). Subjects also received prophylactic trimethoprim and sulfamethoxazole (Septra). All subjects met regularly with diabetes educators with the aim of achieving intensive diabetes control, targeting fasting values between 80 and 120 mg/dL and postprandial values <180 mg/dL.
Autoantibodies.
GAD-65 and insulinoma-associated protein 2 were measured with radioimmunobinding assays. If other autoantibodies were negative, islet-cell autoantibodies were measured with indirect immunofluorescence (22).
C-peptide.
Plasma C-peptide levels were measured (21) at the NorthWest Lipid Laboratories at the University of Washington (Seattle, WA) using a two-site immunoenzymometric assay performed on a Tosoh 600 II autoanalyzer (21). The lower limit of quantification was 0.013 pmol/mL and interassay and intra-assay coefficients of variation were <10%. Results reported as less than the lower limit of detection were imputed as half the lower limit of detection. C-peptide area under the curve (AUC) was calculated using the trapezoidal rule for values through 120 min and expressed as picomoles per milliliter per minute.
Flow cytometry.
For phenotypic stains, peripheral blood mononuclear cells (PBMCs) were thawed and stained for two surface panels and an intracellular panel. Cells were stained with CD4 AmCyan, CD45RO allophycocyanin (APC)-H7, CD25 phycoerythrin (PE), CD127 V450, CXCR3 PE-Cy5, CCR6 PerCP-Cy5.5, CCR7 PE-Cy7, CD38 Alexa700, CD8 eFluor650NC, CRTh2 Alexa647, and CCR4 fluorescein (FITC) (panel 1) and CD3 V450, CD4 AmCyan, CD16 APC-H7, CD38 PerCP-Cy5.5, CD8 eFluor650NC, CD56 PE, CD57 FITC, CD19 PE-Cy7, CD27 Alexa647, and CD95 PE-Cy5 (panel 2) plus a LIVE/DEAD Blue stain. For the intracellular stain, cells were stained with CD25 PE and CD45RO Alexa700 and stimulated for 6 h with phorbol myristic acid (PMA) (500 ng/mL) and ionomycin (500 pg/mL). Brefeldin A (0.5 units/mL) and myosin (0.5 units/mL) were added during the last 4 h, and cells were then fixed and stained with CD4 PE-Cy5, interferon-γ (IFN-γ) V450, IL-17 PerCP-Cy5.5, FOXP3 Alexa647, helios fluorescein isothiocyanate (FITC), and HLA DR eFluor605NC. For phosphostains, PBMCs were thawed, rested, and stained as described previously (23). After stimulation with IL-2 (100 IU/mL) or anti-CD3 (5 μg/mL) and anti-CD28 (1 μg/mL) antibody plus Fab x-linker (10 mg/mL), cells were fixed and permeabilized at designated times. IL-2–stimulated cells were stained with CD56 FITC, CD25 PE, pSTAT5(pY694) Alexa647, and CD4 PerCP. CD3/28-stimulated cells were stained with pAkt(pS473) Alexa488 or pS6(pS235/pS236) Alexa647, CD8 FITC, CD25 PE, and CD4 PerCP. Data were acquired on an LSR II or FACSCalibur and analyzed using FlowJo or Winlist. Samples from different draw dates for the same subject were assayed on the same day whenever possible.
Serum cytokine analysis.
Serum cytokine levels were measured using Searchlight chemiluminescent platform in custom-designed multiplex arrays provided by Aushon Biosystems (Billerica, MA), a Clinical Laboratory Improvement Amendments–certified contract laboratory. The serum analytes tested included IFN-γ, IL-4, IL-5, IL-6, IL-8, IL-10, IL-1β, IL-1Rα, IL-2, IL-12p70, tumor necrosis factor-α, eotaxin, IL-12p40, macrophage inflammatory protein (MIP)-1α, MIP-1β, E-selectin, IL-2RA, insulin-like growth factor–binding protein 3 (IGFBP3), IL-17, IL-13, transforming growth factor-β1 (TGF-β1), and IL-23. Samples (in duplicate) were incubated on array plates prespotted with capture antibodies specific for each cytokine. Plates were washed before adding a cocktail of biotinylated detection antibodies to each well. After incubating with detection antibodies, plates were washed and incubated with streptavidin–horseradish peroxidase conjugate followed by a chemiluminescent substrate and immediately imaged using the Aushon CCD imaging system. All incubations were performed at room temperature with shaking. Data were analyzed using Aushon Array Analyst software, and concentrations were interpolated from a standard curve (run in triplicate) using a four-parameter fit equation. All assays passed the manufacturer’s quality control criteria.
Epigenetic analysis.
Cell pellets were snap frozen, and Epiontis performed DNA isolation and real-time based quantification of Treg-specific demethylation region (TSDR) (24) and CD3 demethylation (25).
Statistics.
Change from baseline (time 0) was determined using a one-way ANOVA using Prism. For analysis of two time points, a paired Student t test was used. No adjustments were made for multiple comparisons. Unless otherwise noted, P values <0.05 were considered significant.
RESULTS
Rapamycin/IL-2 combination therapy results in a transient decrease in C-peptide levels.
Nine adults were enrolled (Table 1). Subject 5 received only 10 of 12 IL-2 doses due to abdominal pain likely related to therapy. Review of 3-month MMTT data in the first seven subjects demonstrated that all had a marked decrease of β-cell function (Fig. 1). Further enrollment was halted, and subjects 8 and 9, who had already completed their IL-2 course, were instructed to stop ongoing rapamycin therapy at days 78 and 28, respectively (Table 1 and Supplementary Fig. 1). Follow-up of subjects through 3 years continues as per protocol. All subjects experienced transient eosinophilia and IL-2 injection site reactions, including firm nodules of 1–3 cm and erythema in some individuals as much as 9 cm in diameter. Additional adverse effects were fatigue and malaise during the first month of treatment.
TABLE 1.
Subject characteristics
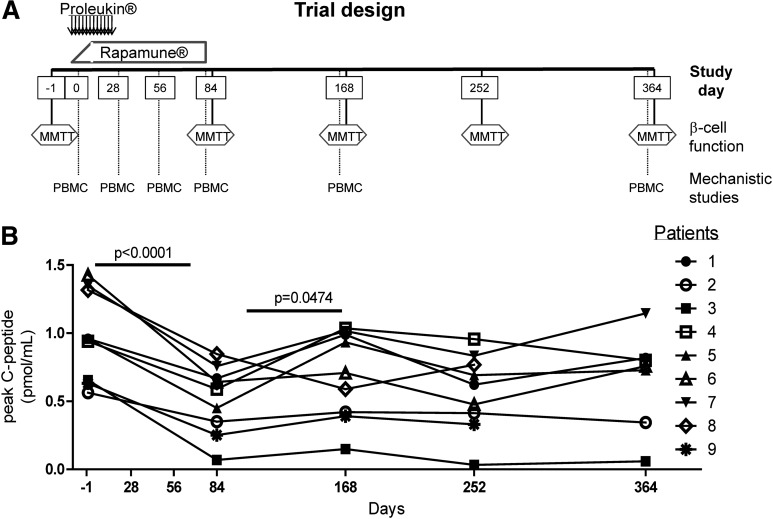
FIG. 1.
Transient decrease in β-cell function with rapamycin/IL-2 combination therapy. Trial design is shown in A for which T1D patients were treated with 4.5 × 106 IU IL-2 s.c. three times per week for 1 month and rapamycin 2–4 mg/day orally for 3 months with target blood rapamycin trough levels of 5–10 ng/mL (see Supplementary Fig. 1). β-Cell function was monitored using an MMTT, and PBMCs were collected for mechanistic studies on the indicated days. B: Peak C-peptide values from the MMTT were assessed for all subjects over time. Statistical significance was determined using a paired Student t test comparing screening and day 84 and days 84–168.
All subjects had a decrease in C-peptide at the first assessment at 3 months, including subjects 8 and 9, who were not receiving rapamycin therapy at the time of testing (day 0 mean = 8.926 pmol/mL and day 84 mean = 4.693 pmol/mL; P < 0.0001) (Fig. 1). This was associated with a stable HbA1c achieved with increasing doses of insulin (Supplementary Fig. 2). The mean rate of AUC C-peptide fall was 0.104 pmol/mL/month or 43% over 3 months. This is markedly greater than the expected rate of decline postdiagnosis in an adult with T1D (3,4,26–28). However, in almost all subjects, the subsequent C-peptide values increased by day 168 (day 84 mean = 4.693 pmol/mL and day 168 mean = 6.321 pmol/mL; P < 0.0474). Based on these data, combination therapy resulted in a transient decrease in β-cell function.
Rapamycin/IL-2 combination therapy in T1D patients transiently increases FOXP3+ natural Tregs.
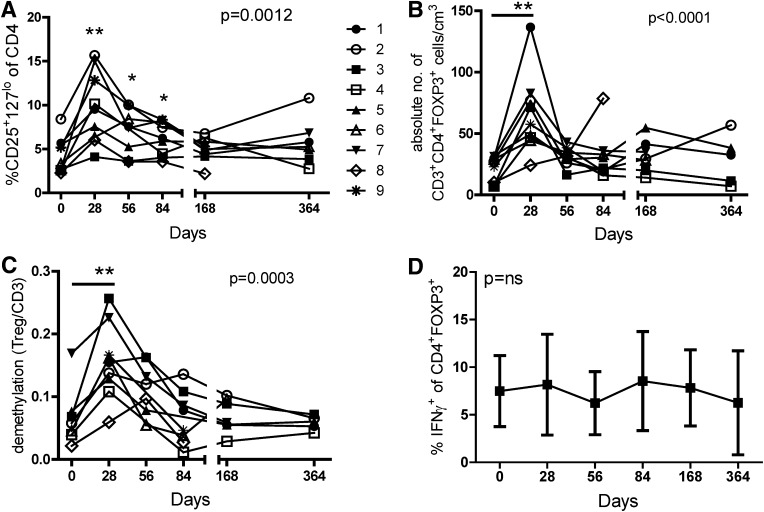
To determine whether Tregs were augmented by combination therapy, we analyzed CD4+CD25+CD127lo cells and FOXP3 expression in CD4+ T cells of peripheral blood (Fig. 2A and B). The frequency of CD4+CD25+CD127lo cells increased in all subjects, declining to baseline levels after completion of therapy. The absolute number of FOXP3+ cells increased from baseline to day 28, falling back to baseline by day 56 when rapamycin therapy was still ongoing.
FIG. 2.
Rapamycin/IL-2 combination therapy transiently increases nTregs in the peripheral blood. Thawed PBMCs were stained for viability, CD4, CD25, CD127, and FOXP3. A and B: The frequency of CD4+ cells that are CD25+CD127lo and the number of cells that are CD3+CD4+FOXP3+ within PBMCs are shown for each patient. C: nTregs quantified using real-time PCR analysis of the Treg-specific demethylated region of the FOXP3 gene relative to the methylated region are expressed as percentage of CD3 cells (www.epiontis.com). D: PBMCs were stimulated with PMA/ionomysin in the presence of brefeldin A/myosin prior to staining for FOXP3 and IFN-γ. Means ± SD are shown in D. Statistical significance was determined by a one-way ANOVA and is shown in each graph. Significant differences from time 0 to each time point were determined using a paired Student t test and are indicated with an asterisk. *P ≤ 0.05 and **P ≤ 0.01.
In humans, FOXP3 is expressed constitutively in Tregs and transiently in activated Teffs. However, the FOXP3 locus remains methylated in Teffs, whereas similar analysis of natural Tregs (nTregs) displays demethylation at the TSDR, making TSDR methylation a more reliable marker of stable nTregs (29). An increased frequency of FOXP3 gene demethylation was observed on day 28 (Fig. 2C) and correlated with the frequency of CD4+FOXP3+ cells measured by flow cytometry, suggesting that the increase in CD4+FOXP3+ cells was primarily due to an increase in the nTreg subset. In comparison, IFN-γ secretion by FOXP3+ cells is often indicative of activated Teffs. A limited amount of IFN-γ was secreted by CD4+FOXP3+ cells, and the frequency of CD4+FOXP3+ cells secreting IFN-γ did not increase with therapy (Fig. 2D). Thus, combination therapy promoted Treg expansion in the periphery.
Enhanced response to IL-2 in CD25+ cells of T1D patients treated with rapamycin/IL-2 combination therapy is maintained after therapy.
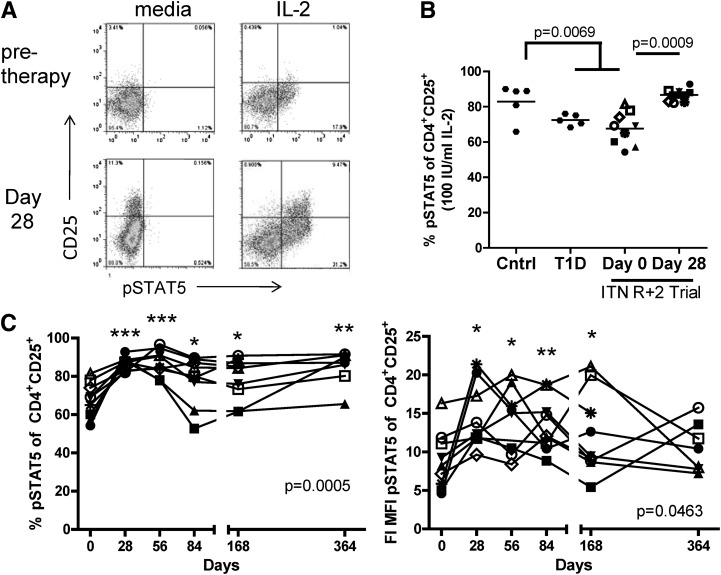
We have recently shown that IL-2 responses in CD4+ T cells of T1D subjects are decreased as compared with controls (23). To determine whether treatment with rapamycin/IL-2 altered responses to IL-2, we assessed the phosphorylation of signal transducer and activator of transcription 5 (pSTAT5) in response to IL-2 (Fig. 3A). The T1D subjects in this trial had reduced IL-2 responsiveness prior to therapy (Fig. 3B). The basal level of pSTAT5 did not change with time (Supplementary Fig. 3), but the response to IL-2 stimulation was enhanced at day 28 (Fig. 3B and C). This increased responsiveness to IL-2 was maintained after IL-2 therapy, as seen by persistently higher responses on day 56, a time point by which the frequency and level of expression of CD25 had fallen (Figs. 3C and 2A). A significant increase in the frequency of CD25+ cells responding to IL-2 was observed in the peripheral blood even as late as 1 year past initiation of therapy.
FIG. 3.
Rapamycin/IL-2 combination therapy results in a persistent enhancement of IL-2 responsiveness in CD4+CD25+ T cells. Thawed PBMCs were stimulated with media alone or 100 IU/mL IL-2 prior to fixation, permeabilization, and staining for CD4, CD25, and pSTAT5. Representative dot plots of CD4+ cells are shown in A. B: The frequency of pSTAT5+ cells of CD4+CD25+ cells (A, top) is shown for control (n = 5) and T1D (n = 5) subjects matched to subjects in the trial (noted by ITN R + 2 Trial) by HLA, age, sex, and genotype (PTPN221858 or PTPN2rs1893217). Day 0 and 28 analyses are shown for patients in the trial. Statistical significance was determined between control and T1D subjects (matched + trial) using a two-sample Student t test and between day 0 and 28 in the trial using a paired Student t test. C: The frequency and fold increase (FI) of pSTAT5 in CD4+CD25+ cells are shown over time for patients in the trial. Statistical significance was determined by a one-way ANOVA and is shown in both graphs. Significance from time 0 to each time point was determined using a paired Student t test and is indicated with an asterisk. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
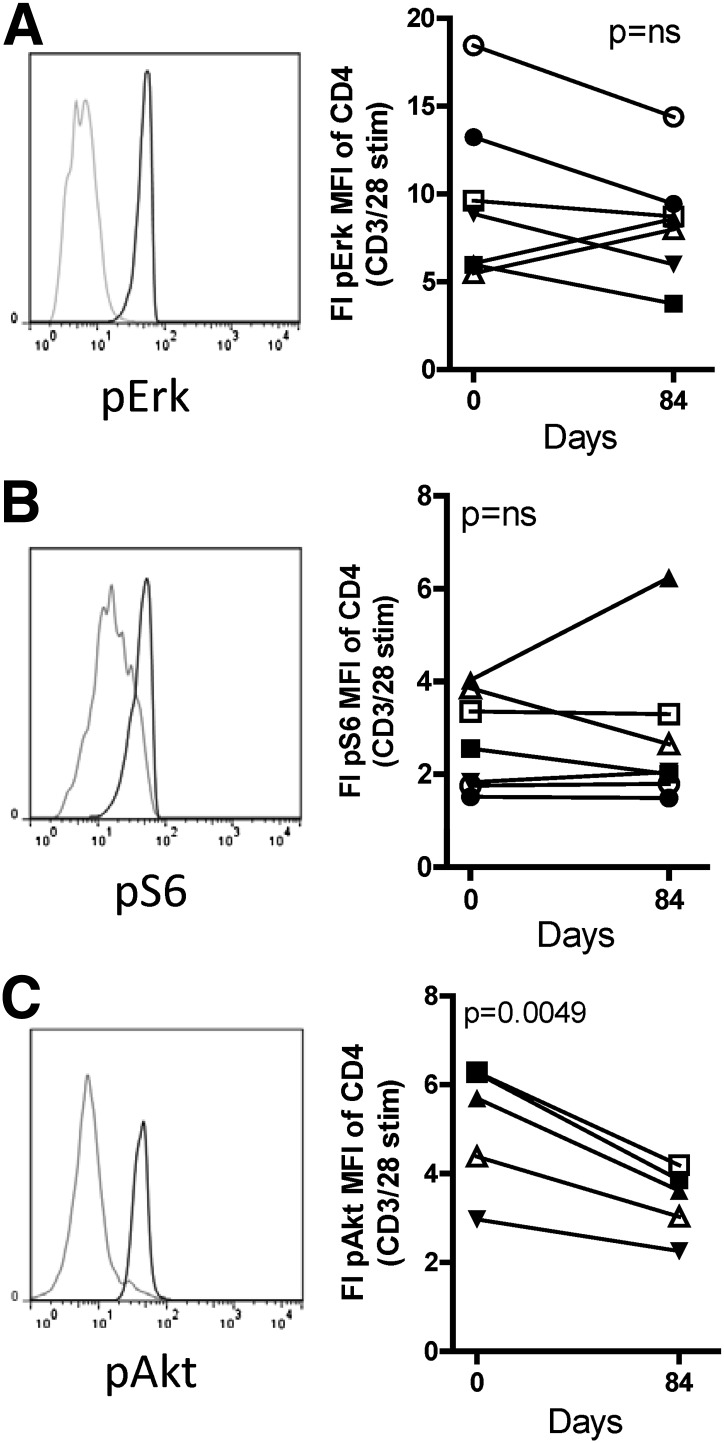
Rapamycin/IL-2 combination therapy in T1D subjects modulates pAkt, but not pS6.
Rapamycin is an mTOR inhibitor, and both S6 and Akt are in the phosphatidylinositol 3-kinase pathway and are phosphorylated after T-cell receptor ligation. The levels of pS6 and pAkt were measured in the presence or absence of anti-CD3/anti-CD28 stimulation. T-cell activation was sufficient in these assays, as demonstrated by consistent phosphorylation of extracellular signal–related kinase (Erk) (Fig. 4A), a molecule that is activated by T-cell receptor engagement but is not targeted by rapamycin. Stimulated levels of pS6 in CD4+ T cells did not change with therapy (Fig. 4B). This lack of a reduction in pS6 after rapamycin therapy was not due to a compensatory increase in total S6 expression (data not shown). After combination therapy, CD4+ cells exhibited a markedly decreased induction of pAkt upon in vitro stimulation with anti-CD3/anti-CD28 (Fig. 4C). Similar responses were observed in both CD4+ and CD8+ T cells (data not shown). The decreased induction of pAkt, but not pS6, suggests that combination therapy resulted in modulation of proximal signaling molecules within the peripheral blood T cells of subjects in our study.
FIG. 4.
Rapamycin/IL-2 combination therapy modulates pAkt, but not phosphorylated (p)Erk or pS6, in CD4+ T cells. Thawed PBMCs were stimulated with media alone or cross-linking of anti-CD3/anti-CD28–bound antibodies with Fab x-linker for 20, 30, or 10 min for Erk, S6, and Akt analysis, respectively. Cells were then fixed, permeabilized, and stained for CD4, CD8, pAkt, pErk, and pS6. Representative histograms are shown for pErk (A), pS6 (B), and pAkt (C) with media (gray line) and anti-CD3/anti-CD28 (black line) stimulation. Fold increase (FI) was determined by the equation (MFI with CD3/CD28 stim) ÷ (MFI with media) for each sample and is compared between day 0 and 84 for pErk (A), pS6 (B), and pAkt (C). Data from all subjects receiving full dosing of rapamycin are included except for pAkt for subjects 1 and 2, for which pAkt data were not acquired. Statistical significance was determined by a paired Student t test.
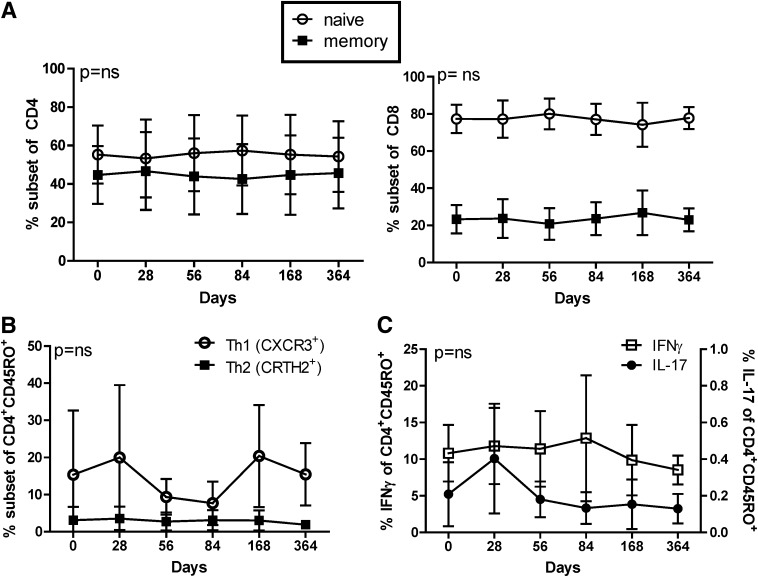
Rapamycin/IL-2 combination therapy in T1D patients does not alter CD4 or CD8 Teff differentiation.
We found no difference in autoantibody titers, absolute white blood cell counts, the frequency of lymphocytes and monocyte populations, or the CD4:CD8 ratio with combination therapy (Supplementary Table 1 and data not shown). No changes were seen in the periphery in the frequency of naive and memory CD4+ or CD8+ T cells, effector and central memory T-cell subsets, or frequency of CRTH2+ or CXCR3+ cells, indicative of Th2 and Th1 cells, respectively (Fig. 5A and B and Supplementary Fig. 4). Likewise, no change in IFN-γ secretion, a cytokine made by Th1 cells, was observed over time (Fig. 5C). Despite some variance, no significant difference was observed over time in IL-17 secretion (Fig. 5C and Supplementary Fig. 5). Activation of CD4+ and CD8+ T cells was measured by expression of CD38 and Fas, and no differences were observed over time (data not shown). Together, these data demonstrate that T-cell activation and memory composition along with CD4+ helper subset differentiation may not have been altered in the peripheral blood during combination therapy in T1D patients.
FIG. 5.
Rapamycin/IL-2 combination therapy did not alter CD4 helper subset differentiation or CD8 memory composition. Thawed PBMCs were stained for viability, CD4, CD8, CD45RO, CXCR3, and CRTH2. A: The frequency of memory CD45RO+ (solid square) and naive CD45RO− (open circles) in the CD4+ (left) and CD8+ (right) T-cell populations is shown. B: The frequency of CXCR3 and CRTH2 of CD4+CD45RO+ cells is shown as approximate markers of Th1 and Th2 cells, respectively. C: PBMCs were stimulated for 6 h with PMA/ionomysin in the presence of brefeldin A/myosin prior to staining for CD4, CD45RO, IL-17, and IFN-γ. Frequencies of IFN-γ+ (open squares, left axis) and IL-17+ (solid circles, right axis) cells of CD4+CD45RO+-gated cells are shown. Means ± SD are shown for all subjects assayed. Statistical significance was determined by a one-way ANOVA and is shown in each plot.
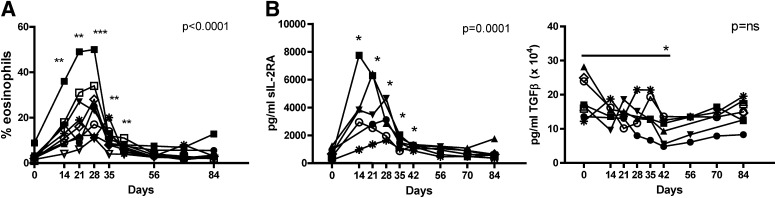
Immune activation occurred upon rapamycin/IL-2 combination therapy.
We observed significant eosinophilia in all subjects as early as day 14 (Fig. 6A), which coincided with IL-2 but not rapamycin therapy. In parallel with the increase in eosinophils was a decrease in the frequency of neutrophils in the peripheral blood (data not shown). Serum cytokine levels were assessed for all patients before and after combination therapy (day 0 vs. 84) and for a subset (n = 6) of patients at earlier time points. Significant levels of soluble IL-2Rα (sIL-2RA) were detected on days 14, 21, and 28, with levels declining back to pretreatment levels by day 35, a week after IL-2 therapy (Fig. 6B). We also observed a subtle decrease in TGF-β secretion. Notably, we did not detect significant amounts of other serum cytokines tested. Thus, a systemic excess of cytokines at and beyond day 14 is unlikely to have contributed to the decreases observed in C-peptide levels. Whether earlier production of cytokines contributed to initiation of inflammation is not known.
FIG. 6.
Rapamycin/IL-2 combination therapy increased eosinophils and sIL-2RA while decreasing serum TGF-β. A: The frequency of eosinophils in whole blood was determined in a standard hematologic assay and is shown for each subject over time. B: Serum analytes were measured using a multiplex Searchlight chemiluminiscent assay. Averages of duplicates for all subjects are shown for day 0 and 84, and subjects 1, 2, 3, 5, and 7 are shown for time points between days 14 and 70. Of the 22 cytokines and cytokine receptors tested, data are shown where statistical differences were observed. Measures are shown for each subject over time, and statistical significance was determined by a one-way ANOVA, noted in the graph. Significance from time 0 to each time point was determined using a paired Student t test and is indicated with an asterisk. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
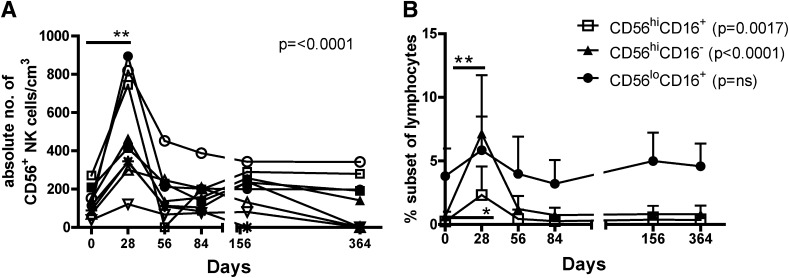
We observed a transient increase in the number of total peripheral blood CD56+ NK cells in all patients on day 28 (Fig. 7A), which declined to pretreatment levels by day 56. Within the CD56+ NK population, we observed a significant increase in CD56hi NK subsets (Fig. 7B) but no change in the CD56loCD16+ subset. CD3+CD56+ NK T-cell frequencies did not change with therapy (Supplementary Fig. 6).
FIG. 7.
Rapamycin/IL-2 combination therapy transiently augments NK cell frequency. Thawed PBMCs were stained for viability, CD56, CD3, and CD16. A: The number of CD56+CD3− NK cells of PBMCs are shown for individual subjects. B: The average ± SD frequencies for CD56hiCD16+ (open squares), CD56hiCD16− (closed triangles), and CD56loCD16+ (closed circles) subsets of CD56+ NK cells within the live lymphocyte gate are shown. Statistical significance was determined by a one-way ANOVA, noted in the graph. Significance from time 0 to each time point was determined using a paired Student t test and is indicated with an asterisk. *P ≤ 0.05, **P ≤ 0.01.
Using sIL-2RA and rapamycin trough levels as a measure of the impact of IL-2 and rapamycin, respectively, we found a direct correlation between sIL-2RA levels and the frequency of multiple cell types (NK, FOXP3, and eosinophils), whereas rapamycin trough levels did not correlate with the frequency of any of these cell populations (Supplementary Fig. 7). These data suggest a prominent role for IL-2 in the induction of phenotypes observed in this clinical trial.
DISCUSSION
Coordinated analysis of both clinical and mechanistic data during trials may provide biomarkers of safety and efficacy. Using this approach, we found that rapamycin/IL-2 combination therapy had the unwanted effect of transiently decreasing β-cell function. This occurred despite the beneficial effects of this therapy in the NOD mouse model of diabetes (20), the beneficial effects of low-dose IL-2 in GVHD (18) and vasculitis (19), and a wealth of in vitro and in vivo data supporting the hypothesis that this therapy would result in coordinated enrichment of Treg responses and inhibition of Teff responses. Treg numbers and IL-2 responsiveness in peripheral blood were boosted in patients in this trial. Yet, the number of NK cells and eosinophils also increased, suggesting that combination therapy promoted an environment that may have been responsible for the transient decrease in β-cell function. These studies test, for the first time, IL-2 and rapamycin therapy in human T1D subjects and highlight the challenges in translating studies from preclinical models. Moreover, this work suggests specific tests that may be informative in future trials to assess safety and efficacy.
On the basis of mouse models (15–17,20), we predicted that combination therapy would halt damage of β-islet cells. However, we observed a significant decline in 2-h AUC C-peptide values that occurred in all subjects at an accelerated rate, with a mean percent change of approximately four times that of comparable published control groups (3,4,26–28). Of importance, β-cell function increased at later time points. This transient decrease in β-cell function may have occurred due to IL-2 and rapamycin dosing/timing issues, combination of rapamycin with IL-2, and/or factors unique to T1D subjects. In two recently reported clinical trials, low doses of IL-2 were beneficial in promoting tolerance in GVHD and vasculitis (18,19). Although administered in different dosing schedules, the overall amount of IL-2 given in those studies over ∼2 months was similar to the amount we used (54 × 106 IU over 1 month) with 52.5 × 106 IU over 9 weeks in the vasculitis trial and the tolerated dose of ∼95 × 106 IU over 8 weeks in the GVHD trial. In our study, IL-2 was given for 1–2 weeks before rapamycin reached targeted blood levels (Supplementary Fig. 1). This is unlike the mouse models where rapamycin was initiated at full dose with IL-2 (21) and may explain some of the early proinflammatory responses, even though IL-2 doses were similar to other low-dose regimens for tolerance induction. Future studies will benefit from including early assessment of both immunological and clinical parameters as biomarkers of safety and mechanisms of action.
Although earlier observations in vitro and in transplant models have suggested that rapamycin may be β-cell toxic (30,31), evidence from this trial suggests that rapamycin may not be the primary or only cause of β-cell damage. C-peptide decline was seen even in two subjects who did not receive the full course of rapamycin, and the most significant changes in mechanistic data occurred within the first month of therapy concordant with IL-2. In addition, in long-standing T1D patients waiting for islet transplant, rapamycin monotherapy at doses similar to those used in our trial did not result in a drop in C-peptide levels (11,32). Nevertheless, in other clinical settings higher doses of rapamycin have resulted in impaired regulation of insulin levels (33). Thus, although we cannot rule out an impact of rapamycin on β-cell function, our evidence points to a detrimental effect of IL-2 or the combination of rapamycin and IL-2 in this patient population.
Rapamycin/IL-2 combination therapy resulted in a significant increase in FOXP3+ Tregs. Whether the function of FOXP3+ cells in our trial was altered by therapy was not addressed. Isolated CD25hi T cells from patients in the low-dose IL-2 trial in GVHD were functional Tregs. Yet, it is interesting that not all GVHD patients showed beneficial effects of therapy even though Tregs were significantly elevated in all study subjects. In addition, the level of Treg enhancement was highly variable in the vasculitis study (19) while all patients improved clinically, suggesting that other cells beyond Tregs may play a role.
Other cells known to be activated by IL-2 include eosinophils and NK cells, both of which increased in our trial. This response was equal to or greater than that observed with low-dose IL-2 treatment of GVHD (18) and vasculitis (19), but was not observed in NOD mice treated with IL-2, most likely due to multiple genetic defects associated with NK cells in these mice (34). The increased NK response to combination therapy occurred primarily in the CD56hi subpopulation, a population that has been shown to play both an effector and regulatory role (35). Whether NK cells from patients in this trial secrete pro- or anti-inflammatory cytokines is not known. In multiple sclerosis patients, there is an increase in CD56bright NK cells that produce IL-10 and are associated with clinical benefit upon daclizumab therapy (36). Yet, a clinical trial of daclizumab plus mycophenolate mofetil did not alter the disease course in new-onset T1D subjects (26). As in our trial, a transient increase in eosinophils has been observed in HIV patients with IL-2 monotherapy (37) and low-dose IL-2 therapy for GVHD (18) but, interestingly, not in the low-dose IL-2 vasculitis study (19). Eosinophils constitutively express the low-affinity IL-2R. Upon activation, they express CD25 and gain the potential to secrete peroxidase and IL-6 (38), suggesting that eosinophilia could also contribute to the proinflammatory responses observed during combination therapy. Future studies should address the functional properties of NK cells and eosinophils that changed upon therapy.
Remarkably, combination therapy resulted in a durable rescue of IL-2R signaling even a full year after initiation of therapy, most dramatically in the CD25+ population. Increased response to IL-2 is most likely not solely due to increased expression of CD25, since CD25 MFI (mean fluorescence intensity) transiently increased with IL-2 therapy (data not shown) and the percentage of CD25+CD127lo Tregs increased during therapy whereas response to IL-2 persisted beyond therapy. Rescue of IL-2 responsiveness is not unprecedented. In some cancer patients treated with high doses of IL-2, pSTAT5 in CD4+ and CD8+ T cells is enhanced (39), and IL-2 therapy for Wiskott-Aldrich syndrome rescues poor IL-2 responsiveness in NK cells (40). Thus, treatment with rapamycin and IL-2 may stably enhance IL-2R complex signaling molecules and/or promote selective outgrowth or homing of IL-2–responsive cells. Even though IL-2 responsiveness was maintained, the increased frequency of FOXP3+ Tregs was transient in the peripheral blood. These FOXP3+ cells may have migrated from the periphery or may have undergone activation-induced cell death upon withdrawal of IL-2.
Rapamycin has been shown to increase memory CD8+ T-cell formation, increase the Th2:Th1 ratio, and decrease activation and proliferation of CD4+ Teffs (10), whereas IL-2, when given alone, leads to an expansion of the CD8+ and CD4+ Teffs (37). Yet, we did not observe such changes in peripheral blood samples. It remains possible that once activated, the relevant cells leave the circulation and move to the tissues or secondary lymphoid populations, or that combination of rapamycin with IL-2 negated the effect of rapamycin alone on T-cell differentiation. Alternatively, T cells of T1D subjects may be relatively resistant to rapamycin due to disease-specific defects in mTOR signaling, although the reduction in pAkt after therapy suggests that rapamycin did affect the Akt/phosphatidylinositol 3-kinase pathway. Thus, the functional composition of the memory T-cell pool may have been altered with combination therapy without causing measurable changes in the frequency of memory or helper T-cell subsets.
Recently, the immunostimulatory effects of rapamycin on innate cells have been recognized (41). Although we did not test monocyte function, we did find a significant decrease in TGF-β secreted by myeloid cells. We also noted increased serum sIL-2RA, a marker of immune activation. This increase is most likely due to IL-2RA shedding upon activation (42). Whether sIL-2RA has any biological effects is unclear, although one report suggested that high sIL-2RA levels block Treg function (43). Future studies will benefit from a broader survey of immune cells, including array technologies, to determine all cell types impacted by therapy.
Overall, these data suggest that Tregs of T1D subjects can be enhanced by IL-2 therapy, providing a foundation upon which to build future treatments designed to augment Tregs. However, rapamycin/IL-2 combination therapy also promoted an environment that presumably resulted in transient β-cell dysfunction. In future studies, approaches need to be identified that dissociate Treg expansion from effector responses. For next-generation trials, markers of IL-2 therapy safety should include early assessment of β-cell function as well as FOXP3 expression, eosinophil and NK frequencies, and changes in sIL-2RA levels.
ACKNOWLEDGMENTS
These studies were supported by the National Institute of Allergy and Infectious Diseases–sponsored Immune Tolerance Network (ITN), the National Institute of Diabetes and Digestive and Kidney Diseases–sponsored Diabetes TrialNet, and an ITN grant to J.H.B.
Glucometers and test strips were donated by LifeScan. L.A.T. has family financial interests in Novartis. No other potential conflicts of interest relevant to this article were reported.
S.A.L. wrote the manuscript and researched data. M.R., P.L.S., and S.A. researched data. S.S., J.B.B., R.G., and A.A. conducted the clinical trial. A.R., S.A., D.P., L.A.T., and J.A.B. reviewed and edited the manuscript and contributed to discussion. M.R.E. and P.J.B. assisted with trial design and conduct, reviewed and edited the manuscript, and contributed to discussion. K.D.B. and S.A.A. provided biostatistical and regulatory support. J.H.B. wrote the manuscript and contributed to discussion. C.J.G. conducted the clinical trial, wrote the manuscript, and contributed to discussion. S.A.L., J.H.B., and C.J.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank all the patients who volunteered to participate in this trial and the clinical staff at Benaroya Research Institute, Naomi Berrie Diabetes Center, and the Harold Schnitzer Diabetes Health Center. Deborah Hefty at Benaroya Research Institute was particularly attentive to the changes in β-cell function occurring in participants.
Footnotes
Clinical trial reg. no. NCT00525889, clinicaltrials.gov.
This article contains Supplementary Material online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0049/-/DC1.
See accompanying commentary, p. 2214.
REFERENCES
- 1.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 2.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 3.Orban T, Bundy B, Becker DJ, et al. Type 1 Diabetes TrialNet Abatacept Study Group Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 2010;10:849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev 2008;7:550–557 [DOI] [PubMed] [Google Scholar]
- 7.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 2009;9:324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgoffe GM, Kole TP, Zheng Y, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009;30:832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 2011;12:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 2010;33:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monti P, Scirpoli M, Maffi P, et al. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes 2008;57:2341–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malek TR. The biology of interleukin-2. Annu Rev Immunol 2008;26:453–479 [DOI] [PubMed] [Google Scholar]
- 13.Turka LA, Walsh PT. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci 2008;13:1440–1446 [DOI] [PubMed] [Google Scholar]
- 14.Hulme MA, Wasserfall CH, Atkinson MA, Brusko TM. Central role for interleukin-2 in type 1 diabetes. Diabetes 2012;61:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010;207:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koulmanda M, Budo E, Bonner-Weir S, et al. Modification of adverse inflammation is required to cure new-onset type 1 diabetic hosts. Proc Natl Acad Sci USA 2007;104:13074–13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008;28:687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011;365:2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011;365:2067–2077 [DOI] [PubMed] [Google Scholar]
- 20.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 2002;51:638–645 [DOI] [PubMed] [Google Scholar]
- 21.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Type 1 Diabetes Trial Net Research Group. European C-Peptide Trial Study Group Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45:926–933 [DOI] [PubMed] [Google Scholar]
- 23.Long SA, Cerosaletti K, Bollyky PL, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes 2010;59:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieczorek G, Asemissen A, Model F, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res 2009;69:599–608 [DOI] [PubMed] [Google Scholar]
- 25.Sehouli J, Loddenkemper C, Cornu T, et al. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics 2011;6:236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb PA, Quinlan S, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet MMF/DZB Study Group Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care 2010;33:826–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherry N, Hagopian W, Ludvigsson J, et al. Protégé Trial Investigators Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet 2011;378:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherrett DK, Bundy B, Becker DJ, et al. Type 1 Diabetes TrialNet GAD Study Group Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011;378:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol 2009;9:83–89 [DOI] [PubMed] [Google Scholar]
- 30.Tanemura M, Saga A, Kawamoto K, et al. Rapamycin induces autophagy in islets: relevance in islet transplantation. Transplant Proc 2009;41:334–338 [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Su D, Qu S, et al. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes 2006;55:2429–2436 [DOI] [PubMed] [Google Scholar]
- 32.Piemonti L, Maffi P, Monti L, et al. Beta cell function during rapamycin monotherapy in long-term type 1 diabetes. Diabetologia 2011;54:433–439 [DOI] [PubMed] [Google Scholar]
- 33.Di Paolo S, Teutonico A, Leogrande D, Capobianco C, Schena PF. Chronic inhibition of mammalian target of rapamycin signaling downregulates insulin receptor substrates 1 and 2 and AKT activation: a crossroad between cancer and diabetes? J Am Soc Nephrol 2006;17:2236–2244 [DOI] [PubMed] [Google Scholar]
- 34.Brauner H, Elemans M, Lemos S, et al. Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol 2010;184:2272–2280 [DOI] [PubMed] [Google Scholar]
- 35.Schleinitz N, Vély F, Harlé JR, Vivier E. Natural killer cells in human autoimmune diseases. Immunology 2010;131:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 2006;103:5941–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein ZP, Porter MM, Gould M, et al. Prolonged administration of low-dose interleukin-2 in human immunodeficiency virus-associated malignancy results in selective expansion of innate immune effectors without significant clinical toxicity. Blood 1995;86:3287–3294 [PubMed] [Google Scholar]
- 38.Hoenstein R, Admon D, Solomon A, Norris A, Moqbel R, Levi-Schaffer F. Interleukin-2 activates human peripheral blood eosinophils. Cell Immunol 2001;210:116–124 [DOI] [PubMed] [Google Scholar]
- 39.Varker KA, Kondadasula SV, Go MR, et al. Multiparametric flow cytometric analysis of signal transducer and activator of transcription 5 phosphorylation in immune cell subsets in vitro and following interleukin-2 immunotherapy. Clin Cancer Res 2006;12:5850–5858 [DOI] [PubMed] [Google Scholar]
- 40.Orange JS, Roy-Ghanta S, Mace EM, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest 2011;121:1535–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turnquist HR, Cardinal J, Macedo C, et al. mTOR and GSK-3 shape the CD4+ T-cell stimulatory and differentiation capacity of myeloid DCs after exposure to LPS. Blood 2010;115:4758–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robb RJ, Kutny RM. Structure-function relationships for the IL 2-receptor system. IV. Analysis of the sequence and ligand-binding properties of soluble Tac protein. J Immunol 1987;139:855–862 [PubMed] [Google Scholar]
- 43.Maier LM, Anderson DE, Severson CA, et al. Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol 2009;182:1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]