Abstract
Obesity, type 2 diabetes, and cardiovascular disease correlate with infiltration to adipose tissue of different immune cells, with uncertain influences on metabolism. Rats were fed a diet high in carbohydrates and saturated fats to develop diet-induced obesity over 16 weeks. This nutritional overload caused overexpression and secretion of phospholipase A2 group IIA (pla2g2a) from immune cells in adipose tissue rather than adipocytes, whereas expression of adipose-specific phospholipase A2 (pla2g16) was unchanged. These immune cells produce prostaglandin E2 (PGE2), which influences adipocyte signaling. We found that a selective inhibitor of human pla2g2a (5-(4-benzyloxyphenyl)-(4S)-(phenyl-heptanoylamino)-pentanoic acid [KH064]) attenuated secretion of PGE2 from human immune cells stimulated with the fatty acid, palmitic acid, or with lipopolysaccharide. Oral administration of KH064 (5 mg/kg/day) to rats fed the high-carbohydrate, high-fat diet prevented the overexpression of pla2g2a and the increased macrophage infiltration and elevated PGE2 concentrations in adipose tissue. The treatment also attenuated visceral adiposity and reversed most characteristics of metabolic syndrome, producing marked improvements in insulin sensitivity, glucose intolerance, and cardiovascular abnormalities. We suggest that pla2g2a may have a causal relationship with chronic adiposity and metabolic syndrome and that its inhibition in vivo may be a valuable new approach to treat obesity, type 2 diabetes, and metabolic dysfunction in humans.
Obesity is a complex chronic condition in which excess adiposity predisposes the interacting metabolic and immune systems to continuous stress (1–4). Among the dynamic components of adipose tissue are adipocytes and many different immune cells, including macrophages, monocytes, T cells, and mast cells, that contribute to adipocyte function (5–7). Stress caused by nutritional overload is associated with initial infiltration of neutrophils, T cells, and mast cells into adipose, followed during chronic overload by macrophages, leading to adipocyte dysfunction and metabolic and cardiovascular abnormalities that characterize metabolic syndrome (4). Genetic ablation or chemical intervention that reduces immune cell infiltration or action in adipose tissue can prevent or treat diet-induced obesity in rodents (5–8). Recent studies implicate novel roles for immune cells, such as macrophages, in regulating lipolysis during diet-induced obesity (8). Weight loss induced by calorie restriction is also associated with transient recruitment of macrophages to white adipose tissue, indicating a role for macrophages as transporters of fatty acids during lipolysis (8). Macrophages were predicted to facilitate trafficking of lipids from white adipose tissue, probably to the liver for metabolism, based on the transient recruitment patterns and expression of scavenger receptors and lipid-handling genes (8,9). However, precise mechanisms for macrophage regulation of adipose tissue lipolysis remain unknown. Whether macrophages and other immune cells exert endocrine or paracrine control of adipocytes to regulate lipolysis in diet-induced obesity has not been reported (9).
The turnover rate of adipocytes is similar for lean and obese individuals (10), but the rate of lipid storage and removal from adipocytes differs between healthy humans and people with obesity or hyperlipidemia (11). The triglyceride content is renewed an average of six times during the 10-year life span of human adipocytes, whereas rates of triglyceride storage increase and their removal decreases in obese versus lean individuals (11). Thus, increased lipid storage in adipocytes, combined with decreased removal, contributes to development of obesity and adipocyte dysfunction in humans (11,12). Pharmaceutical interventions are under investigation to decrease fat stores in adipose tissue and improve adipocyte function by stimulating lipolysis and oxidation of released fatty acids (13).
The lipolysis of triglycerides to free fatty acids and glycerol is tightly regulated in adipose tissue by hormones or enzymes (14). Inflammatory cells, such as mast cells, neutrophils, and macrophages, express phospholipase A2 (PLA2) enzymes that are involved in various physiologic processes, including lipid metabolism and cellular signaling (14,15). Adipose-specific phospholipase A2 group XVI (pla2g16) and prostaglandin E2 (PGE2) may play important autocrine and paracrine roles in ob/ob and db/db knockout mice in regulating lipolysis through a PGE2–prostaglandin EP3 receptor–cAMP pathway (14,16). PGE2 is strongly linked to antilipolytic responses in adipocytes by acting through the Gαi-coupled EP3 receptor (14,17,18). However, secretory PLA2 group IIA (pla2g2a) is mainly recognized for its role in chronic inflammatory diseases and generation of PGE2 and other eicosanoids after immune cell activation (15,19,20).
This study has specifically investigated the role of pla2g2a, and the therapeutic potential of its inhibition, in adipose tissue during diet-induced obesity in a rat model relevant to human disease. The findings support a new hypothesis that inhibition of pla2g2a may reverse and protect against adiposity and metabolic dysfunction in diet-induced obese rats and suggest a mechanism of promoting lipolysis to enhance fat utilization and energy expenditure. The pharmacologic responses of a selective pla2g2a inhibitor (5-(4-benzyloxyphenyl)-(4S)-(phenyl-heptanoylamino)-pentanoic acid [KH064]) were not related to direct effects on adipocytes but rather to decreasing PGE2 release from immune cells (e.g., macrophages, T cells, monocytes, and mast cells) also resident in adipose tissue. This inhibitor stimulated lipolysis in adipose tissue, suggesting a novel mechanism for immune cells regulating lipolysis through the PGE2-EP3-cAMP pathway in diet-induced obesity.
RESEARCH DESIGN AND METHODS
Animals and diets.
Male Wistar rats were bred at The University of Queensland Biological Resources facility. All experimental protocols were approved by the Animal Experimentation Ethics Committee of The University of Queensland. The cornstarch (CS) and high-carbohydrate high-fat (HCHF) diets have been previously described (21). KH064 (5 mg/kg/day suspended in olive oil) was administered daily by oral gavage to HCHF rats, starting at week 8 of the study protocol. C57BL/6 mice, used for gene expression studies, were fed with a high-fat diet for 16 weeks.
Adipose tissue analysis.
Postmortem retroperitoneal, mesenteric, and epididymal fat depots were collected and weighed immediately. The retroperitoneal depot was defined as adipose tissue located behind the kidney, along the back of the abdomen. All molecular analyses have been done in retroperitoneal whole adipose tissue as a major component of abdominal fat. Retroperitoneal rat whole adipose tissue was digested using collagenase B and was differentially centrifuged to derive floating adipocyte and stromal vascular cell (SVC) fractions. To assess lipolysis, retroperitoneal whole adipose tissue explants were incubated in Kreb’s Ringer bicarbonate HEPES for 2 h, with or without PGE2. Glycerol levels were measured using Free Glycerol Reagent (Sigma-Aldrich).
Gene expression.
RNA was extracted using Qiazol reagent according to the RNeasy mini kit (Qiagen). RT-PCR was performed as previously described (22). Primer sequences are listed in Supplementary Table 1.
Immunoblot.
Protein levels were detected using antibodies against pla2g2a (Abcam), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Sigma-Aldrich), phospho-hormone-sensitive lipase (HSL) (Ser563), and HSL (Cell Signaling). Bands were analyzed using ImageJ 1.40e software.
Cell culture.
Human mastocytoma cell line (HMC-1) cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM). Jurkat E6.1 and human acute monocytic leukemia cell line (THP-1) cells were cultured in RPMI. Human peripheral blood mononuclear cells (PBMCs) and monocyte-derived macrophages (HMDMs) were cultured in IMDM. 3T3-L1 fibroblasts were differentiated 2 days after confluence, and fully differentiated adipocytes were used for experiments such as the cAMP-Glo assay (Promega).
PBMC and HMDM isolation.
PBMCs and HMDMs were harvested from buffy coat of anonymous human donors (Australian Red Cross Blood Service, Brisbane). For HMDMs, cluster of differentiation (CD) 14+ monocytes were isolated using magnetic sorting (Miltenyi Biotech), then differentiated using macrophage-colony stimulating factor.
Enzyme-linked immunosorbent assay.
Cells were pretreated with KH064 (10 μmol/L) for 15 min before experiments. Culture supernatant, serum, and adipose tissue homogenate PGE2 and insulin concentrations were determined using a PGE2 metabolite and a rat insulin kit, respectively.
Physiologic parameters.
Oral glucose tolerance and clearance tests were previously described. For glucose tolerance, a glucose load of 2 g/kg body weight was used for glucose tolerance and an insulin load of 0.3 IU/kg was used for insulin tolerance (21). Plasma lipids and enzyme concentrations were measured by The University of Queensland Veterinary Pathology Service (21). Systolic blood pressure was measured as described (23).
Echocardiographic studies, heart preparation, and histopathologic analysis.
Echocardiographic examination was performed after 16 weeks as described (21). Evaluation of the left ventricular function of all rats in all treatment groups after 16 weeks was performed as described (21). Histopathologic analysis of the heart, liver, and adipose was performed as described (21,23).
Total lipid content.
Extraction of tissue and fecal lipids was undertaken by manual solvent extraction as described (24).
Drug.
The inhibitor KH064 of pla2g2a was synthesized, purified, and enzymatically characterized as described (compound 2b in [25]). It binds in the active site of human pla2g2a (25) and is a potent inhibitor of enzymatic activity (half-maximal inhibitory concentration [IC50] 0.029 μmol/L). This enzyme is similar to human macrophage pla2g5 enzyme against which KH064 is 100 times less potent (IC50 2 μmol/L (26)). The rat homolog of human pla2g2a was not commercially available. KH064 does not bind or inhibit cPLA2 or iPLA2 enzymes (unpublished data from D.P.F.). We have reported pharmacokinetics for KH064 given to rats intravenously (27) and orally (28). At a 5 mg/kg p.o. dose (as used herein), KH064 was detectable in plasma within 15 min at relatively constant concentrations for 6 h with Cmax ∼0.2 μg/mL, Tmax ∼2 h, and ∼4% oral bioavailability (area under the curve orally/area under the curve intravenously). KH064 was ∼0.05 μg/mL in plasma 18–24 h after oral dosing, suggesting partitioning to tissues (28). KH064 has been administered to mice at 300 mg/kg/p.o. in a single dose with no substantive toxic effects, and it has no significant off-target effects in a screen against 25 enzymes and 30 human G protein-coupled receptors (unpublished data from D.P.F.). KH064 is progressing to clinical development.
Statistical analysis.
Data were plotted and analyzed using GraphPad Prism v5.00 software (GraphPad Software, San Diego, CA). Statistical differences in pairwise comparisons between treatments were assessed using the Student t test, and changes in body weight were assessed by two-way ANOVA (*P <0.05, **P < 0.01, and ***P < 0.001). All values of independent parameters are mean ± SEM (n ≥ 3 independent experiments) unless stated otherwise.
RESULTS
Pla2g2a but not pla2g16 is upregulated in rat adipose by HCHF feeding.
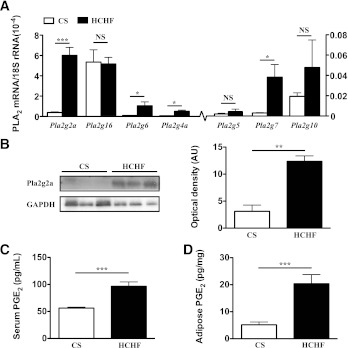
Our first objective was to investigate which phospholipase could be a potential therapeutic target to prevent adiposity and metabolic dysfunction. Increased PLA2 expression in adipose tissue might dampen lipolysis through PGE2-EP3-Gαi-cAMP signaling, promoting adipocyte and metabolic dysfunction together with cardiovascular symptoms of metabolic syndrome (14). Rats were fed a HCHF diet to induce adiposity and symptoms of metabolic syndrome (21). Relative to rats fed a CS diet, those receiving the HCHF diet for 16 weeks became obese, gaining 54 ± 4% weight from weeks 0 to 16 and 112 ± 17% total visceral fat compared with CS rats (21). We measured mRNA expression in adipose tissue of PLA2 isozymes recognized for their roles in inflammation (pla2g2a, pla2g4a, pla2g5, pla2g7, and pla2g10) or lipid metabolism (pla2g6 and pla2g16). Expression of pla2g2a, pla2g6, pla2g4a, pla2g7, and pla2g10 genes was extremely low in CS-fed rats but significantly elevated in HCHF-fed obese rats. Among these, PLA2 enzymes were somewhat upregulated in response to HCHF feeding; pla2g2a mRNA expression was strikingly elevated by ∼20-fold (Fig. 1A). Correspondingly, pla2g2a protein expression was also substantially increased in adipose tissue from HCHF-fed obese rats compared with CS-fed normal rats (Fig. 1B). Plasma and whole adipose tissue PGE2 concentrations were elevated by HCHF compared with CS feeding, correlating with increased adiposity (Fig. 1C and D). We compared pla2g16 mRNA expression in rat adipose tissue because this enzyme reportedly regulates adipocyte function and lipid metabolism in mice and is overexpressed in ob/ob mice adipose tissue. Pla2g16 was expressed in rat adipose tissue, but expression was unchanged by HCHF feeding (Fig. 1A and Supplementary Fig. 1A). In an isolated experiment, pla2g16 gene expression was unchanged in adipose tissue from lean mice and diet-induced obese mice after 16 weeks (Supplementary Fig.1B).
FIG. 1.
RT-PCR gene expression of different PLA2 enzymes and protein expression of pla2g2a in rat adipose tissue. A: Quantitative comparison of mRNA expression level of PLA2 enzymes in adipose tissue from rats fed CS vs. HCHF diets for 16 weeks. Values of PLA2 mRNA levels were normalized relative to the housekeeping gene 18S rRNA. B: Immunoblot of pla2g2a protein levels in adipose tissue with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as a loading control. Each lane represents adipose tissue homogenate from a single rat. Optical density of protein bands was determined using ImageJ software. Serum (C) and adipose (D) homogenate concentrations of PGE2 from CS vs. HCHF fed rats. Error bars represent means ± SEM (n = 3–5 animals). *P < 0.05, **P < 0.01, ***P < 0.001.
KH064 attenuates adiposity in diet-induced obese rats.
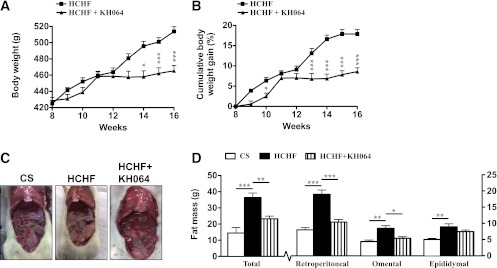
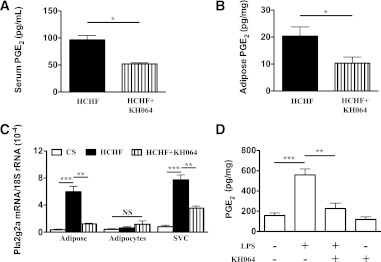
KH064 is an orally active, potent, and isoform-selective inhibitor of pla2g2a. We have reported a crystal structure for this inhibitor in complex with pla2g2a (25) and anti-inflammatory activity for this compound (25–28). The increased adiposity exhibited by rats fed a HCHF diet for 16 weeks (Fig. 2) was attenuated by oral administration of KH064 (5 mg/kg/day) between weeks 8 and 16, with marked prevention of body weight gain (weeks 8–16 HCHF, 19 ± 1%; +KH064, 9 ± 1%; Fig. 2A and B) and total visceral fat deposition (+KH064, Fig. 2C–E). Treatment with KH064 also attenuated retroperitoneal and omental fat rather than epididymal fat deposition (Fig. 2E). Further, administration of KH064 daily between weeks 8 and 16 to CS-fed rats, which expressed only low pla2g2a, did not exhibit any overt symptoms of toxic side effects or cause changes in body weight (data not shown). Besides improvements in adiposity, KH064 treatment from weeks 8 to 16 attenuated increases in plasma and whole adipose tissue PGE2 concentrations in HCHF rats (Fig. 3A and B). Adipose tissue immunohistochemistry demonstrated that treatment with KH064 also prevented the significant increase in crown formation and macrophage infiltration measured by ED1 staining (Supplementary Fig. 1C and D). Further, investigating the polarization of infiltrated and resident macrophages in adipose tissue using inflammatory/surface marker gene expression to profile M1- versus M2-macrophages (29) suggested that HCHF-fed rats had increased expression of M1-specific genes in adipose tissue in contrast to CS-fed rats (Supplementary Fig. 1E and F). Treatment with KH064 normalized this trend to a more M2-like population in the adipose tissue comparable to CS-fed rats (Supplementary Fig. 1E and F).
FIG. 2.
KH064 modulates diet-induced adiposity in vivo in rats. A: Daily body weights for HCHF-fed rats (n = 10) alone or with daily oral treatment with KH064 (5 mg/kg; HCHF+KH064, n = 10) from weeks 8 to 16. B: Cumulative percentage weight gain for HCHF-fed rats (n = 10) alone or with daily oral treatment with KH064 (5 mg/kg; HCHF+KH064, n = 10) from weeks 8 to 16. C: Representative photographs show comparison of visceral fat in rats at 16 weeks after different treatments. D: Total adiposity and depot specific adiposity for rats from different treatment groups (n = 6). Error bars represent means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. (A high-quality color representation of this figure is available in the online issue.)
FIG. 3.
KH064 treatment modulates PGE2 concentrations in Wistar rats and SVCs. PGE2 concentrations in serum (A) and whole adipose tissue (B) from rats fed a high carbohydrate high fat (HCHF) diet alone or with drug (KH064 5 mg/kg/day). C: Pla2g2a gene expression in whole adipose tissue, adipocyte, and SVC fractions. D: Ex vivo treatment with KH064 inhibits LPS-induced PGE2 production in SVCs. Error bars represent mean ± SEM of least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Pla2g2a overexpression and inhibitor action in immune cells, not adipocytes.
Separation of whole adipose tissue into adipocyte and SVC fractions confirmed that pla2g2a was mainly expressed in the SVC fraction rather than adipocytes in adipose tissue. Furthermore, HCHF feeding induced overexpression of pla2g2a, but only in the SVC (Fig. 3C). In contrast, pla2g16 was predominantly expressed in the adipocyte fraction from whole adipose tissue (Supplementary Fig. 1B), and its expression levels were not affected by HCHF feeding (as shown in Fig. 1A).
The pla2g2a enzyme is an important generator of inflammatory lipid mediators during immune cell activation, including PGE2 produced by cyclooxygenase (15,19,20). We focused on PGE2 because it is implicated as a major paracrine antilipolytic factor in adipocytes (14,30). We found that PGE2 inhibited cAMP production in 3T3-L1 adipocytes and established the involvement of EP3 receptor and pertussis toxin–sensitive Gαi-proteins in this process (Supplementary Fig. 2A and B). In line with our in vitro results, exogenous addition of PGE2 to rat whole adipose tissue explants decreased glycerol release consistent with reduced lipolysis (Supplementary Fig. 2C). To establish that pla2g2a was primarily expressed in the SVC and that this was where KH064 was acting, we pretreated SVC with KH064 before lipopolysaccharide (LPS) stimulation. LPS stimulated PGE2 production in the SVC, and this was inhibited by KH064 (Fig. 3D). Further, at a relatively high concentration (1 mmol/L), KH064 did not inhibit any PLA2 enzymatic activity in adipose tissue from CS-fed rats, confirming that KH064 did not inhibit pla2g16 or other PLA2 enzymes in adipose tissue (Supplementary Fig. 3). These results clearly trace the pharmacologic responses of KH064 on pla2g2a to the immune cell–rich SVC in whole adipose tissue and not the adipocyte fraction.
Regulation of lipolysis via pla2g2a and PGE2 in immune cells.
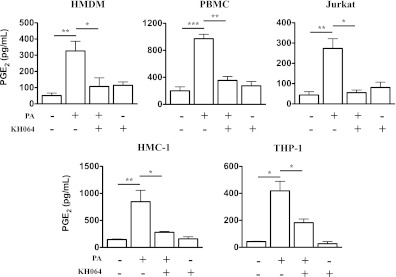
Many types of resident and infiltrated immune cells, including macrophages, monocytes, T cells and mast cells, contribute to adipocyte dysfunction (5–7). However, their relative roles and importance in lipid homeostasis and adipocyte function in obesity are not yet clear. In this study, we investigated whether some or all of these cells regulate lipolysis via pla2g2a/PGE2 in adipose. Because we were not able to isolate infiltrated rat adipose tissue immune cells in sufficient quantity or purity for in vitro studies, we chose five primary or cultured human cell types that are closely associated with adiposity and metabolic dysfunction. The five immune cell types—HMDM, PBMC, HMC-1, Jurkat, and THP-1—all showed increased PGE2 production after stimulation with palmitic acid, the most common and abundant nutritional fatty acid in Western-style diets that plays a major role in inducing adipocyte and metabolic dysfunction in obese subjects (31). Treatment of each cell type with KH064 markedly reduced (two- to fivefold) this increased PGE2 production (Fig. 4). To support the notion that KH064 was inhibiting palmitic acid-induced PGE2 production via the pla2g2a enzyme in these cell types, LPS was separately used to elicit PGE2 production in these five immune cell types (Supplementary Fig. 4). HMDM, PBMC, and HMC-1 showed increased PGE2 production after LPS stimulation, and KH064 treatment markedly reduced the increased PGE2 production in these cells (Supplementary Fig. 4). However, there was no significant PGE2 increase in Jurkat and THP-1 cells after stimulation with LPS. This suggests that, in addition to macrophages (8), other immune cell types, such as monocytes, T cells, and mast cells, may also secrete antilipolytic factors, such as PGE2, to reduce lipolysis and free fatty acid concentrations, thereby promoting adiposity.
FIG. 4.
In vitro KH064 inhibits palmitic acid–induced PGE2 production in human immune cells. The production of PGE2 in culture supernatants was analyzed by ELISA. HMDM, PBMC, Jurkat, HMC-1, and THP-1 secrete PGE2 elicited by palmitic acid (PA) and pretreatment of KH064 inhibits this palmitic acid–induced PGE2 production. Error bars represent mean ± SEM of least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Pla2g2a and PGE2 inhibition restores lipolysis in vivo.
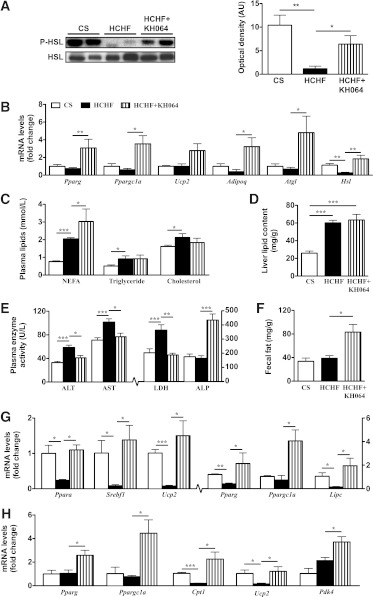
One approach to improve adipocyte function is to decrease fat stores in adipose tissue by stimulating lipolysis and oxidation of released fatty acids (13). Because the pla2g2a inhibitor KH064 normalized lipolysis and attenuated adiposity, we investigated whether inhibiting PGE2 in vivo stimulated enhanced release of fat from adipose toward better fat utilization and oxidation in the liver and skeletal muscle. Phosphorylation of HSL through cAMP-mediated activation of protein kinase A is a key mediator of increased lipolysis in adipose tissue (14). HCHF-fed rats decreased phosphorylation of HSL (Ser563) in adipose compared with CS-fed rats (Fig. 5A). Treatment with KH064 from weeks 8 to 16 normalized or increased lipolysis in adipose of HCHF rats by preventing this decrease in HSL phosphorylation, without altering total HSL protein expression (Fig. 5A). In addition, genes involved in lipid metabolism and the lipolytic cascade, such as peroxisome proliferator-activator receptor-γ (Pparg), Ppargc1a, uncoupling protein 2 (Ucp2), adiponectin (Adipoq), adipose triglyceride lipase/desnutrin (Atgl), and Hsl in adipose tissue were all normalized or increased with KH064 treatment compared with CS-fed and HCHF-fed rats, respectively (Fig. 5B). Plasma nonesterified fatty acids were increased with KH064 treatment compared with untreated HCHF animals at week 16, consistent with increased lipolysis from adipose tissue stores (Fig. 5C).
FIG. 5.
In vivo responses of KH064 (5 mg/kg/day p.o., weeks 8–16) on the regulation of metabolic parameters that were elevated in rats fed HCHF vs. CS diets for 16 weeks. A: Immunoblot of phospho-HSL (Ser563) in adipose tissue with total HSL as the loading control. Optical density of protein bands was determined using ImageJ software. B: RT-PCR gene expression of genes in adipose tissue of rats fed CS and HCHF diets with and without KH064 treatment. C: Plasma concentrations for lipids nonesterified fatty acids (NEFA), triglycerides, and total cholesterol in rats of different groups (n = 5–10). D: Liver total lipid content in rats of different groups (n = 5). E: Plasma liver enzymes in rats of different groups (n = 5–10). F: Total soluble fecal fat in rats of different groups (n = 5). RT-PCR gene expression of different metabolic genes in liver (G) and skeletal muscle (H). Values of mRNA levels were normalized relative to 18S rRNA. Error bars represent means ± SEM (n = 3–5 animals). *P < 0.05, **P < 0.01, ***P < 0.001.
During lipolysis, either through acute weight loss or metabolic disease, macrophages have been proposed as transporters of fatty acids from adipose and peripheral tissues to liver for metabolism and energy production, similar to involvement of macrophages in the reverse cholesterol transport pathway during atherosclerosis (8,9). HCHF rats showed increased total lipids and steatosis in the liver at 16 weeks compared with CS-fed rats (Fig. 5D and Supplementary Fig. 5). Activities of the plasma liver enzymes, aspartate aminotransferase, and alanine aminotransferase were higher in HCHF-fed compared with CS-fed rats, suggesting mild liver dysfunction (Fig. 5E). Treatment with KH064 from weeks 8 to 16 attenuated this increase in liver enzymes (Fig. 5E). Further, the increased lipid content in liver was not attenuated with KH064 treatment from weeks 8 to 16 (Fig. 5D). Histology analysis showed that livers from KH064-treated rats had accumulated fat droplets (Supplementary Fig. 5 and Fig. 5E). Plasma concentrations of alkaline phosphatase increased with KH064 treatment (Fig. 5E). Extractable fecal lipid content increased with KH064 treatment, suggesting decreased absorption of fat from the intestines or increased excretion of fat (Fig. 5F). Furthermore, genes involved in lipid metabolism and energy expenditure in the liver, such as Ppara, sterol regulatory element binding transcription factor 1 (Srebf1), Ucp2, Pparg, and hepatic lipase (Lipc), were suppressed in HCHF rats but increased or normalized in HCHF rats treated with KH064 (Fig. 5G). In skeletal muscle, carnitine palmitoyltransferase 1 (Cpt1) and Ucp2 genes involved in energy expenditure were suppressed in HCHF rats but increased or normalized in HCHF rats treated with KH064 (Fig. 5H). Other genes involved in fatty acid oxidation and mitochondrial biogenesis, including Pparg, Ppargc1a, and pyruvate dehydrogenase kinase 4 (Pdk4) in adipose, liver, and skeletal muscle (32,33), were upregulated in KH064-treated compared with untreated HCHF-fed rats (Fig. 5B, G, and H). However, further investigation is needed on the exact mechanisms by which KH064 alters the expression of lipid-handling genes in liver and skeletal muscle and its direct responses on these metabolically relevant tissues.
Pla2g2a inhibitor KH064 promotes lipolysis and protects against diet-induced metabolic syndrome.
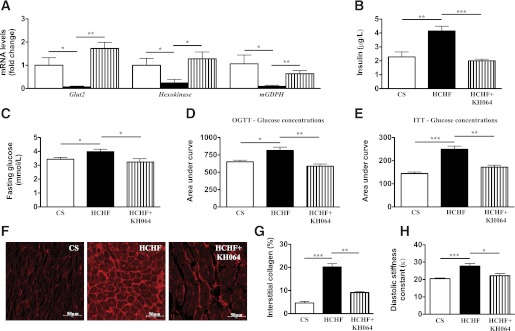
Attenuation of adipocyte dysfunction or adiposity improves metabolic and cardiovascular symptoms of metabolic syndrome in animals and clinical studies (4,34–37). In this study, alterations in metabolic parameters in HCHF-fed compared with CS-fed rats included decreased expression of genes involved in glucose metabolism in the pancreas (Fig. 6A), increased plasma insulin concentrations (Fig. 6B), impaired glucose and insulin tolerance (Fig. 6C–E), increased systolic blood pressure (Supplementary Table 2 and Supplementary Fig. 6), and abnormalities in cardiac structure and function (Fig. 6F–H). Treatment with KH064 attenuated changes in glucose metabolism genes in the pancreas, such as glucose transporter 2 (glut2), hexokinase, and mitochondria glycerophosphate dehydrogenase (mGPDH) (38,39), while normalizing glucose and insulin tolerance (Fig. 6C–E). Furthermore, most of the changes in cardiovascular structure and function in HCHF-fed rats were attenuated by KH064 (Fig. 6A–H, Supplementary Table 2, and Supplementary Fig. 6), including excessive collagen deposition in the left ventricle of the heart in HCHF rats.
FIG. 6.
In vivo and ex vivo responses to KH064 (5 mg/kg/day p.o. for weeks 8–16) of the regulation of metabolic and cardiovascular parameters that were elevated in rats fed HCHF vs. CS diets for 16 weeks. A: RT-PCR analysis of glucose metabolism genes in rat pancreas of different groups. B: Plasma concentrations of insulin in rats of different groups (n = 5). C: Fasting blood glucose concentrations in rats of different groups (n = 6 for all groups). D: Oral glucose tolerance tests (OGTT) in rats of different groups (n = 6 for all groups). E: Insulin tolerance test (ITT) in rats of different groups (n = 6 for all groups). F: Representative images (original magnification ×40) of interstitial collagen deposition in the left ventricle. G: Quantitative measurement of the area of collagen deposition in the interstitial region of the left ventricle (n = 4 for all groups). H: Diastolic stiffness constant of rats in different groups. Error bars represent mean ± SEM (n = 3–5 animals). *P < 0.05, **P < 0.01, ***P < 0.001. (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
This study reports important new evidence for involvement of secretory phospholipase A2 enzyme, pla2g2a, in diet-induced adiposity and metabolic and cardiovascular dysfunction. Phospholipases are important in pathogenesis of chronic inflammatory diseases and thought to be important in lipid metabolism. Here we show that the specific isozyme pla2g2a is an important mediator in the cross talk between immune and metabolic systems in adipose tissue, relevant to lipid and energy homeostasis, metabolic, and cardiovascular function. In HCHF-fed rats, pla2g2a was upregulated in the SVC but not in the adipocyte fraction of adipose tissue. The selective pla2g2a inhibitor (KH064) (25), administered orally to HCHF-fed rats, was found herein to inhibit the development of obesity, adiposity, insulin resistance, and glucose intolerance. The pla2g2a inhibitor also attenuated changes in other features of metabolic and cardiovascular dysfunction induced by nutritional overload.
These in vivo effects of KH064 were linked to preventing PGE2 release in adipose tissue from immune cells and not adipocytes. Immune cells infiltrate adipose tissue during obesity and metabolize fatty acids. Our hypothesis is that these immune cells overexpress pla2g2a, generating metabolites of phospholipids, such as PGE2, to act on secondary target cells (adipocytes) and inhibit lipolysis via a PGE2–EP3–cAMP pathway. An inhibitor of pla2g2a has been shown here to inhibit in vitro palmitate- or LPS-stimulated release of PGE2 from macrophages, T cells, monocytes, and mast cells, known to reside in adipose tissue of obese humans (5,6,40). The differential responses of immune cells to LPS- and palmitate-induced secretion of PGE2 may involve other, as yet unknown, signaling mechanisms. Palmitate-induced production of PGE2 may involve fatty acid receptors, such as FFA1 (GPR40), FFA2 (GPR43), FFA3 (GPR41), or GPR120 (41). Nonetheless, in vivo KH064 decreased plasma and adipose PGE2 concentrations, normalized and stimulated lipolysis in adipose tissue, and protected against adiposity in diet-induced obese rats. This suggests a novel role for immune cells in regulating lipolysis through EP3-Gαi-cAMP signaling during diet-induced obesity. PGE2 regulates adipocyte dysfunction and contributes to antilipolytic pathways via EP3 receptors, thereby decreasing cAMP concentrations (14). This is the first study to show that modulating PGE2 secretion, through inhibition of pla2g2a from key immune cells resident in and infiltrated to adipose, reverses diet-induced adiposity and metabolic syndrome. However, we cannot rule out improvements in metabolic parameters arising from synergistic responses from KH064 acting on other metabolically relevant tissues along with adipose, from decreased intestinal fat absorption and altered lipid metabolism, or through unknown off-target effects on nuclear receptors such as constitutive active/androstane receptor (CAR) or pregnane X receptor (PXR).
Some other PLA2 isozymes (pla2g16, pla2g6b, pla2g4a) may be important in metabolism because their genetic knockout prevented development of obesity (14,42–44). However, both pla2g16−/− and pla2g6b−/− phenotypes exhibited severe defects in insulin secretion. In pla2g16−/− mice, insulin resistance was observed in normal and high-fat diets (14), whereas pla2g6b−/− mice were refractory to glucose-stimulated insulin release (43). Similarly, contradictory evidence on deposition of lipids in the liver was reported; pla2g16−/− mice showing increased fat deposition in the liver, whereas pla2g4a−/− and pla2g6b−/− had decreased fat deposition in liver compared with wild types after high fat feeding (43–45). There is growing evidence suggesting that immune cells transport lipids from adipose to liver for metabolism, but those studies did not investigate whether deposition of lipids was transient or on-going or caused liver damage. Moreover, pla2g16 was upregulated in genetically modified mice (ob/ob and db/db) and in acute-fed fasted mice (14). In our much longer-duration study, pla2g16 gene expression was unchanged in adipose from rats and mice (Supplementary Fig.1A) after 16 weeks of HCHF feeding. Our findings differ from those reported for pla2g16 in fasted acute-fed mice (14). It is conceivable that pla2g16 might act early in a proinflammatory pathway required for immune cell infiltration. In the absence of pla2g16, any increase in adipose tissue inflammation and immune cell infiltration might have been blocked to prevent an increase in adipose tissue PLA2 activity. However, in our much longer chronic obesity experiments, it is clear that pla2g2a is unique among sPLA2 isoforms examined in being dramatically overexpressed in HCHF-fed rats compared with CS-fed rats, and importantly, the pla2g2a-selective inhibitor attenuated adiposity and most symptoms of metabolic syndrome in these rats.
Activating inflammatory lipid mediators associated with adipokines and cytokines to alter adipocyte and immune cell function has now become the prevailing hypothesis to explain mechanisms of metabolic and adipocyte dysfunction (4,46). Nutrient- and pathogen-sensing systems in humans may have evolved together, possibly for more efficient management of energy homeostasis, metabolic function, and immunity by coordinating energy storage, transport, and metabolism (1,3). We speculate that recruiting immune cells into adipose during obesity, and associated metabolic dysfunction, is not only an inflammatory response to stress but also a mechanism to regulate energy metabolism. Weight loss by calorie restriction is associated with macrophage recruitment to white adipose tissue and regulation of lipid trafficking and lipolysis (8,9). Our hypothesis is supported by recent studies in which there was increased local release of fatty acids during fasting, inducing recruitment of immune cells and secretion of antilipolytic factors such as PGE2 to reduce free fatty acid concentrations (8,9). Our results indicate that resident and infiltrating immune cells in adipose have important signaling roles in regulating energy metabolism, such as lipolysis and oxidation of released fatty acids, and these signaling networks may be overloaded and dysfunctional in obesity. Pharmacologic modulation of immune cells can improve diet-induced adiposity and symptoms of metabolic syndrome. Contrary to current opinion, increasing or normalizing lipolysis as a pharmaceutic strategy or elevating plasma free fatty acids in obese subjects may not necessarily translate into metabolic dysfunction or insulin resistance (47). More dynamic and refined studies are required to clarify relationships and roles of specific fatty acids in obesity and metabolic dysfunction, with greater emphasis on consequences of impaired or dysfunctional adipocyte fat storage, release, and function on adipokines/cytokines that affect metabolism (47).
In summary, pla2g2a has been shown to be by far the most upregulated Pla2 isozyme in adipose tissue of diet-induced obese rats. This protein is localized to immune cells in adipose tissue rather than to adipocytes. The study raises the possibility that systemic pla2g2a inhibition may decrease fat stores by preventing PGE2 biosynthesis from immune cells in adipose tissue to stimulate lipolysis and fatty acid oxidation. Importantly, KH064 is a potent and specific inhibitor of human pla2g2a and prevents diet-induced adiposity, insulin resistance, and metabolic and cardiac dysfunction in rats. These results indicate that modulation of adipose tissue homeostasis, by blocking an endocrine and paracrine stimulus from immune cells rather than adipocytes, may be a novel approach to treat diet-induced metabolic syndrome in humans.
ACKNOWLEDGMENTS
The authors thank the Australia & New Zealand (ANZ) Trustee’s Medical Research in Queensland for providing financial assistance via a PhD scholarship to A.I. and the Institute for Molecular Bioscience and The University of Queensland for a PhD scholarship to J.L. The authors also thank the Australian Research Council for funding (Grant DP1093245) and a Federation Fellowship (FF0668733) to D.P.F., the National Health and Medical Research Council (Grant 631534) for funding the research, and a Senior Principal Research Fellowship (APP1027369) to D.P.F.
No potential conflicts of interest relevant to this article were reported.
A.I. and J.L. performed experiments, collected data, and drafted the manuscript. H.P., J.Y.S., and J.W. assisted in data collection. R.C.R. synthesized the inhibitor. J.B.P. and J.P.W. provided advice on cellular studies. D.P.F. and L.B. directed the research, contributed intellectually, and developed the manuscript. D.P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1179/-/DC1.
REFERENCES
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–445 [DOI] [PubMed] [Google Scholar]
- 3.Horng T, Hotamisligil GS. Linking the inflammasome to obesity-related disease. Nat Med 2011;17:164–165 [DOI] [PubMed] [Google Scholar]
- 4.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol 2010;6:71–82 [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med 2009;15:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 [DOI] [PubMed] [Google Scholar]
- 8.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest 2010;120:3466–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Red Eagle A, Chawla A. In obesity and weight loss, all roads lead to the mighty macrophage. J Clin Invest 2010;120:3437–3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]
- 11.Arner P, Bernard S, Salehpour M, et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 2011;478:110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch L. Adipose lipid turnover-a new target in metabolic disease. Nat Rev Endocrinol 2011;7:694. [DOI] [PubMed] [Google Scholar]
- 13.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res 2006;53:482–491 [DOI] [PubMed] [Google Scholar]
- 14.Jaworski K, Ahmadian M, Duncan RE, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med 2009;15:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott DL, White SP, Browning JL, Rosa JJ, Gelb MH, Sigler PB. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science 1991;254:1007–1010 [DOI] [PubMed] [Google Scholar]
- 16.Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J Biol Chem 2008;283:25428–25436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong P, Coleman RA, Humphrey PP. Prostanoid-induced inhibition of lipolysis in rat isolated adipocytes: probable involvement of EP3 receptors. Prostaglandins 1992;43:559–566 [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Moustaid-Moussa N. Secretory, endocrine and autocrine/paracrine function of the adipocyte. J Nutr 2000;130:3110S–3115S [DOI] [PubMed] [Google Scholar]
- 19.Wery JP, Schevitz RW, Clawson DK, et al. Structure of recombinant human rheumatoid arthritic synovial fluid phospholipase A2 at 2.2 A resolution. Nature 1991;352:79–82 [DOI] [PubMed] [Google Scholar]
- 20.Reddy ST, Herschman HR. Transcellular prostaglandin production following mast cell activation is mediated by proximal secretory phospholipase A2 and distal prostaglandin synthase 1. J Biol Chem 1996;271:186–191 [DOI] [PubMed] [Google Scholar]
- 21.Panchal SK, Poudyal H, Iyer A, et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 2011;57:611–624 [DOI] [PubMed] [Google Scholar]
- 22.Suen JY, Gardiner B, Grimmond S, Fairlie DP. Profiling gene expression induced by protease-activated receptor 2 (PAR2) activation in human kidney cells. PLoS One 2010;5:e13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer A, Fenning A, Lim J, et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Br J Pharmacol 2010;159:1408–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poudyal H, Panchal SK, Waanders J, Ward L, Brown L. Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J Nutr Biochem 2012;23:153–162 [DOI] [PubMed] [Google Scholar]
- 25.Hansford KA, Reid RC, Clark CI, et al. D-Tyrosine as a chiral precusor to potent inhibitors of human nonpancreatic secretory phospholipase A2 (IIa) with antiinflammatory activity. ChemBioChem 2003;4:181–185 [DOI] [PubMed] [Google Scholar]
- 26.Levick S, Loch D, Rolfe B, et al. Antifibrotic activity of an inhibitor of group IIA secretory phospholipase A2 in young spontaneously hypertensive rats. J Immunol 2006;176:7000–7007 [DOI] [PubMed] [Google Scholar]
- 27.Arumugam TV, Arnold N, Proctor LM, et al. Comparative protection against rat intestinal reperfusion injury by a new inhibitor of sPLA2, COX-1 and COX-2 selective inhibitors, and an LTC4 receptor antagonist. Br J Pharmacol 2003;140:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodruff TM, Arumugam TV, Shiels IA, et al. A potent and selective inhibitor of group IIa secretory phospholipase A2 protects rats from TNBS-induced colitis. Int Immunopharmacol 2005;5:883–892 [DOI] [PubMed] [Google Scholar]
- 29.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mater MK, Thelen AP, Jump DB. Arachidonic acid and PGE2 regulation of hepatic lipogenic gene expression. J Lipid Res 1999;40:1045–1052 [PubMed] [Google Scholar]
- 31.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 2005;25:10684–10694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 2007;26:1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880 [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 37.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kjørholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes 2005;54:2755–2763 [DOI] [PubMed] [Google Scholar]
- 39.Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci USA 1990;87:6492–6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoddart LA, Smith NJ, Milligan G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol Rev 2008;60:405–417 [DOI] [PubMed] [Google Scholar]
- 42.Song H, Wohltmann M, Bao S, Ladenson JH, Semenkovich CF, Turk J. Mice deficient in group VIB phospholipase A2 (iPLA2gamma) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet. Am J Physiol Endocrinol Metab 2010;298:E1097–E1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ii H, Hatakeyama S, Tsutsumi K, Sato T, Akiba S. Group IVA phospholipase A2 is associated with the storage of lipids in adipose tissue and liver. Prostaglandins Other Lipid Mediat 2008;86:12–17 [DOI] [PubMed] [Google Scholar]
- 44.Mancuso DJ, Sims HF, Yang K, et al. Genetic ablation of calcium-independent phospholipase A2gamma prevents obesity and insulin resistance during high fat feeding by mitochondrial uncoupling and increased adipocyte fatty acid oxidation. J Biol Chem 2010;285:36495–36510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ii H, Yokoyama N, Yoshida S, et al. Alleviation of high-fat diet-induced fatty liver damage in group IVA phospholipase A2-knockout mice. PLoS One 2009;4:e8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iyer A, Brown L. Lipid mediators and inflammation in glucose intolerance and insulin resistance. Drug Discov Today Dis Mech 2010;7:e191–e197 [Google Scholar]
- 47.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]