Abstract
CCN2, a secreted profibrotic protein, is highly expressed in diabetic nephropathy (DN) and implicated in its pathogenesis; however, the actions of CCN2 in DN remain elusive. We previously demonstrated that CCN2 triggers signaling via tropomyosin receptor kinase A (TrkA). Trace expression of TrkA is found in normal kidneys, but its expression is elevated in several nephropathies; yet its role in DN is unexplored. In this study we show de novo expression of TrkA in human and murine DN. We go on to study the molecular mechanisms leading to TrkA activation and show that it involves hypoxia, as demonstrated by ischemia–reperfusion injury and in vitro experiments mimicking hypoxia, implicating hypoxia as a common pathway leading to disease. We also expose renal cells to hyperglycemia, which led to TrkA phosphorylation in mesangial cells, tubular epithelial cells, and podocytes but not in glomerular endothelial cells and renal fibroblasts. In addition, we report that hyperglycemia caused an induction of phosphorylated extracellular signal–related kinase 1/2 and Snail1 that was abrogated by silencing of TrkA or CCN2 using small interfering RNA. In conclusion, we provide novel evidence that TrkA is activated in diabetic kidneys and suggest that anti-TrkA therapy may prove beneficial in DN.
Diabetic nephropathy (DN) is characterized by excessive deposition of extracellular matrix (ECM) proteins in the mesangium. Mesangial expansion leads to obliteration of glomerular capillaries, followed by tubulointerstitial fibrosis and subsequent loss of organ function (1). A main feature of kidney fibrosis is uncontrolled type I collagen deposition, which is triggered by transforming growth factor-β (TGF-β) (2). High glucose (HG) is involved in the pathogenesis of DN. It induces TGF-β and connective tissue growth factor (CTGF, CCN2) in cultured renal cells and in vivo (3–5). Previous work in our laboratory showed that increased expression of CCN2 correlates with progression of human DN, as shown by in situ hybridization and immunohistochemical analyses of biopsy specimens from patients with different stages of DN (6). CCN2 is also induced by TGF-β, hypoxia (low oxygen), and advanced glycation-end products, all of which are stimulants that result in kidney damage (6–9). Moreover, CCN2 overexpression in transgenic mice with streptozotocin-induced DN led to worsening of the disease, thus indicating the latter may play a profibrotic role after primary injury in the context of diabetes (10).
More evidence that CCN2 is a profibrotic protein comes from studies of genetically modified animals. Sonnylal et al. (11) showed that transgenic mice overexpressing CCN2 under the collagen 1 α 2 promoter (COL1A2) showed a higher susceptibility to carbon tetrachloride-induced liver fibrosis. In addition, mice with a fibroblast/smooth muscle cell–specific deletion of CCN2 were more resistant to bleomycin-induced skin fibrosis than wild-type controls (12).
Thus genetic evidence supports the idea that CCN2 may be involved in driving fibrogenesis in vivo (13). However, we recently showed that the role of CCN2 during fibrosis, at least in the kidney, is more complex than previously thought. We used transgenic CCN2 mice injured with aristolochic acid, which targets kidney tubules and causes tubular death and atrophy, followed by fibrosis, and showed that modest elevation of CCN2 expression did not correlate with more kidney fibrosis, suggesting that CCN2 effects are highly dosage- and context-specific (14). Moreover, experiments with CCN2 knockout mice demonstrated that CCN2 was not required for skin development (15), supporting the importance of obtaining a clearer understanding of the role of CCN2 in physiologic and pathologic situations, in order to use this information to inform new therapeutic interventions.
CCN2 is secreted, and therefore to initiate cellular responses, it is thought to require interaction(s) with plasma membrane receptors. We previously showed that CCN2 activated and coimmunoprecipitated with the tropomyosin receptor kinase A (TrkA) in human mesangial cells (HMCs) (16). TrkA was originally described as a transmembrane receptor for the neurotrophin, nerve growth factor (17). We showed that pharmacologic inhibition of TrkA inhibited CCN2-stimulated signaling in mesangial cells, suggesting the receptor may be essential for some CCN2-mediated effects (16). In accord with this, pharmacologic inhibition of TrkA inhibited CCN2-stimulated induction of monocyte-chemoattractant protein-1 (MCP-1), fractalkine, regulated upon activation, normal T-cell expressed, and secreted (RANTES), and interlukin-8 (IL-8) in human renal cells (18,19). Moreover, although only trace TrkA is expressed in normal kidneys, its expression becomes prominent in human IgA nephropathy, lupus nephritis, and glomerulonephritis (20,21). However, the expression and activation of TrkA in DN is completely unexplored.
Hypoxia is a well-established feature of the diabetic kidney: the decreased bioavailability of nitric oxide leads to cytochrome c oxidase activation, which initiates mitochondrial respiration and results in O2 consumption (22). In addition, sodium potassium ATPase activity is increased during DN, and this is O2-dependent (23). Hypoxia-inducible factor (HIF) is a transcriptional complex that responds to O2 changes; HIF is activated when O2 is low (23,24) and alters transcription of target genes. Therefore, because the kidney may be exposed to hypoxia during DN, we investigated whether low oxygen can activate the TrkA receptor as part of a common pathway leading to end-stage renal disease in diabetes.
RESEARCH DESIGN AND METHODS
Cell cultures, recombinant CCN2, and inhibitors.
Human primary mesangial, epithelial, and endothelial cells, purchased from Lonza and ScienCell Research Laboratories, were cultured according to manufacturers’ instructions. Transformed HMCs (tHMC) were described previously (4) and were grown accordingly. A human podocyte line, grown at 33°C and differentiated at 37°C, was a kind gift by Dr. M.A. Saleem (Bristol, U.K.) (25). TK173 renal fibroblastic line was a gift from Prof. F. Strutz (Gottingen, Germany), and primary human renal fibroblasts were from Dr. J. Norman (London, U.K.). HK2 cells were purchased from American Type Culture Collection and grown according to the manufacturer’s instructions. The tHMC cells were transfected 24 h after seeding as previously described (2). An expression vector containing the CCN2 coding sequence was transfected into tHMC and supernatants were collected (4). Human recombinant CCN2 was purified using Ni-NTA Magnetic Agarose Beads (Qiagen) following the manufacturer’s instructions. HK2 cells were treated with dimethyloxalylglycine (DMOG) (Frontier Scientific) or desferrioxamine (DFO) (Sigma) 24–48 h before analysis for TrkA expression. GW441756, a potent TrkA inhibitor (half-maximal inhibitory concentration = 2 nmol/L), was obtained from Axon Medchem (cat. no. Axon1251). A neutralizing antibody to CCN2 was obtained from PeproTech and used according the manufacturer’s instructions.
Western blotting and immunohistochemistry.
Cells were lysed with radioimmunoprecipitation assay lysis buffer supplemented with protease/phosphatase inhibitors (Invitrogen). Protein concentration was estimated using the BCA Protein Assay kit (Pierce) following the manufacturer’s instructions. SDS-PAGE and transfer was done as previously described (2). The following primary antibodies were used: rabbit polyclonal TrkA antibody (Upstate), phospho-specific antibodies TrkA Tyr490 and Tyr674/675 (Cell Signaling Technology and Santa Cruz), and CCN2, Snail, and phosphorylated extracellular signal–related kinase (pERK; Santa Cruz). Detection was performed using the ECL Plus Detection System (Amersham Pharmacia Biotech), followed by autoradiography. Cluster of differentiation 31 (CD31) was from Abcam (ab28364). Immunohistochemistry was performed as previously described (14). Scoring for Snail was performed using ImagePro software. CD31-positive glomerular cells were counted in 10 glomeruli per mouse and scores were plotted as 3,3'-diaminobenzidine–positive cells.
Real-time RT-PCR.
Total RNA was isolated using TRIzol (Invitrogen), and 2 μg total RNA was reverse transcribed using a one-step reverse transcription kit (Roche). Specific primers for CCN2, TrkA, and β-actin were designed (Primer 3 software) and purchased from Eurofins. Semiquantitative PCR was performed using SYBR green reagent (Applied Biosystems) in an ABI real-time PCR device in accordance with Applied Biosystems’ instructions. RNA was retrieved from formalin-fixed paraffin-embedded tissues from kidney biopsies from three healthy control individuals and four patients diagnosed with DN. Eight slices of 10-μm-thick sections were used for each RNA extraction. Removal of wax was performed in a 1.5-mL tube with xylene/ethanol. RNA was extracted in homogenized tissue using TRIzol reagent according to the manufacturer’s instructions. Purity of RNA was determined by obtaining the 260-to-280 ratio for each sample with NanoDrop ND-1000. Moreover, TrkA primers span exons, lowering the potential of amplification of genomic DNA. Fold change was calculated using the comparative ΔΔCT method.
Human tissues.
The study was performed on histologic sections from biopsy specimens that were surplus to those required for diagnostic purposes and was approved by the Research Ethics Committee at the Hammersmith Hospital. Human control kidney sections were purchased from ProSci Incorporated.
Animals.
Heterozygote DBA/2-Ins2Akita mice and wild-type littermates were purchased from The Jackson Laboratory (Bar Harbor, ME). Beginning at 8 weeks of age, blood glucose was recorded at monthly intervals using a Glucometer Optium Xceed (Pharmex). Blood samples were obtained by puncturing the tail vein with a 21-gauge needle. Approximately 2 μL blood was collected directly onto the testing strip for measurement. Urine was collected from mice that were placed in individual metabolic cages for 24 h with free access to food and water. Urinary creatinine concentration and albumin were measured using the creatinine companion and an indirect competitive enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Exocell, Philadelphia, PA).
At age 22 weeks, mice were killed and their kidneys collected, decapsulated, and incubated in formalin before they were embedded in paraffin. Sections were stained with periodic acid–Schiff (PAS), hematoxylin-eosin (H&E), or specific immunohistochemical staining for TrkA (Upstate) or CD31 (Abcam).
Ischemia–reperfusion injury was performed as described previously (26). Briefly, the left renal artery of mice under inhalation isoflurane anesthesia was clamped with an atraumatic vascular clamp for 45 min, followed by reperfusion for 24 h. The right uninjured kidney was used as a control in all experiments. All mouse experiments were done under the authority of a U.K. Home Office license.
Statistical and bioinformatics analyses.
Data were analyzed with GraphPad Prism software with the one-way ANOVA test with the Bonferroni post hoc test. Conservation between different species (with a human reference sequence) was performed using Web-based genome browser, Vista (http://pipeline.lbl.gov/cgi-bin/gateway2). The sequence of the TrkA promoter was analyzed for potential binding hypoxia response elements (HRE) sites with Genomatix transcription factor Web-based software (www.genomatix.de), and HRE were confirmed by manually searching for the “CGTG” motif within the promoter of TrkA.
RESULTS
Diabetes drives TrkA expression in affected kidneys.
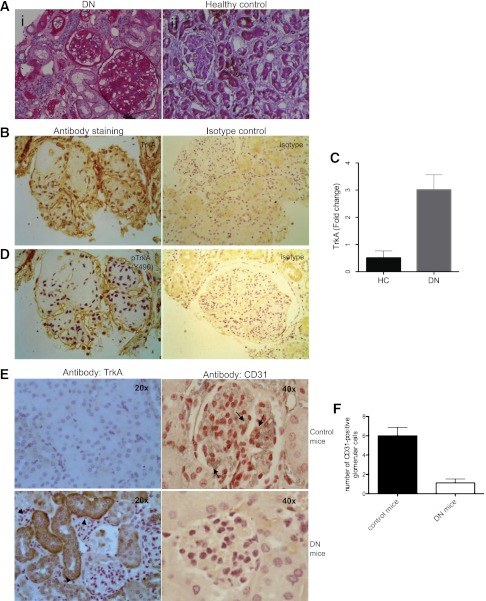
Experiments performed previously in our laboratory identified CCN2 as a protein whose expression is significantly elevated in injured glomeruli during DN. Because there was ambiguity about how CCN2, which is a secreted cytokine, triggers intracellular signaling, our laboratory discovered an interaction between CCN2 and TrkA that is vital for ERK signaling via CCN2. In this study we decided to investigate the expression of TrkA in kidneys from patients with established DN and compare this expression with control tissue. Histologic examination of specimens from five individuals with DN showed glycogen deposition, casts, cellular infiltrate, and diffuse fibrosis (Fig. 1A). Immunohistochemical detection of TrkA was performed, and although we only detected trace TrkA in healthy human tissue, TrkA was highly expressed in all of the specimens of patients with DN that were studied (Fig. 1B, left panel). The specificity of the antibody stained was confirmed by performing isotype control staining, which resulted in weak staining (Fig. 1B, right panel). TrkA was expressed in tubular epithelial cells and in glomerular cells of affected kidneys. This is in accord with our previous finding that CCN2 is expressed in injured glomeruli and may suggest that released CCN2 activates TrkA and, via this, initiates downstream signaling. TrkA expression was also checked by real-time PCR, and we report that TrkA expression is low in healthy human control (HC, n = 3) kidneys, whereas TrkA mRNA expression is higher in patients with DN (n = 4) compared with HC (Fig. 1C). The RNA absorbance 260-to-280 ratio, which illustrates the purity of RNA, was 1.8 ± 0.17. We then used a phospho-specific antibody to study whether TrkA was activated by studying the phosphorylated form of TrkA. We report that TrkA was phosphorylated (tyrosine Y490) in DN patients (Fig. 1D). Phosphorylation of TrkA at Y490 is associated with initiating receptor signaling through the ERK/mitogen-activated protein kinase pathway (16).
FIG. 1.
Diabetes drives TrkA expression in affected kidneys. A: Five biopsy specimens of patients with DN (i) and tissues from human control individuals (ii) were stained with PAS; representative photomicrographs are shown. B: Immunohistochemistry for TrkA was performed in all biopsy specimens and representative microphotographs of DN and control tissue are shown. C: Real-time PCR for TrkA expression in specimens of tissues from kidneys of HC patients (n = 3) and DN patients (n = 4) was performed. Results are shown as fold change normalized to one of the HC samples. D: Phosphorylated form of TrkA (Y490) was detected with specific antibody in DN but was not present, or barely detectable, in control specimens (blue-purple, hematoxylin counterstain of nuclei; brown, specific stain of TrkA). E: A murine model of DN (DBA/2-Ins2Akita, lower panel) and DBA/2 control (upper panel) mice were probed immunohistochemically for CD31 expression, a marker of endothelial cells (brown, CD31; blue-purple, hematoxylin nuclear counterstain) and for TrkA (brown, TrkA; blue-purple, hematoxylin nuclear counterstain). Data for n = 6 mice; representative photomicrographs are shown. The arrows indicate CD31-positive cells in glomeruli; arrowheads indicate TrkA staining in tubules. F: CD31-postive glomerular cells were counted in control mice and compared with CD-31 positivity in glomeruli of DN mice. The error bars in C and F show the SD. (A high-quality digital representation of this figure is available in the online issue.)
After establishing that TrkA was activated in human DN, we studied TrkA in a mouse model of DN. The previously characterized DBA/2 mice, heterozygous for the insulin 2 (C96Y) mutation (Akita), were used for this (27,28). The mice developed kidney disease, as illustrated by their albumin-to-creatinine ratio of 91 ± 21.3 for controls vs 370 ± 81.3 for diabetic mice (P = 0.002). Strong tubular epithelial expression of TrkA was detected in diabetic but not in control mice (Fig. 1E, left panel).
Because loss of endothelial cells has been reported during DN (29), we studied the expression of CD31 (endothelial cell marker) in glomeruli of DN and control mice. We report that CD31 was expressed in glomeruli of control kidneys, whereas diabetic mice showed a marked reduction in glomerular CD31-positive cells, suggestive of endothelial cell death (Fig. 1E, right panel). Quantification of numbers of CD31-positive cells was performed (Fig. 1F). The expression of CD31 in renal cortical vessels (marking vascular endothelial cells) did not change. The Akita mouse strain on a DBA/2 genetic background became hyperglycemic early on; however, we experienced problems with these mice because they became sick and lost significant weight, and the experiment had to be terminated at age 6 months. Therefore, using the Akita mouse strain on a DBA/2 background would be advisable if shorter-term glucose effects are the main aim of the study.
HG-induced TrkA phosphorylation in mesangial cells is CCN2-dependent.
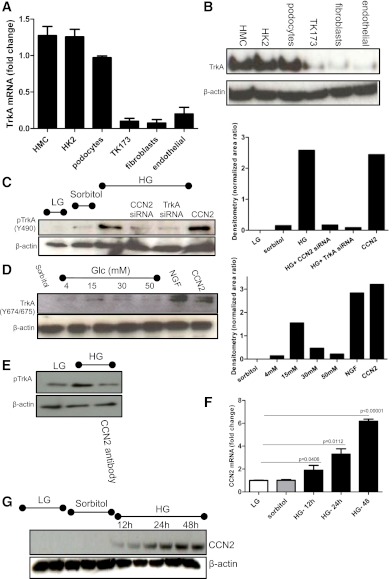
Having established that diabetes drives TrkA expression in vivo, we decided to study this in vitro using a model of hyperglycemia. We tested whether human kidney primary and transformed cell lines express TrkA. We report that TrkA is expressed in cultures of serum-starved mesangial and proximal tubular epithelial cells, as well as in podocytes, but is not expressed in renal fibroblasts or renal glomerular endothelial cells, as shown by mRNA and protein (Fig. 2A and B, respectively). We then studied the effects of glucose in mediating phosphorylation of TrkA (Y490). We exposed cells to HG (30 mmol/L), low glucose (LG, 4 mmol/L), or to an osmotic control (26 mmol/L sorbitol and 4 mmol/L glucose). This did not alter total TrkA expression, as determined by Western blotting; however, HG led to a significant increase of TrkA phosphorylation at positions Y490 (Fig. 2C) and Y674/675 (Fig. 2D). We also stimulated cells with recombinant human CCN2 and recombinant nerve growth factor as positive controls for the experiment (Fig. 2D). We report that CCN2 stimulated the receptor at Y490 to a similar level as that observed with HG stimulation (Fig. 2C); however, CCN2 led to more phosphorylation than glucose at Y674/675, suggesting a dose-dependent activation of the receptor (Fig. 2C and D, quantification, lower panels).
FIG. 2.
HG-induced TrkA phosphorylation is CCN2-dependent. A: Real-time PCR was performed to detect TrkA mRNA in samples collected from HMCs, human tubular epithelial cells (HK2), human renal transformed and differentiated podocytes (podocytes), human transformed renal fibroblasts (TK173), human primary renal fibroblasts (fibroblasts), and human primary glomerular endothelial cells (endothelial). Data are expressed as fold change relative to expression of TrkA in podocytes (n = 5). Error bars show the SD. B: Western blot was performed to detect TrkA protein expression in cells described; a representative blot is shown (n = 3). C: Phosphorylated (Y490) TrkA and actin loading control protein expression was detected by Western blot in human primary mesangial cells; a representative blot is shown (n = 3). Quantification of the Western blot is shown (right-hand side panel). D: Phosphorylated TrkA at position Y674/675 was detected by Western blot and a representative blot is shown (n = 3). Quantification of the Western blot is shown (right-hand side panel). E: pTrkA was studied in LG and HG in the presence or absence of neutralizing antibody. F: Real-time PCR was performed to detect CCN2 mRNA expression in mesangial cells in response to HG and LG stimulation. Data are represented as fold change (n = 5). G: Western blot for CCN2 in HMC stimulated for increasing time with HG or LG or sorbitol as the osmotic control (n = 3). (A high-quality color representation of this figure is available in the online issue.)
We then investigated whether CCN2 is involved in the HG-induced phosphorylation of TrkA. To study this, we silenced CCN2 using specific small interfering RNA (siRNA) sequences and found that in the absence of CCN2, HG failed to induce phosphorylation of TrkA, suggesting that CCN2 is required for this activation (Fig. 2C, CCN2 siRNA). HG also failed to stimulate phosphorylation of TrkA in the presence of a neutralizing antibody to CCN2 (Fig. 2E). To confirm that CCN2 was produced in response to HG stimulation, we studied CCN2 production under LG and HG concentrations. We report that HG led to a time-dependent upregulation of CCN2 mRNA and protein (Fig. 2F and G, respectively), suggesting that some of the HG effects can be attributed to CCN2 production. Collectively, we conclude that HG led to TrkA phosphorylation in a CCN2-dependent manner.
TrkA is required for HG-induced ERK phosphorylation and Snail production.
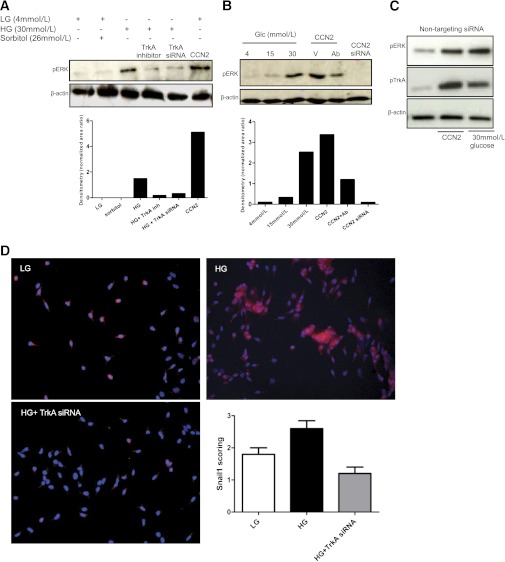
Isono et al. (30) reported that HG-stimulated ERK1/2 activation led to TGF-β1 upregulation in mesangial cells and contributed to increased extracellular matrix expression. We confirmed that HG induces phosphorylation of ERK1/2 in mesangial cells and, importantly, showed that activation of ERK was inhibited by silencing TrkA with siRNAs or pharmacologic inhibition (Fig. 3A) or by inhibiting CCN2 with specific siRNA against CCN2 or a neutralizing antibody to CCN2 (Fig. 3B). Scrambled siRNAs had no effects on pERK or pTrkA status in mesangial cells stimulated with HG or CCN2 (Fig. 3C). We thus propose that activation of ERK1/2, in response to HG in HMCs requires upregulation of CCN2 and activation of its receptor, TrkA. HG stimulation led to a significant upregulation of the transcription factor Snail1, at the level of protein, in HK2 epithelial cells. The induction of Snail1 by HG was abrogated using selective silencing of TrkA (Fig. 3D, lower panel), suggesting that HG induces Snail via activation of the TrkA receptor pathway. Induction of Snail1 protein by HG has not been reported previously; however, glucose-depleted cancer cells undergo Snail1-dependent necrosis, hence implicating Snail1 in metabolic stress-induced death (31). Moreover, a previous study showed mRNA for Snail1 was induced by glucose in epithelial cells (32). We also studied the expression of Snail1 in mesangial cells and report it was below detection level in cells maintained in LG or HG.
FIG. 3.
TrkA is required for HG-induced ERK phosphorylation and Snail production. A: Western blot analysis for pERK and total β-actin was performed in mesangial cells treated with LG, sorbitol control, HG, or CCN2. TrkA was inhibited with specific siRNA or with pharmacologic inhibition (100 nmol/L GW441756). A representative blot is shown (n = 3). Quantification of the Western blot is shown in the lower panel. B: Western blot of pERK is shown for mesangial cells treated with different concentrations of glucose, or transfected with a CCN2 vector (V) alone, or together with CCN2 neutralizing antibody (Ab) treatment, or with silencing of CCN2 using a specific siRNA. A representative blot is shown (n = 3). Quantification of the Western blot is shown in the lower panel. C: Western blotting of pTrkA, pERK, and β-actin loading control in cells transfected with nontargeting siRNA is shown. D: Snail1 immunofluorescence was performed in HK2 cells using rabbit anti-Snail1 primary antibody and anti-rabbit Cy3 conjugated secondary antibody (pink–red, Snail1; blue, DAPI nuclear counterstain). HG stimulated Snail1 production and TrkA siRNA inhibited the HG-stimulated Snail1 expression. Representative photomicrographs are shown (n = 5). Scoring of Snail immunofluorescence is shown. The error bars indicate the SD. (A high-quality digital representation of this figure is available in the online issue.)
Hypoxia induces TrkA and may lead to kidney damage in vivo.
Hypoxia has been proposed to lead to accumulation of extracellular matrix and fibrosis. In the kidney, during DN, it is suggested that certain regions are hypoxic. For this reason, we hypothesized that a potential common pathway that may lead to TrkA expression in vivo is hypoxia. Genes that are upregulated by hypoxia have distinct consensus sites on their promoters, HREs. HIF1α has been shown to interact with HRE in relevant promoters to activate transcription of the given gene. The classical HRE motif is 5′-ACGTG-3′. Increased expression of HIF1α has been reported in mesangial cells of diabetic mice (33).
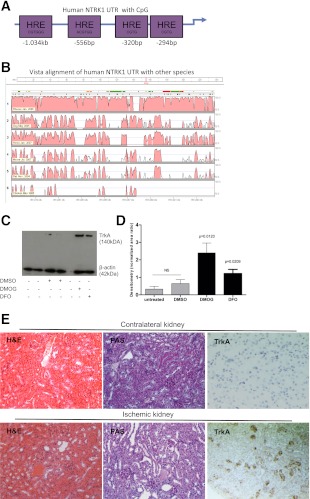
To test whether TrkA contained HRE sites, we performed bioinformatics analyses of the promoter of TrkA (i.e., up to 1.5 kb upstream of the transcriptional start site) using the Vista genome browser and Genomatix Web-based transcription factor software. We report that the TrkA promoter contains four canonical HRE sites that are spread within its promoter. The exact sites of the HREs are shown schematically (Fig. 4A). We also studied the conservation of base pairs between human (reference sequence), monkey (Rhesus), dog, horse, mouse, rat, and chicken. This homology search showed that the promoter of TrkA is highly conserved between the human and the mouse, suggesting the existence of important regulatory sequences (Fig. 4B) that could be studied using a murine model.
FIG. 4.
Hypoxia induces TrkA and may lead to kidney damage in vivo. A: Bioinformatics analysis of the promoter of TrkA was performed and four HRE sites were found and are diagrammatically presented. kb, kilobases; bp, base pairs. B: Vista Genome browser analysis was performed to find homology between the human and the mouse promoter sequence for TrkA. C: Western blot analysis for TrkA and β-actin was performed in HK2 cells treated with DFO or DMOG. Representative blot is shown (n = 4). D: Densitometry of TrkA protein expression was performed (n = 4). E: A mouse model of ischemia–reperfusion was used to study the expression of TrkA in vivo. H&E and PAS were performed to assess renal damage after ischemic injury. TrkA was detected, immunohistochemically, in ischemic kidneys but was absent in the contralateral control kidneys (right side, brown stain; n = 5). UTR, untranslated regions. The error bars show the SD. (A high-quality digital representation of this figure is available in the online issue.)
We then went on to mimic the effects of low oxygen, using small molecules, and studied the effects of hypoxia on TrkA expression in human proximal tubular cells (HK2). TrkA is expressed in serum-starved conditions, as previously shown (Fig. 2A), but not in the presence of serum unless the cells are otherwise challenged. HK2 cells, grown in 5% serum, were exposed to DMOG, a cell-permeable, competitive inhibitor of HIF-α-prolyl hydroxylase (a stabilizer of HIF1) (34), or DFO, an iron-chelator shown to induce HIF accumulation (35), or DMSO as a negative control. Accumulation of HIF1α with DMOG or DFO led to a significant increase of TrkA protein observed by Western blotting (Fig. 4C). These data are quantified in Fig. 4D.
Having established that the TrkA promoter contains sites for HIF1α binding that are conserved between the human and the mouse, we then studied whether hypoxia may lead to an increase of TrkA in vivo by performing murine ischemia–reperfusion injury. As expected, normal contralateral (uninjured) kidneys did not express TrkA; however, the kidneys that were subjected to hypoxia showed damage that was associated with increased TrkA expression (Fig. 4E). The renal damage observed in these mice is mainly localized in the tubules, as seen by tubular dilatation and necrosis (Fig. 4E, PAS and H&E staining). Consistent with tubular damage, the expression of TrkA was also mainly localized in the tubules (Fig. 4E, brown stain, TrkA; purple–blue, hematoxylin nuclear counterstain). In conclusion, these data indicate that TrkA is a hypoxia-inducible gene that is upregulated in the kidney by ischemia–reperfusion injury and in vitro by mimicking of hypoxia.
DISCUSSION
TrkA is a member of the Trk family of plasma membrane receptors (TrkA, TrkB, TrkC). These receptors interact with neurotrophins, as homodimers or as heterodimers with the pan neurotrophin receptor, p75NTR. Neurotrophins, such as nerve growth factor, brain-derived neurotrophic factor, neurotrophin 3 and 4, and their receptors, are critical for the development, survival, and function of neurons (36). Previous work performed in our laboratory has revealed that mesangial cell TrkA was activated by CCN2 and co-immunoprecipitated with it in crosslinking experiments (16), suggesting a role for TrkA activation in the kidney. Moreover, ephrin A5, another non-neurotrophin protein, was found to interact with the TrkB receptor (37). Therefore, it is evident that Trk receptors are also activated by selected non-neurotrophin ligands in non-neuronal tissues, perhaps implicating their involvement in other functions.
Our finding that TrkA is expressed in kidneys of DN patients but not in healthy control subjects is important because we previously showed that CCN2 is also markedly increased in DN kidneys. Therefore, we put forward the idea that HG induces CCN2 expression, which triggers phosphorylation of TrkA and initiates intracellular signaling that may result in fibrosis. CCN2 exerts several actions on mesangial cells in vitro that are likely to drive glomerulosclerosis in DN (7), and silencing of CCN2 in mice, with the use of antisense oligonucleotides, ameliorates the development of kidney changes in murine models of type 1 and 2 diabetes (38). Moreover, a polymorphism in the promoter of CCN2 has been associated with scleroderma, a condition that involves fibrosis of multiple organs (39). However, we recently showed that enhanced expression of CCN2 in murine aristolochic acid nephropathy did not lead to enhanced fibrosis and postulated that this might be due to dosage effects (14). Therefore, although the role of CCN2 in fibrogenesis is established, new findings highlight the importance of understanding how CCN2 interacts with other molecules to initiate signaling. Our observation that TrkA is expressed, although not activated, in several renal cell types in LG culture conditions in vitro contrasts with in vivo findings where it is not expressed in normoglycemic controls. Because we established that TrkA expression in vivo is upregulated in response to stressful conditions (e.g., hyperglycemia, hypoxia), it seems likely that the in vitro expression observed may be a response to stresses arising from cell culture itself.
Our present study provides evidence for a pathway in which HG causes cellular damage via TrkA, which is required for the induction of the Snail1 transcription factor. Induction of Snail is already implicated in metabolic stress conditions because Snail is necessary for glucose depletion–induced necrosis of cancer cells (31). Snail is a transcription factor that is expressed in human fibrotic but not in control kidney tissue. Activation of Snail in adult transgenic mice is sufficient to induce tubular dilatation and kidney fibrosis (40). Although this was attributed to epithelial-mesenchymal transition, a phenomenon known to be induced by Snail in vitro, there is now debate about whether epithelial-mesenchymal transition occurs in vivo (41). Thus, understanding the role of TrkA-induced Snail in vivo in HG conditions is highly important and will require further experimental work.
We report upregulation of TrkA in kidneys of a previously described murine model of DN (27,28). Mice with the Akita mutation on a DBA/2 background had prolonged hyperglycemia and proteinuria, in keeping with previously published data (28). They lost weight and became sick early on, requiring termination of the experimental protocol earlier than anticipated. Histologic examination of the kidneys revealed that there was no fibrosis at this stage of disease; however, there was marked proteinuria and mesangial expansion accompanied by endothelial cell loss as detected by reduced expression of CD31, a marker of endothelial cells. Loss of endothelial cells in human and rat DN is established and is thought to be an important early pathogenic factor leading to glomerular damage (29). It is of interest that TrkA was already expressed in such kidneys, suggesting an early involvement of TrkA in possibly driving some renal changes before fibrosis. Our in vivo studies on diabetic mice posed the question about whether hyperglycemia may directly stimulate TrkA in renal cells, and our in vitro experiments shed light on this question by showing that HG led to TrkA activation in a CCN2-dependent manner. Hence, we propose that TrkA may be one of the pathogenic factors induced by HG and serves as a receptor for some of the effects of CCN2.
The effects of HG in the kidney are multifactorial, and TrkA represents one aspect of this series of events that results in end-stage renal disease. In search for a common pathway that may lead to a number of transcriptional changes in the kidney that may cause damage, we investigated hypoxia. Hypoxia activates HIF transcription factors that alter transcription of several target genes. We showed that TrkA was activated by hypoxia not only in cells but also in vivo using a mouse model of ischemia–reperfusion. Moreover, because it is very likely that certain regions of the kidney during DN become hypoxic early during disease development, it is possible to hypothesize that hypoxia may be a common pathway that leads to activation of TrkA, CCN2, and other proteins that synergistically lead to tissue damage. Therefore, inhibiting hypoxia-inducible factors and/or preventing tissue hypoxia or some of the target molecules, such as TrkA, may prove beneficial in preventing kidney fibrosis.
ACKNOWLEDGMENTS
This work was funded by a Diabetes UK project grant.
No potential conflicts of interest relevant to this article were reported.
M.F. designed and performed the experiments, researched the data, and wrote and reviewed the manuscript. N.H. and R.H. performed the experiments. G.B.-G., F.W.T., and J.D. reviewed the manuscript. T.C. analyzed immunohistochemistry and reviewed the manuscript. R.M.M. proposed the project, designed the experiments, and wrote and reviewed the manuscript. R.M.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 2003;14:1358–1373 [DOI] [PubMed] [Google Scholar]
- 2.Fragiadaki M, Ikeda T, Witherden A, Mason RM, Abraham D, Bou-Gharios G. High doses of TGF-β potently suppress type I collagen via the transcription factor CUX1. Mol Biol Cell 2011;22:1836–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riser BL, Denichilo M, Cortes P, et al. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol 2000;11:25–38 [DOI] [PubMed] [Google Scholar]
- 4.Wahab NA, Yevdokimon N, Weston BS, et al. Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem J 2001;359:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest 1994;93:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahab NA, Schaefer L, Weston BS, et al. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia 2005;12:2650–2660 [DOI] [PubMed] [Google Scholar]
- 7.Mason RM. Connective tissue growth factor(CCN2), a pathogenic factor in diabetic nephropathy. What does it do? How does it do it? J Cell Commun Signal 2009;3:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy M, Godson C, Cannon S, et al. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem 1999;274:5830–5834 [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Zhang A, Li R, et al. High glucose promotes the CTGF expression in human mesangial cells via serum and glucocorticoid-induced kinase 1 pathway. J Huazhong Univ Sci Technolog Med Sci 2008;28:508–512 [DOI] [PubMed] [Google Scholar]
- 10.Yokoi H, Mukoyama M, Mori K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int 2008;73:446–455 [DOI] [PubMed] [Google Scholar]
- 11.Sonnylal S, Shi-Wen X, Leoni P, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum 2010;62:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Shi-wen X, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum 2011;63:239–246 [DOI] [PubMed] [Google Scholar]
- 13.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal 2010;4:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fragiadaki M, Witherden AS, Kaneko T, et al. Interstitial fibrosis is associated with increased COL1A2 transcription in AA-injured renal tubular epithelial cells in vivo. Matrix Biol 2011;30:396–403 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Leask A. CCN2 is not required for skin development. J Cell Commun Signal 2011;5:179–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahab NA, Weston BS, Mason RM. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J Am Soc Nephrol 2005;16:340–351 [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Jing SQ, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell 1991;65:189–197 [DOI] [PubMed] [Google Scholar]
- 18.Wang X, McLennan SV, Allen TJ, Twigg SM. Regulation of pro-inflammatory and pro-fibrotic factors by CCN2/CTGF in H9c2 cardiomyocytes. J Cell Commun Signal 2010;4:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu SH, Lu C, Dong L, Chen ZQ. Signal transduction involved in CTGF-induced production of chemokines in mesangial cells. Growth Factors 2008;26:192–200 [DOI] [PubMed] [Google Scholar]
- 20.Antonucci MT, Bonofiglio R, Papalia T, et al. Nerve growth factor and its monocyte receptors are affected in kidney disease. Nephron Clin Pract 2009;111:c21–c28 [DOI] [PubMed] [Google Scholar]
- 21.Bonofiglio R, Antonucci MT, Papalia T, et al. Nerve growth factor (NGF) and NGF-receptor expression in diseased human kidneys. J Nephrol 2007;20:186–195 [PubMed] [Google Scholar]
- 22.Edlund J, Fasching A, Liss P, Hansell P, Palm F. The roles of NADPH-oxidase and nNOS for the increased oxidative stress and the oxygen consumption in the diabetic kidney. Diabetes Metab Res Rev 2010;26:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock JS, Carmines PK. Diabetic nephropathy: nitric oxide and renal medullary hypoxia. Am J Physiol Renal Physiol 2008;294:F28–F29 [DOI] [PubMed] [Google Scholar]
- 24.Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol 2005;90:791–797 [DOI] [PubMed] [Google Scholar]
- 25.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 2002;13:630–638 [DOI] [PubMed] [Google Scholar]
- 26.Stokman G, Leemans JC, Claessen N, Weening JJ, Florquin S. Hematopoietic stem cell mobilization therapy accelerates recovery of renal function independent of stem cell contribution. J Am Soc Nephrol 2005;16:1684–1692 [DOI] [PubMed] [Google Scholar]
- 27.Brosius FC, 3rd, Alpers CE, Bottinger EP, et al. Animal Models of Diabetic Complications Consortium Mouse models of diabetic nephropathy. J Am Soc Nephrol 2009;20:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurley SB, Mach CL, Stegbauer J, et al. Influence of genetic background on albuminuria and kidney injury in Ins2(+/C96Y) (Akita) mice. Am J Physiol Renal Physiol 2010;298:F788–F795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly DJ, Buck D, Cox AJ, Zhang Y, Gilbert RE. Effects on protein kinase C-beta inhibition on glomerular vascular endothelial growth factor expression and endothelial cells in advanced experimental diabetic nephropathy. Am J Physiol Renal Physiol 2007;293:F565–F574 [DOI] [PubMed] [Google Scholar]
- 30.Isono M, Cruz MC, Chen S, Hong SW, Ziyadeh FN. Extracellular signal-regulated kinase mediates stimulation of TGF-beta1 and matrix by high glucose in mesangial cells. J Am Soc Nephrol 2000;11:2222–2230 [DOI] [PubMed] [Google Scholar]
- 31.Kim CH, Jeon HM, Lee SY, et al. Implication of snail in metabolic stress-induced necrosis. PLoS ONE 2011;6:e18000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumual S, Saad S, Tang O, et al. Differential regulation of Snail by hypoxia and hyperglycemia in human proximal tubule cells. Int J Biochem Cell Biol 2010;42:1689–1697 [DOI] [PubMed] [Google Scholar]
- 33.Kishi S, Abe H, Akiyama H, et al. SOX9 protein induces a chondrogenic phenotype of mesangial cells and contributes to advanced diabetic nephropathy. J Biol Chem 2011;286:32162–32169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudecova S, Lencesova L, Csaderova L, et al. Chemically mimicked hypoxia modulates gene expression and protein levels of the sodium calcium exchanger in HEK 293 cell line via HIF-1α. Gen Physiol Biophys 2011;30:196–206 [DOI] [PubMed] [Google Scholar]
- 35.Ren X, Dorrington KL, Maxwell PH, Robbins PA. Effects of desferrioxamine on serum erythropoietin and ventilatory sensitivity to hypoxia in humans. J Appl Physiol 2000;89:680–686 [DOI] [PubMed] [Google Scholar]
- 36.Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 2006;110:175–191 [DOI] [PubMed] [Google Scholar]
- 37.Hutchins BI, Li L. EphrinA and TrkB interact to promote axon branching. J Neurosci 2009;29:4329–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J 2007;21:3355–3368 [DOI] [PubMed] [Google Scholar]
- 39.Fonseca C, Lindahl GE, Ponticos M, et al. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N Engl J Med 2007;357:1210–1220 [DOI] [PubMed] [Google Scholar]
- 40.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 2006;25:5603–5613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int J Exp Pathol 2011;92:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]