Abstract
T-cell regulation in adipose tissue provides a link between inflammation and insulin resistance. Because of alterations in adipose tissue T-cell composition in obesity, we aimed to identify the antigen-presenting cells in adipose tissue of obese mice and patients with insulin resistance. Dendritic cells (DCs) and T cells were studied in mice and in two cohorts of obese patients. In lean mice, only CD11c+ DCs were detected in adipose tissue. Adoptive transfer of naive CD4+ T cells in Rag1−/− mice led to a predominant Th1 response in adipose tissue. In contrast, during obesity DCs (human CD11c+CD1c+ and mouse CD11chighF4/80low) accumulated in adipose tissue. CD11chighF4/80low DCs from obese mice induced Th17 differentiation. In patients, the presence of CD11c+CD1c+ DCs correlated with the BMI and with an elevation in Th17 cells. In addition, these DCs led to ex vivo Th17 differentiation. CD1c gene expression further correlated with homeostatic model assessment-insulin resistance in the subcutaneous adipose tissue of obese patients. We show for the first time the presence and accumulation of specific DCs in adipose tissue in mouse and human obesity. These DCs were functional and could be important regulators of adipose tissue inflammation by regulating the switch toward Th17 cell responses in obesity-associated insulin resistance.
Obesity has become a major worldwide health problem. It is associated with an increased risk in developing of type 2 diabetes, hypertension, and nonalcoholic fatty liver disease (NAFLD), which reduces life expectancy and leads to huge economic and social consequences (1). Obesity is characterized by low-grade chronic inflammation, as evidenced by an increased systemic concentration of proinflammatory molecules such as interleukin (IL)-6 (2). Thus, cytokine production can be deregulated in obesity, contributing substantially to insulin resistance (3).
In both humans and rodents, macrophage accumulation in adipose tissue (AT) in obese conditions correlates with insulin resistance (4–7). These macrophages might be a source of proinflammatory cytokines such as tumor necrosis factor -α and IL-6 that inhibit insulin action in adipocytes and provide a potential link between inflammation and insulin resistance (4,5). A recent study reported that a specific subset of F4/80+ macrophages is recruited into AT in diet-induced obesity in mice. These macrophages express a low level of CD11c (CD11clow) and produce a high level of proinflammatory cytokines involved in the development of insulin resistance (8). Depletion of CD11c+ cells by conditional ablation based on transgenic expression of the diphtheria toxin receptor under the control of CD11c promoter normalized insulin sensitivity in obese and insulin-resistant animals (9). Such an approach probably depletes dendritic cells (DCs) since CD11c is considered to be a specific marker of mouse DCs (10). However, few data are available concerning either DCs in AT or their potential role in the inflammatory process associated with obesity and insulin resistance (11,12).
DCs are key participants in innate and adaptive immune responses (13). The prevalent model of DC migration is a unidirectional pathway whereby precursor DCs arise from bone marrow progenitors, reach the blood, and traffic into secondary lymphoid organs and peripheral tissues where they contribute to the front line of defense against pathogens (14). DCs are a heterogeneous population of cells including the conventional/myeloid DCs (cDCs) and the plasmacytoid DCs (pDCs), which were originally isolated from the spleen but which are found in almost all tissues in a steady state (15,16). Recently, a novel DC population called inflammatory DCs (inf-DCs) was described to be generated from inflammatory monocytes while absent in a steady state and to be related to infection or inflammation (17,18). Furthermore, DCs are well recognized for their role in the priming and differentiation of naive CD4+ T cells. Upon antigenic stimulation, naive CD4+ T cells become effector T helper Th1, Th2, or Th17 cells, or even regulatory T cells (Tregs) depending on their cytokine microenvironment (19–21).
Recent studies have reported that T cells are also regulated in AT and might contribute to obesity-induced inflammation (22). In AT of lean mice, the major T-cell population corresponds to Tregs (23). Under high-fat diet (HFD), the number of AT Tregs decreases, whereas Th1 cells increase followed by the recruitment of inflammatory macrophages into AT (24). Moreover, it was suggested that AT T cells exhibited specific rearrangements in T-cell receptor, suggesting the presence in AT of DCs that presents antigens and induces T-cell polarization. Thus, our aim was to identify and characterize DCs in AT and to study their potential role in AT T-cell polarization in lean and obese mice and patients.
Here, we defined human CD11c+CD1c+ DCs and mouse CD11chighF4/80low DCs as inflammatory DCs in AT during obesity-associated insulin resistance, and we showed their potential capacity to induce Th17 cell responses.
RESEARCH DESIGN AND METHODS
Study population.
For flow cytometric analysis, subcutaneous AT was obtained from healthy women undergoing elective procedures for fat removal for aesthetic purposes (n = 24 individuals, age = 44.6 ± 2.5 years, BMI = 28.3 ± 1.2 kg/m2). This protocol was approved by the Institutional Research Board of INSERM and the Toulouse University Hospital. For gene expression, morbidly obese patients (n = 20) undergoing bariatric surgery were recruited through the Department of Digestive Surgery and Liver Transplantation (Nice hospital) as described previously (25–27). Before surgery, fasting blood samples were obtained to measure glucose, insulin, and HbA1c. Insulin resistance was calculated using the homeostatic model assessment (HOMA-IR) index. Abdominal subcutaneous AT was obtained during surgery. Control subcutaneous AT was obtained from four lean subjects (2 females and 2 males; age 37.2 ± 11.5 years; BMI 21.1 ± 0.7 kg/m2) undergoing lipectomy for cosmetic purposes. Informed written consent was obtained from all subjects for this study, which was set up in accordance with French legislation regarding Ethics and Human Research (Huriet-Serusclat). The Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale de Nice approved the study (07/04:2003, N° 03.017).
Animals.
Wild-type C57BL/6 male mice (7–10 weeks of age) from Janvier (Le Genest-St-Isle, France) had free access to water and were fed an HFD containing 45% fat (D12451; Research Diet) for 15 weeks. As expected these mice exhibited increased weight. Obese db/db mice 8 weeks old were purchased from Janvier (Le Genest-St-Isle, France). Both obese models displayed marked insulin resistance as evaluated by a higher fasting glucose level (db/db: 275 ± 37 g/L vs. db/+: 109 ± 17 g/L, P = 0.0081; HFD: 187 ± 12 g/L vs. normal diet [ND]: 133 ± 6 g/L, P = 0.0137; five mice per group) and area under the curve (AUC) of the glucose tolerance test compared with the db/+ and lean mice (ND) (db/db: AUC 24,342 ± 3,345 vs. db/+: AUC 9,767 ± 1,909, P = 0.0367; HFD: AUC 22,313 ± 1,551 vs. ND: AUC 13,381 ± 2,830, P = 0.0216; five mice per group), respectively. C57BL/6 recombinase-activating gene deficient (Rag1−/−) mice and ovalbumine (OVA)-specific T-cell receptor (OT2) mice were obtained from Charles River Laboratories. The guidelines of laboratory animal care were followed, and the local Ethical Committee approved the animal experiments.
Transfer of naive T cells into Rag1−/− mice.
Naive OVA-specific CD4+ T cells were isolated from the spleen of OT2 mice and fluorescence-activated cell sorter (FACS)-sorted based on CD62L+CD44− expression, and 106 cells were transferred into Rag1−/− mice immunized previously intraperitoneally with 50 μg of OVA protein.
Preparation of the stromal vascular fraction from adipose tissue.
In mice studies, white epididymal AT pads were removed, immediately cut into small pieces, and rinsed in a buffer containing 120 mmol/L NaCl, 4 mmol/L KH2PO4, 1 mmol/L MgSO4, 750 µM CaCl2, 10 mmol/L NaHCO3, and 30 mmol/L HEPES (pH 7.4). Explants were incubated at 37°C for 30 min in 15 mL of the above buffer supplemented with 1% BSA, 280 μM glucose, and 15 mg of type-1 collagenase (Worthington Biochemical Corporation). Adipocytes were then collected by filtration and floatation. The stromal vascular fraction (SVF) was collected by centrifugation for 15 min at 260g. In human studies, AT was immediately processed after removal and digested using collagenase (250 units/mL in PBS1×, 2% BSA [pH 7.4]) for 30 min under constant shaking. After centrifugation (300g, 10 min), the pellet containing the SVF was resuspended in erythrocyte lysis buffer (155 mmol/L NH4Cl, 5.7 mmol/L K2HPO4, 0.1 mmol/L EDTA) for 10 min. Finally and after successive filtration through 100-, 70-, and 40-µm sieves, the cells were resuspended in PBS plus 2% FCS. The SVF was analyzed by flow cytometry or used to separate the different cellular fractions. Cells were stained as described previously (28).
Antibodies and flow cytometric analysis.
Rat antibodies mouse-CD3 (17A2), -CD4 (H129.19), -CD8 (53–6.7), -CD11b (M1/70), -CD11c (HL3), -CD45, -CD62 L (Mel-14), -CD86 (GL1), -CD103 (M290), -IAd (2G9), -IL-4 (BVD6–2462), -IL-10 (JES5–16E3), -IL-17 (TC11–18H10.1), and -interferon (IFN)-γ (XMG1.2) were purchased from Becton-Dickinson. Antibodies against F4/80 and CX3CR1 were purchased from e-biosciences and LS Biosciences, respectively. Mouse antibodies against human CD4 (SK3) and CD45RA (HI100) were purchased from BD Biosciences.
For the intracellular cytokine assay, two rounds of 5 days of stimulation of 2 × 105 naive OVA-specific T cells from OT2 mice were performed with different 5 × 104 DCs sorted from the SVF of AT as mentioned, in the presence of OVA peptide (0.6 µM). Fourteen days later, cells were stimulated for 4 h by ionomycin and phorbol 12-myristate 13-acetate in the presence of stop Golgi (BD Biosciences) according to the manufacturer’s instruction, and stained for intracellular cytokines as described previously (18). Human CD4+ T cells (1 × 105) sorted from the blood of healthy donors (FACSAria; BD Biosciences) were cultured for 6 days with allogeneic CD1c+ DCs (1 × 104) sorted from AT of obese patients. Cultures were restimulated on day 7 with ionomycin and phorbol 12-myristate 13-acetate and stained for intracellular cytokines.
Human DCs were analyzed using a blood DC enumeration kit (Miltenyi-Biotec) following the manufacturer’s instructions. Fractions of freshly isolated CD34−CD14−CD3+ cells, defined as T lymphocytes (patient age = 42.2 ± 1.7 years, BMI = 28.8 ± 1.1 kg/m2, n = 35), were used for intracellular IL-17 assay.
Flow cytometry was performed on a FACSCanto or FACSAria and analyzed using FACS-Diva version 6.0 software (BD Biosciences).
Cytology.
DCs sorted from AT of lean and obese mice were cytocentrifuged (Cytospin2, Shandon) and stained with May-Grünwald-Giemsa.
Real-time quantitative PCR analysis.
Total RNA was extracted from human tissues using an RNeasy Mini Kit (Qiagen, Contraboeuf, France). One microgram of total RNA was reverse-transcribed, and real-time PCR was performed (ABI PRISM 7500). The TaqMan gene expression assays were purchased from Applied Biosystems: CD1c (Hs00233509_m1), CD83 (Hs00188486_m1), Glut4 (SLC2A4) (Hs00168966_m1), RPLP0 (Ribosomal Phosphoprotein Large P0, Hs99999902_m1), INFγ (Hs00174143_m1), and IL17A (Hs00936345_m1). Gene expression values were normalized to the expression value of the housekeeping gene RPLP0 or 18S rRNA and calculated based on the comparative cycle threshold Ct method (2−ΔCt) as described by the manufacturer's protocols.
Statistical analysis.
The statistical significance of the differential gene expression between groups was determined using the nonparametric Mann-Whitney test with the ΔCt of each group. Other data were statistically analyzed using the Mann-Whitney test. Correlations were analyzed using the Spearman’s rank correlation test. P < 0.05 was considered as significant.
RESULTS
Homing of naive T cells into adipose tissue.
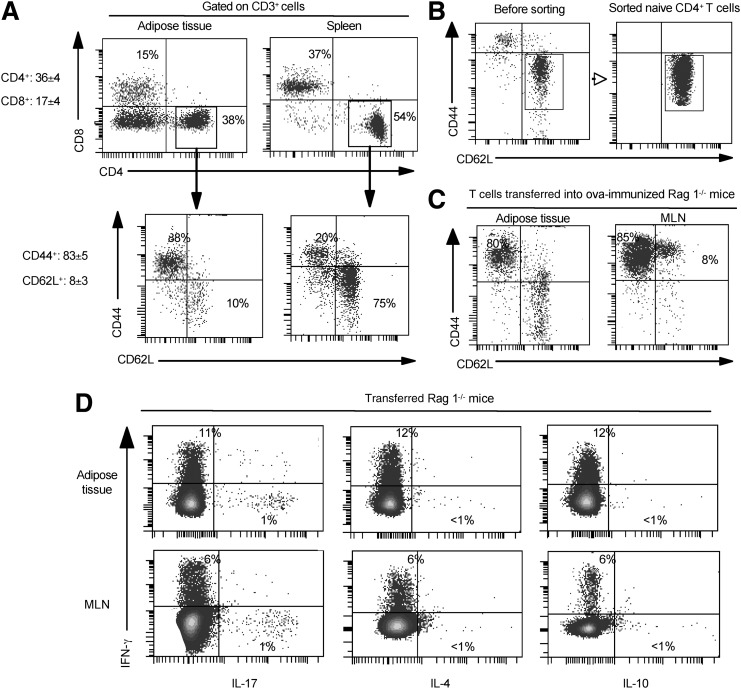
AT consists of adipocytes but also stromal and vascular cells including vascular endothelial and immune cells. We first analyzed the T-cell populations present in the SVF from epididymal AT of lean C57BL/6 mice. Flow cytometric analysis of T cells for CD3, CD4, and CD8 expression revealed three CD3+ populations: CD4+CD8−, CD4−CD8+, and CD4−CD8− cells. The majority of these T cells had a memory phenotype (CD44+CD62L−) (83.3 ± 4.5%), and only 8% were naive T cells (CD44−CD62L+). As expected (Fig. 1A), the majority of CD4+ T cells from the spleen were naive cells (CD44−CD62L+). These results prompted us to determine the homing and activation status of naive T cells in AT.
FIG. 1.
Phenotype of fat-associated CD4+ T cells and homing of naive T cells into AT. A: Epididymal fat pads as well as the spleen were isolated from lean C57BL/6 mice, and the SVF was stained for CD3, CD4, CD8, CD44, and CD62 L. Results are representative of 7 experiments. B: Naive OVA-specific CD4+ T cells were isolated from the spleen of OT2 mice, were FACS-sorted based on CD62L+CD44− expression, and 106 cells were transferred into Rag1−/− mice immunized previously intraperitoneally with 50 μg of OVA protein. C: Phenotypic analysis of transferred naive OVA-specific CD4+ T cells, after 1 week, in mesenteric lymph nodes (MLN) and in AT. D: Cytokine profiles of activated T cells by intracytoplasmic analysis for IL-4, IL-10, IL-17, and IFN-γ. The results of 3 independent experiments are shown.
To achieve this, adoptive transfer of naive OVA-specific CD4+ T cells (from OT2 mice) into mice depleted in T and B cells (Rag1−/− mice) and immunized with OVA was performed (Fig. 1B). After one week, the majority of transferred CD4+ naive T cells were activated (CD44+CD62L−) in AT (Fig. 1C) and in mesenteric lymph nodes used as a positive control. Whether activation of these cells occurred directly in AT or in the lymphoid organs before their migration into the AT remains to be determined. These activated T cells differentiated into Th1 and Th17 cells according to the relative intracytoplasmic expression level of IFN-γ and IL-17 in both AT and mesenteric lymph nodes (Fig. 1D). In contrast, these cells did not express either IL-4 or IL-10 (Fig. 1D). Taken together, these results suggest the presence of active antigen-presenting cells in AT.
Phenotypic characterization of adipose tissue DCs.
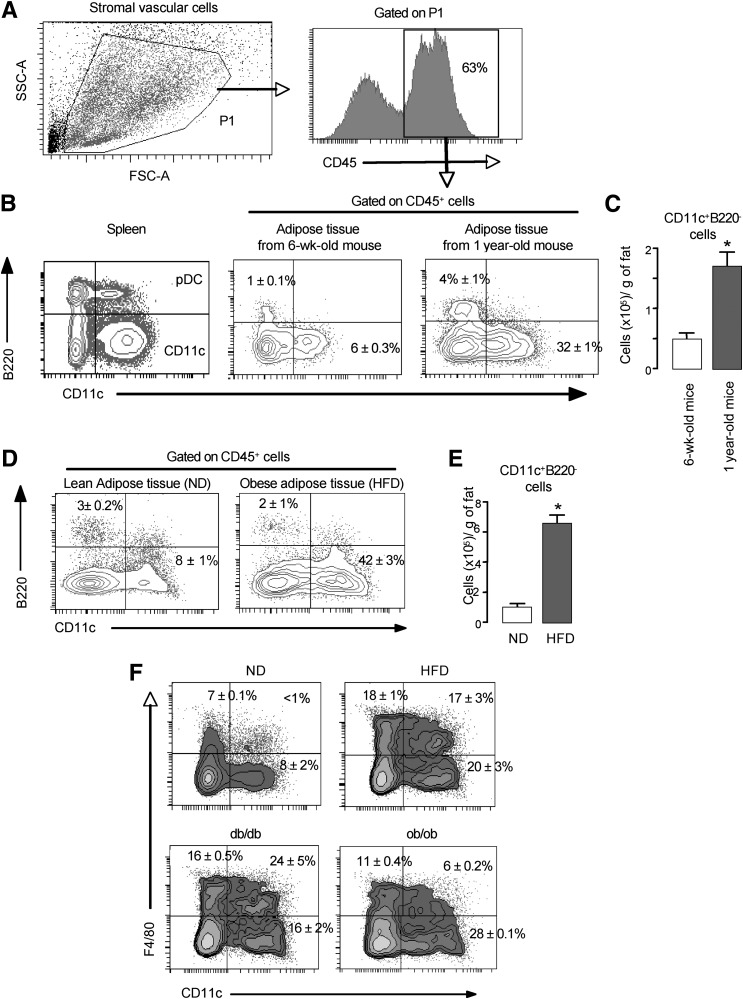
In mice, two major DC subsets have been described to activate T cells: the CD11clowB220+ pDCs and the CD11chighB220neg cells that include cDCs and other DC populations including CD4+ and CD8+ DC as described previously (15). To characterize the DCs from AT, we first evaluated the cell surface expression level of the CD11c and B220 markers in the CD45+ cells from SVF of AT and spleen of lean mice by flow cytometric analysis (Fig. 2A and B). Although two main DC subsets were present in the spleen, only the CD11chighB220neg DCs were detected in SVF of lean mice AT (Fig. 2B). The frequency and absolute number of this population increased with age in AT (Fig. 2B and C) as expected (29), and these cells were able to induce T-cell proliferation in a mixed lymphocyte reaction (MLR) (data not shown), indicating that they are functional. We then investigated whether obesity affected the AT DCs content. After 15 weeks of a 45% HFD, mice displayed obesity and intolerance to glucose (see research design and methods). The SVF of AT from these insulin-resistant obese mice exhibited a marked increase in the level of CD11chighB220neg DCs (Fig. 2D and E). To further characterize these CD11chighB220neg DCs, we evaluated the expression of the F4/80 marker, which is widely used as a marker of macrophages. FACS analysis revealed that the SVF of lean mice did not contain double positive F4/80+ CD11c+ cells (Fig. 2F). HFD challenge led to an increase in CD11chighF4/80neg cells but also to the emergence of an atypical cell population CD11chighF4/80low that might be equivalent to the one already described (8) (Fig. 2F). Similar cell subsets were also observed in the AT of obese diabetic, leptin receptor–deficient db/db, and lepin-deficient ob/ob mice (Fig. 2F).
FIG. 2.
Characterization of DCs in AT from lean and obese mice. Epididymal fat pads as well as spleen were isolated from young (6 weeks old), old (1 year old), ND, HFD, ob/ob, and db/db mice, and the SVF was stained for CD45, CD11c, and B220. Cells were gated on CD45+ cells as indicated (A). B: Flow cytometric analysis of CD11c and B220 on the SVF CD45+ cells from epididymal fat tissue of young (6 weeks old) and old (1 year old) mice. C: The numbers of CD11c+ cells were calculated from FACS data and the SVF cell counts and expressed per gram of epididymal fat tissue. D: Flow cytometric analysis of CD11c and B220 on the SVF CD45+ cells from epididymal fat tissue of ND and HFD mice. E: The number of CD11c+ cells was calculated from FACS data and the SVF cell counts and expressed per gram of epididymal fat tissue. F: Flow cytometric analysis of CD11c and F4/80 on B220 on the SVF CD45+ cells from epididymal fat tissue of ND, HFD, db/db, and ob/ob mice. A representative result of 5 independent experiments with 5 mice per group and with similar results is shown. *P < 0.05. SSC-A, side scatter-aire; FSC-A, forward scatter-aire.
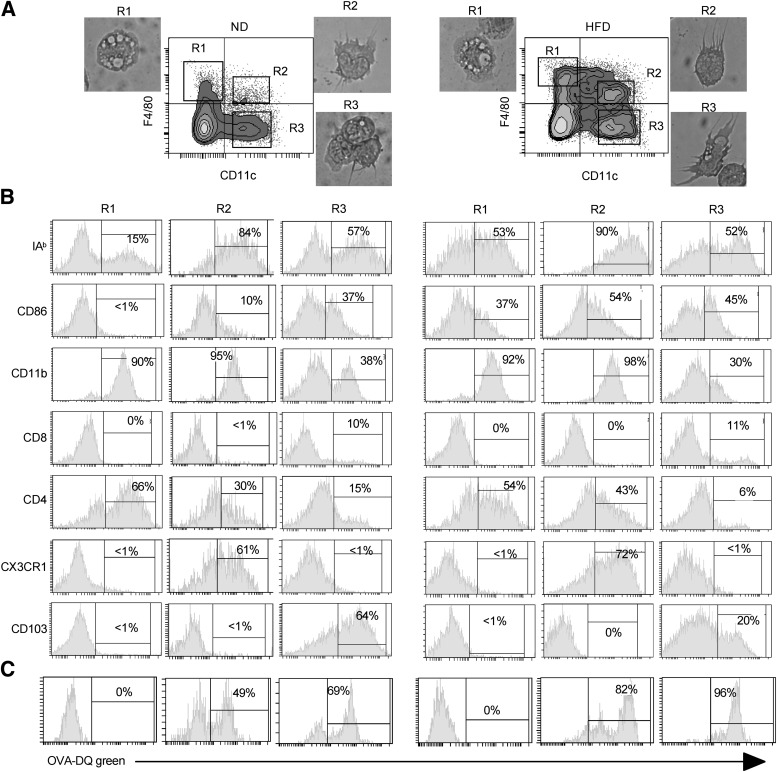
Ex vivo–purified AT CD11c+ cells from lean and obese mice were used to further characterize these cells. The ability to induce proliferation of allogeneic CD4+ T cells in a MLR is one of the definitive features of DCs. The two sorted AT CD11c+ populations (F4/80neg and F4/80low) displayed a typical dendritic morphology (Fig. 3A) and typical dendritic activity since they induced the proliferation of naive CD4+ T cells from BALB/c mice, in a MLR (data not shown). This result suggested that these two types of DCs play an important role in inflammation associated with obesity.
FIG. 3.
Identification of atypical CD11chigh F4/80low DCs. A: Sorted cell populations based on the expression of CD11c and F4/80 on B220 on the SVF CD45+ cells from epididymal fat tissue of ND and HFD mice, as indicated, were cytocentrifuged and stained with May-Grünwald-Giemsa. B: Phenotypic analysis by flow cytometry of AT DCs gated on CD45+ cells, from lean C57BL/6 and HFD mice, was performed by triple immunofluorescent staining after gating of different cell populations as indicated in A. C: Sorted cell populations were incubated with OVA-DQ, and 2 h later the cells were analyzed for the uptake and processing of DQ by flow cytometry. OVA-DQ fluoresced green when processed in acidified lysosomes. A representative result of 3 independent experiments with 5 mice per group and with similar results is shown.
We next examined the expression of a set of DC markers described previously by flow cytometric analyses. As shown in Fig. 3B, all CD11c+ cells expressed major histocompatibility complex I-A molecules and the CD86 costimulatory molecule. Recently, CD103 and CX3CR1, in combination with CD11c, have been reported to define two DC populations. The CD103+ DCs induce the differentiation of Foxp3+CD4+ Treg cells (30), and CX3CR1+ DCs were identified as cells that preferentially support the differentiation of Th17/Th1 cells (31) in the gut. In AT, the majority of DCs were CD103+ and CX3CR1− in lean mice and the atypical CD11chighF4/80low DCs were CD103− and CX3CR1+ in obese mice (Fig. 3B). This CD11chighF4/80low population was also present in the AT from lean mice with an equivalent phenotype but at a very low frequency (<1%) (Fig. 3A) and absolute number (4,000 ± 200 cells/g of fat in lean mice vs. 350,000 ± 10,000 cells/g of fat in obese mice). Besides, in AT, we showed the presence of a DC population expressing CD4+, the role of which remains to be determined in obesity.
Finally, CD11chighF4/80neg and CD11chighF4/80low cells sorted from AT were also capable of processing the antigen BODIPY fluorochrome-conjugated OVA (OVA-DQ green) since cell fluorescence was detected (Fig. 3C). Taken together, CD11chighF4/80neg cells and CD11chighF4/80low cells from AT of lean and obese mice present the characteristic features of DCs.
CD11chighF4/80lowCX3CR1pos DCs induce Th17 differentiation.
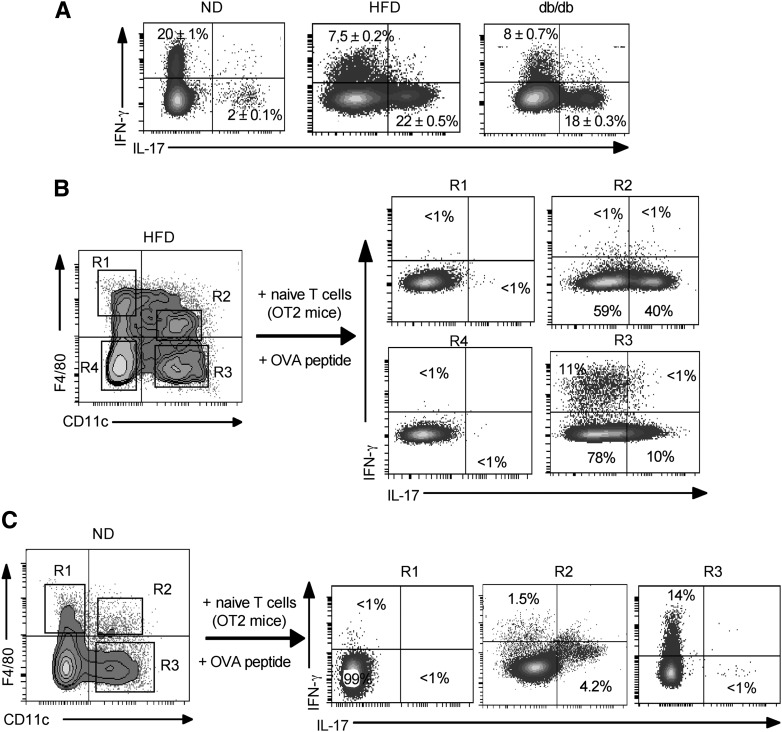
Before evaluating the role of these DCs in T-cell differentiation, we characterized the nature of the CD4+ T cells present in AT of lean, HFD, and db/db mice. Intracytoplasmic cytokine expression analysis showed the presence of more IFN-γ–secreting Th1 cells than IL-17–secreting Th17 cells in lean mice AT (Fig. 4A). In obese HFD and db/db mice, we observed a switch from a Th1 toward a Th17 response (Fig. 4A). This result prompted us to determine the role of each DC subset in the induction of CD4+ T cells.
FIG. 4.
CD11chigh F4/80low CX3CR1pos DCs induce Th17 differentiation in vitro. A: Analysis of cytokine expression of fat pad–associated CD4+ T cells of lean and obese mice by intracellular cytokine assay. B and C: 2 × 105 naive OVA-specific CD4+ T cells were differentiated for two rounds of 5 days of stimulation with 5 × 104 sorted cells from the SVF of lean and obese mice as indicated. After 2 weeks, T cells were collected, stimulated, and stained for intracellular cytokine assay using specific antibodies as indicated. The experiment was repeated three times with similar results.
To analyze the influence of the different DC subsets on the priming and differentiation of naive OVA-specific T cells (from OT2 mice) in vitro, two rounds of 5 days of stimulation of T cells were performed with CD11chighF4/80neg and CD11chighF4/80low DCs sorted from AT of lean and obese mice. As shown in Fig. 4B, ex vivo–purified CD11chighF4/80low DCs from obese mice induced exclusively the differentiation of Th17 cells that produced high levels of IL-17 (Fig. 4B, R2). In contrast, the sorted CD11chighF4/80neg DCs primed both Th1 and Th17 cells, which produced low levels of IFN-γ and IL-17, respectively (Fig. 4B, R3). In lean mice, sorted CD11chighF4/80neg DCs induced only the differentiation of Th1 cells (Fig. 4C, R3). In contrast, CD11chighF4/80low induced only a weak Th17 response (Fig. 4C, R2). However, we did not detect IL-4 or IL-10 in our cocultures (data not shown). Taken together, these results indicate that the induction of Th17 cells in obesity is mainly a result of the emergence and expansion of CD11chighF4/80low DCs.
Characterization of DC and T-cell phenotype in the subcutaneous AT of human patients.
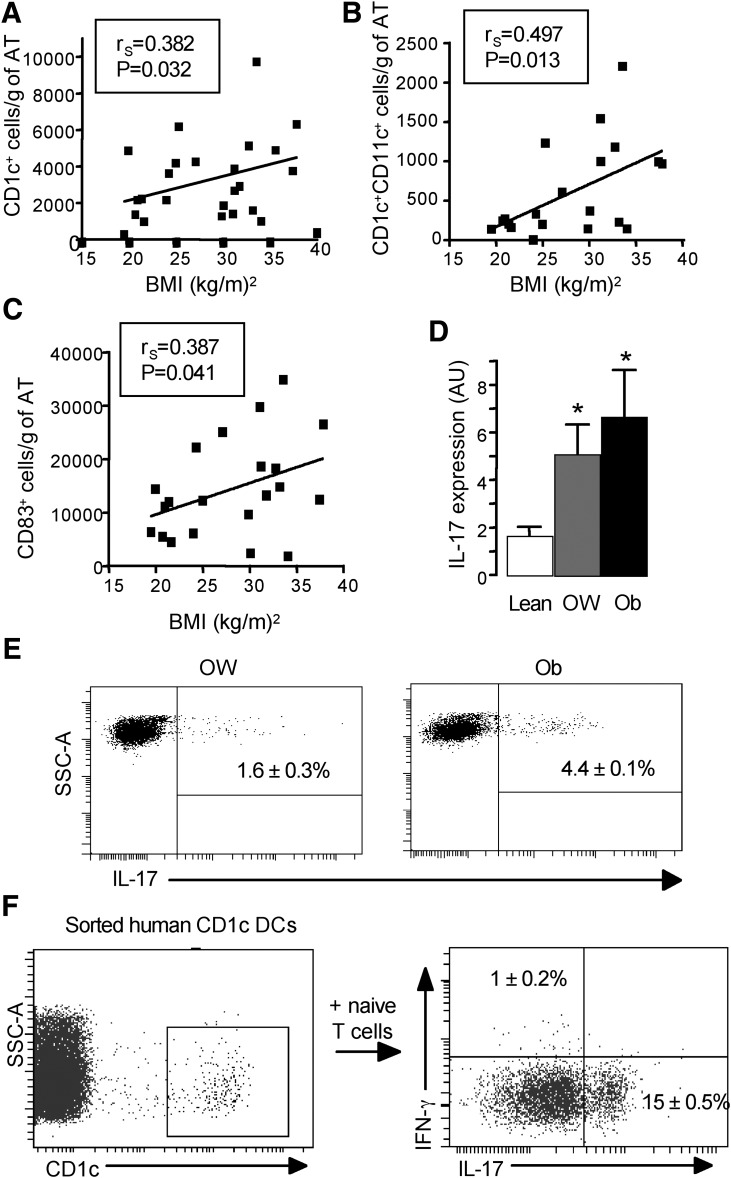
We then extended our study to humans and analyzed the DCs present in the subcutaneous AT from lean, overweight, and obese patients. FACS analyses were performed on freshly isolated SVF from human subcutaneous AT of healthy individuals using human dendritic markers including CD1c, CD11c, and CD83. Positive cell subsets were identified in SVF of human AT and the number of CD1c+, CD1c+CD11c+, and CD83+ cells was found to be positively associated with BMI (Fig. 5A-C). We then determined the expression level of IL-17 in T cells as a function of obesity. To do this, the gene expression level of IL-17 was evaluated in CD3+ cells purified from SVF of freshly harvested AT that was depleted in CD34+ progenitor and capillary endothelial cells and CD14+ monocytes/macrophages. The expression of IL-17 in AT was strongly increased in overweight and obese patients compared with lean subjects (Fig. 5D). The intracellular expression of IL-17 evaluated by flow cytometric analysis was also found in CD4+ T cells in AT of obese patients (Fig. 5E). Finally, the capacity of CD1c+ DCs from obese patients to drive Th17 differentiation was tested in vitro in an allogeneic MLR. As shown in Fig. 5F, CD1c+ DCs sorted from obese patients were able to induce Th17 cell differentiation in vitro.
FIG. 5.
Characterization of human dendritic cells and the expression level of IL-17 in purified T cells as a function of the BMI. FACS analyses were performed on total SVF (patient age = 44.6 ± 2.5 years, BMI ranging from 19.5 to 38 kg/m2, n = 20 to 24). A: Cell number of CD1c+ cells per gram of AT (P < 0.05, Spearman r = 0.3826, n = 24). B: Cell number of CD1c+ CD11c+ cells per gram of AT (P < 0.05, Spearman r = 0.4947, n = 20). C: Cell number of CD83+ cells per gram of AT (P < 0.05, Spearman r = 0.3870, n = 21). D: The gene expression level of IL-17 was evaluated by real-time PCR on immunoselected CD3+ T cells of the AT SVF of lean (n = 10 to 12), overweight (OW; n = 6 to 7), and obese (Ob; n = 12 to 14) patients. Results are means ± SEM. *P < 0.05. E: Intracellular cytokine analysis for IL-17 on immunoselected CD3+ T lymphocytes of the AT SVF of OW (n = 6 to 7) and Ob (n = 12 to 14) patients. F: Allogeneic CD4+ T cells purified from blood of healthy donors (n = 3) were cultured for 6 days with sorted CD1c+ DCs from the CD19−CD14− cells of the SVF of obese patients (n = 3). CD4+ T cells were collected, stimulated, and stained for intracellular IL-17 and IFN-γ cytokines. The experiment was repeated three times with similar results. SSC-A, side scatter-aire; AU, arbitrary unit.
Elevated CD1c expression in subcutaneous AT correlated with HOMA-IR in morbidly obese patients.
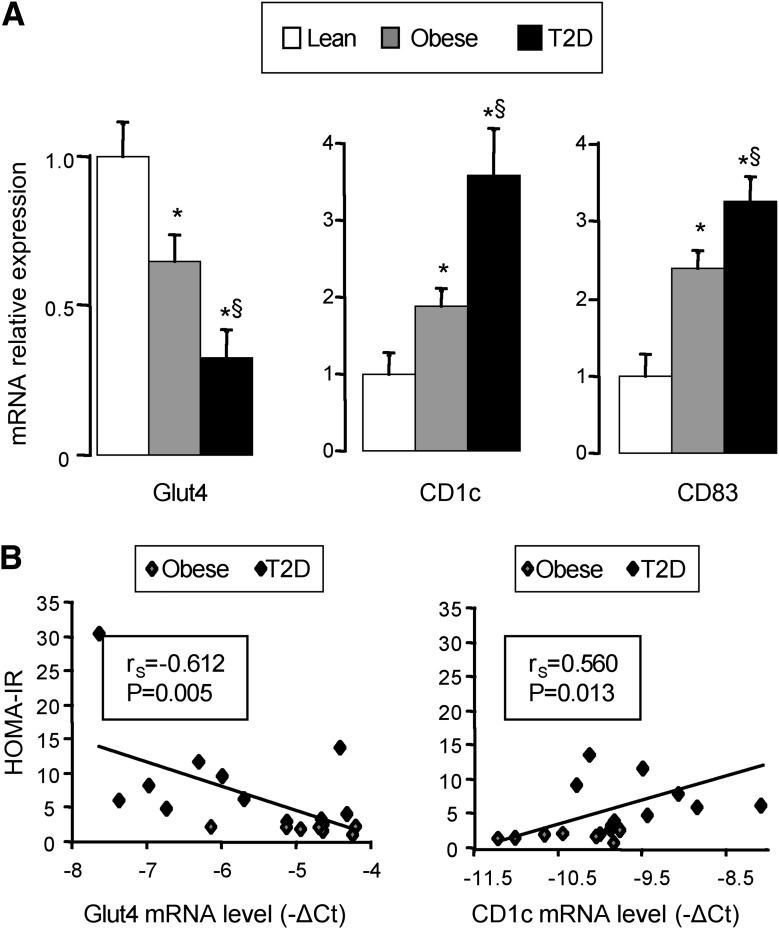
To determine the link between the accumulation of DCs and insulin resistance, we next evaluated the gene expression of Glut4, CD1c, and CD83 in AT from morbidly obese patients without (obese, n = 10, BMI = 42.8 ± 2 kg/m2) or with type 2 diabetes (n = 10; BMI = 46.8 ± 2 kg/m2; not significant vs. obese patients) compared with AT from four lean subjects (BMI = 21.1 ± 0.7 kg/m2). All diabetic patients were mainly women (diabetic: 9 women and 1 man; obese patients: 10 women; not significant), insulin resistant as evaluated by the HOMA-IR (diabetic patients = 1.4 ± 2.6; obese patients = 2.1 ± 0.2, P < 0.05), and had an elevated level of HbA1c (diabetic patients = 8.1 ± 0.7%; obese patients = 5.4 ± 0.1, P < 0.05), fasting glucose (diabetic patients = 10 ± 1.2 mmol/L; obese patients = 5 ± 0.1 mmol/L, P < 0.05), and insulin (diabetic patients = 26.1 ± 4.7 mmol/L; obese patients = 9.6 ± 1.1 μmol/L, P < 0.05). Glut4 mRNA expression was strongly decreased in the AT of diabetic obese patients compared with obese patients and with lean control patients (Fig. 6A). Moreover, Glut4 expression negatively correlated with HOMA-IR (Fig. 6B). In contrast, when compared with lean subjects, CD1c and CD83 gene expression increased progressively with obesity and then with diabetes (Fig. 6A). In AT, CD1c expression correlated negatively with Glut4 expression (rs = −0,613, P = 0.001) and positively with HOMA-IR (Fig. 6B). These results indicate that AT was enriched in activated CD1c+ DCs and that their accumulation directly correlated with insulin resistance.
FIG. 6.
Glut4, CD1c, and CD83 expression in subcutaneous AT in lean and morbidly obese patients without or with type 2 diabetes (T2D). A: Glut4, CD1c, and CD83 mRNA expression was analyzed by real-time quantitative PCR in subcutaneous AT obtained from lean subjects (Lean; n = 4), morbidly obese patients without type 2 diabetes (obese; n = 10), and morbidly obese patients with type 2 diabetes (T2D; n = 10). Data are presented as relative mRNA normalized to RPLP0 mRNA and are expressed as means ± SEM. *P < 0.05 compared with lean; §P < 0.05 compared with obese patients without type 2 diabetes. B: Correlation between HOMA-IR and Glut4 or CD1c mRNA expression levels (−ΔCt) was analyzed using the Spearman’s rank correlation test for morbidly obese patients (n = 19).
DISCUSSION
AT inflammation is now considered to be a crucial event leading to metabolic syndrome, diabetes, artherosclerotic cardiovascular, and liver disease. It has also been established that AT is not only involved in energy storage but also functions as an endocrine organ that secretes various bioactive substances referred to as adipokines with pro- or anti-inflammatory properties (32,33). Nevertheless, how inflammation is initiated and maintained in AT is still elusive.
Here, we showed that DCs residing in AT of lean mice may be capable of taking up and presenting antigens to naive CD4+ T cells in an antigen-specific manner. Upon antigenic stimulation, naive CD4+ T cells become effector T cells with a predominance of Th1 cells over Th17 cells. However, the naive T cells could be either activated directly in lymphoid organs before their migration into the AT or directly in the AT. This point remains to be determined.
Moreover, intracytoplasmic cytokine analysis of CD4+ T cells purified from lean mice AT confirmed this observation. In contrast, the AT of insulin-resistant obese and type 2 diabetic mice displayed a switch from a Th1 toward a Th17 responses. Our data are supported by the recent report from Dosch’s group demonstrating the importance of obesity in the expansion of CD4+ Th17 cells (34). We have also shown for the first time that the number of Th17 cells increases in AT of overweight and obese patients versus lean subjects. Although an antiadipogenic role for IL-17 has been described (35), emerging studies indicate that obesity selectively promotes the expansion of CD4+ Th17 cells in AT, exacerbating autoimmunity in murine models of colitis and experimental autoimmune encephalomyelitis (34,35). Taken together, these observations indicate that IL-17 may play an important role in metabolic processes. However, future investigations are necessary to explore this role more in details.
In this study, we identified a potential role of DCs in the regulation of AT inflammation and its potential consequence on insulin resistance in mice and humans. In mice, we reported that insulin-resistant and type 2 diabetic mice display an increase in cDCs (CD11chighF4/80neg cells) but also the emergence of atypical DCs expressing CD11chighF4/80low markers. Furthermore, the CD11chighF4/80low cells had all the features of DCs including their morphology, the expression of specific surface markers, and the capacity to process antigens. These atypical DCs also expressed CX3CR1, suggesting their preferential role in the differentiation of Th17 cells, as recently demonstrated in gut (31). Indeed, the CD11chighF4/80lowCX3CR1+ DCs induced Th17 differentiation in AT of obese mice. In lean mice, the CD11c+ DCs also led to differentiation of naive CD4+ T cells into Th17 cells but to a very lower extent. However, the majority of CD11c+ DCs in lean AT were CD103+, suggesting a role on the induction of fat Foxp3+CD4+ Treg cells providing anti-inflammatory signals to block AT inflammation as described previously ([30,36] and data not shown).
Our study is the first detailed analysis of human DCs in AT. Human DCs are a heterogeneous cell population that comprises ∼1% of circulating peripheral blood mononuclear cells. They can be subdivided into the CD11c−CD303+ plasmacytoid DCs and CD11c+ myeloid DCs, which can be further subdivided into two subsets based on differential expression of CD1c and CD141. Moreover, each CD1c+ and CD141+ DC has a unique gene expression profile distinct from monocytes and monocyte-derived DCs predicting distinct functions (37–39). We first observed that the levels of human CD11c+CD141+ DCs were the same in overweight/obese and lean patients and that plasmacytoid DCs (CD11c−CD303+) were not detected in human AT (data not shown). In contrast, the number of human CD11c+CD1c+ DCs correlated with the BMI and gene expression of CD1c in subcutaneous AT, which increased in obese patients and even more so in diabetic patients compared with lean subjects. CD1c expression strongly correlated with HOMA-IR in patients with a high BMI. The accumulation of human CD11c+CD1c+ DCs was associated with an increase in human Th17 cells in AT, suggesting a role of these DCs in the induction of Th17 cells. Indeed, our results show that CD1c+ DCs sorted from obese patients were able to induce Th17 cells in vitro. In this sense, Hänsel et al. showed recently that dermal CD1c+ DCs from patients with psoriasis were capable of driving a Th17 cell response in vitro (40).
Collectively, our study defines human CD11c+CD1c+ and mouse CD11chighF4/80low DCs as inflammatory DCs in AT in obesity-associated insulin resistance and identifies their potential capacity to induce Th17 cell responses. Finally, understanding the role of IL-17 in metabolic processes and its direct clinical implications is clearly the next challenge for the field.
ACKNOWLEDGMENTS
This work was supported by CNRS (A.W.), INSERM, the University of Nice, the Programme Hospitalier de Recherche Clinique (CHU of Nice), charities (SFD/BMS and AFEF/Schering-Plough to P.G.) and the European Union Commission (Collaborative Project ADAPT [www.adapt-eu.net] for V.B., C.D., and A.Bou. from Unit 1048, Toulouse). A.Ber. was successively supported by the Programme Hospitalier de Recherche Clinique (CHU of Nice) and the Association pour la Recherche sur le Cancer (France). P.G. is the recipient of an Interface Grant from CHU of Nice.
No potential conflicts of interest relevant to this article were reported.
A.Ber., T.C., and A.Bou. researched data, reviewed the manuscript, and contributed to discussions. D.R., V.B., C.D., S.B., R.A., A.I., and J.G. researched data. C.B.-W. contributed to discussions and reviewed the manuscript. A.T. researched data and contributed to discussions. P.G. researched data and wrote and reviewed the manuscript. A.W. designed the research, researched data, and wrote and reviewed the manuscript. A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Y. Le Marchand-Brustel (INSERM U895, Nice) for critical reviewing of the manuscript. The authors acknowledge the flow cytometry facility of UMR 576 (Nice) and the C3M animal room facility (INSERM, U895, Nice).
REFERENCES
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202–1219 [DOI] [PubMed] [Google Scholar]
- 2.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 2002;51:3120–3127 [DOI] [PubMed] [Google Scholar]
- 3.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harkins JM, Moustaid-Moussa N, Chung YJ, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr 2004;134:2673–2677 [DOI] [PubMed] [Google Scholar]
- 6.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005;54:2277–2286 [DOI] [PubMed] [Google Scholar]
- 7.Bertola A, Deveaux V, Bonnafous S, et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes 2009;58:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen MT, Favelyukis S, Nguyen AK, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 2007;282:35279–35292 [DOI] [PubMed] [Google Scholar]
- 9.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 2008;8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol 2007;179:6255–6262 [DOI] [PubMed] [Google Scholar]
- 11.Wu H, Perrard XD, Wang Q, et al. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol 2010;30:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yekollu SK, Thomas R, O’Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-κB and improves insulin resistance in obese mice. Diabetes 2011;60:2928–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252 [DOI] [PubMed] [Google Scholar]
- 14.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol 2000;1:199–205 [DOI] [PubMed] [Google Scholar]
- 15.O’Keeffe M, Hochrein H, Vremec D, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J Exp Med 2002;196:1307–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 2003;18:605–617 [DOI] [PubMed] [Google Scholar]
- 17.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007;7:19–30 [DOI] [PubMed] [Google Scholar]
- 18.Augier S, Ciucci T, Luci C, Carle GF, Blin-Wakkach C, Wakkach A. Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance. J Immunol 2010;185:7165–7173 [DOI] [PubMed] [Google Scholar]
- 19.Mocci S, Coffman RL. Induction of a Th2 population from a polarized Leishmania-specific Th1 population by in vitro culture with IL-4. J Immunol 1995;154:3779–3787 [PubMed] [Google Scholar]
- 20.Wakkach A, Cottrez F, Groux H. Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J Immunol 2001;167:3107–3113 [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531–562 [DOI] [PubMed] [Google Scholar]
- 22.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med 2009;15:846–847 [DOI] [PubMed] [Google Scholar]
- 23.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertola A, Bonnafous S, Anty R, et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS ONE 2010;5:e13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anty R, Bekri S, Luciani N, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol 2006;101:1824–1833 [DOI] [PubMed] [Google Scholar]
- 27.Anty R, Iannelli A, Patouraux S, et al. A new composite model including metabolic syndrome, alanine aminotransferase and cytokeratin-18 for the diagnosis of non-alcoholic steatohepatitis in morbidly obese patients. Aliment Pharmacol Ther 2010;32:1315–1322 [DOI] [PubMed] [Google Scholar]
- 28.Blin-Wakkach C, Wakkach A, Rochet N, Carle GF. Characterization of a novel bipotent hematopoietic progenitor population in normal and osteopetrotic mice. J Bone Miner Res 2004;19:1137–1143 [DOI] [PubMed] [Google Scholar]
- 29.Mori MA, Liu M, Bezy O, et al. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes 2010;59:2960–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007;204:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol 2010;184:2026–2037 [DOI] [PubMed] [Google Scholar]
- 32.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939–949 [DOI] [PubMed] [Google Scholar]
- 33.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol 2003;14:561–566 [DOI] [PubMed] [Google Scholar]
- 34.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol 2009;39:2629–2635 [DOI] [PubMed] [Google Scholar]
- 35.Ahmed M, Gaffen SL. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev 2010;21:449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cipolletta D, Kolodin D, Benoist C, Mathis D. Tissular T(regs): a unique population of adipose-tissue-resident Foxp3+CD4+ T cells that impacts organismal metabolism. Semin Immunol 2011;23:431–437 [DOI] [PubMed] [Google Scholar]
- 37.Lindstedt M, Lundberg K, Borrebaeck CA. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J Immunol 2005;175:4839–4846 [DOI] [PubMed] [Google Scholar]
- 38.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood 2002;100:4512–4520 [DOI] [PubMed] [Google Scholar]
- 39.Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010;207:1247–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansel A, Gunther C, Ingwersen J, et al. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J Allergy Clin Immunol 2011;127:787–794 [DOI] [PubMed] [Google Scholar]