We strive but struggle to translate immune therapies that have been shown to be effective in preclinical models of autoimmune diabetes into use with patients. Only a small proportion of these therapies are actually tested in humans, and of these, efficacy (even short-term) has been achieved in less than a handful (1). A striking example of this struggle is provided by a clinical trial reported in the current issue of Diabetes (2). After setting everything up correctly with convincing data in preclinical models (3,4), an attractive hypothesis (5), and safety studies in animals, the Immune Tolerance Network (ITN) conducted a single-arm trial of combination therapy with interleukin (IL)-2 (4 weeks) together with rapamycin (12 weeks) in patients who had recently developed type 1 diabetes. Treatment successfully led to a respectful increase in circulating regulatory T cell (Treg) numbers. However, the therapy failed to halt β-cell loss and even transiently exacerbated loss of β-cell function. Interestingly, IL-2 seems to have been responsible for both the increased Treg numbers and the loss of β-cell function. It also seems that IL-2 acted on effector arms of innate immunity and that this may have led to the negative effects on the β-cell.
The rationale for IL-2 therapy in type 1 diabetes is relatively strong (Fig. 1), with reproducible genetic associations with genes of the IL-2 pathway (6) and functional defects in IL-2 signaling (7–9), plus successful use of IL-2 in preclinical models of autoimmune diabetes (3,4) and therapeutic benefit in patients with immune-mediated disorders (10,11). One can always find ways to be critical, however. Of note, it would be important to demonstrate functional IL-2 signaling defects in preclinical or new-onset type 1 diabetes rather than genotyped controls (7,8) or in patients with long-standing diabetes (9). Nevertheless, IL-2 therapy did what it was supposed to do—there was a consistent, robust increase in circulating numbers of Tregs during IL-2 therapy and persistent improvement in Treg/IL-2 responsiveness well after cessation of therapy. This outcome has become a supreme goal of immunotherapy in type 1 diabetes. No other therapy has come close to achieving this so convincingly. Of course, in view of the associated impairment in β-cell function, one should now ask whether more and better Tregs is still a Holy Grail for type 1 diabetes. The investigators did not openly question the paradigm and instead went on to identify what may have nullified potential clinical benefits of increasing Treg number and function.
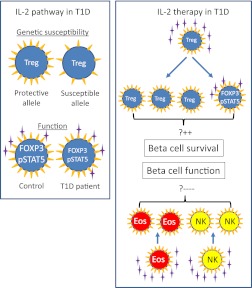
FIG. 1.
Il-2 pathway in the pathogenesis of type 1 diabetes (T1D) (left box) and observed effects of IL-2/rapamycin therapy in patients with type 1 diabetes (right box). Susceptibility to type 1 diabetes is conferred by numerous genes including the IL-2 receptor α (CD25) gene. Tregs in subjects with type 1 diabetes–susceptible alleles express less CD25 (yellow triangles) than those from subjects with protective alleles (7). Moreover, Tregs from patients with type 1 diabetes are less responsive to IL-2 (purple stars) with lower FOXP3 expression and less STAT5 signaling than Tregs from control subjects (9). During IL-2/rapamycin treatment (2), IL-2 increased the number of circulating Tregs and led to a rescue of their IL-2 responsiveness in patients. This is presumed to prevent loss of β-cells and increase β-cell survival and function (upper part of the right box). However, treatment also expanded other CD25 bearing cells of innate immunity such as eosinophils (Eos) and natural killer (NK) cells and this is presumed to be responsible for the marked impairment in β-cell function observed in the study (lower part of the right box).
What could have gone wrong? Tregs constitutively express high levels of the high-affinity IL-2 receptor. However, cells of the innate immune system such as eosinophils and natural killer–cell subsets express intermediate-affinity IL-2 receptor and are activated by IL-2. IL-2 therapy did not ignore these IL-2 receptor–bearing cells and in fact led to pronounced transient increases in circulating eosinophils and activated natural killer cells, along with increases in soluble IL-2 receptor concentration. The authors consider these effects on innate immunity to be a likely reason for the exacerbated impairment of β-cell function. This is likely, but this is only guilt by association. If true, one must consider that activation of the innate immune system outweighs the importance of Treg numbers and function. Although the findings of the study do not prove that innate immunity is key in disease pathogenesis, they do suggest that its activation should probably be avoided.
In looking for reasons why there was no clinical benefit, it should not be ignored that IL-2 therapy was given concomitantly with rapamycin therapy. At first sight, rapamycin does not appear to have been particularly harmful. Most of the undesired inflammatory effects occurred during the period of IL-2 therapy and disappeared during rapamycin monotherapy. It is notable, however, that although rapamycin was initially considered anti-inflammatory, it has recently been shown to promote inflammatory pathways (12). Thus, rapamycin may well have contributed to the activation of innate immunity in the first place. Moreover, it has been reported that the addition of rapamycin reversibly hinders efficacy of anti-CD3 therapy in preclinical models of diabetes (13), and rapamycin has similar detrimental effects when added to low-dose IL-2 therapy in NOD mice (E. Piaggio, personal communication). β-Cell function (and normoglycemia) returned quickly after rapamycin withdrawal in these mice. The ITN investigators also suggest that in their trial β-cell function improved after removal of both drugs. However, there were neither β-cell function measures at the end of the 4-week IL-2 therapy nor did the trial include patients who only received IL-2. Thus, we cannot make firm conclusions with respect to rapamycin’s contribution to the impaired β-cell function observed in this report. Perhaps just as critical for the future of IL-2 therapy is whether impairment of β-cell function during treatment really is transient. The total decline in c-peptide observed 12 months after starting treatment was <30%. Reassuringly, this is the same or even less than that observed in others trials. Indeed, optimistically, one could hope that impairment was completely reversible and that with a durable effect on Treg, there will be a net gain for patients.
One practical aspect of the study worth highlighting was the ability to recognize detrimental effects on β-cell reserve with a nine patient, no control group study. For this, we can applaud the efforts of TrialNet in conducting and reporting several trials in similar patients and establishing rather tight expectations in C-peptide outcomes after diabetes onset (14). Without contemplating the costs that led to this achievement, it clearly helped the ITN investigators and their Data and Safety Monitoring Board in correctly closing out the study. It is hoped that investigators, industry, and regulatory authorities will recognize these benefits and consider more short-term, well monitored, pilot immune therapy trials in type 1 diabetes.
We (re)learned a great deal about translation from this small clinical study: 1) Pilot trials can be extremely valuable; 2) mechanistic studies can be worth their weight in gold; 3) achieving mechanistic goals does not equal clinical efficacy; 4) “off-target” drug effects should not be ignored; and 5) even with a very convincing rationale, translation is a struggle. Can patients be exposed to IL-2? The compelling evidence for involvement of the IL-2 pathway in type 1 diabetes will rightly lead to more efforts with IL-2 therapy. However, it will be necessary to quickly establish whether functional β-cell loss occurs under IL-2 alone and whether functional loss really is transient or if there is also β-cell loss. If transient, we look forward to finding out how we can obtain positive effects on Tregs without negative effects on effector arms of immunity. The road is slightly shorter, but more winding.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2340.
REFERENCES
- 1.Gallagher MP, Goland RS, Greenbaum CJ. Making progress: preserving beta cells in type 1 diabetes. Ann N Y Acad Sci 2011;1243:119–134 [DOI] [PubMed] [Google Scholar]
- 2.Long SA, Rieck M, Sanda S, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 2012;61:2340–2348 [DOI] [PMC free article] [PubMed]
- 3.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 2002;51:638–645 [DOI] [PubMed] [Google Scholar]
- 4.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010;207:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hulme MA, Wasserfall CH, Atkinson MA, Brusko TM. Central role for interleukin-2 in type 1 diabetes. Diabetes 2012;61:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainbow DB, Esposito L, Howlett SK, et al. Commonality in the genetic control of Type 1 diabetes in humans and NOD mice: variants of genes in the IL-2 pathway are associated with autoimmune diabetes in both species. Biochem Soc Trans 2008;36:312–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dendrou CA, Plagnol V, Fung E, et al. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat Genet 2009;41:1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg G, Tyler JR, Yang JH, et al. Type 1 Diabetes-Associated IL2RA Variation Lowers IL-2 Signaling and Contributes to Diminished CD4+CD25+ Regulatory T Cell Function. J Immunol 2012;188:4644–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long SA, Cerosaletti K, Bollyky PL, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes 2010;59:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus- host disease. N Engl J Med 2011;365:2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011;365:2067–2077 [DOI] [PubMed] [Google Scholar]
- 12.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008;29:565–577 [DOI] [PubMed] [Google Scholar]
- 13.Valle A, Jofra T, Stabilini A, Atkinson M, Roncarolo MG, Battaglia M. Rapamycin prevents and breaks the anti-CD3-induced tolerance in NOD mice. Diabetes 2009;58:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachin JM, McGee PL, Greenbaum CJ, et al. Type 1 Diabetes Trial Network Sample size requirements for studies of treatment effects on beta-cell function in newly diagnosed type 1 diabetes. PLoS ONE 2011;6:e26471. [DOI] [PMC free article] [PubMed] [Google Scholar]