Abstract
Purpose
Female breast cancer survivors, a large and growing population, experience impaired physical functioning after treatment. Survivors living in impoverished neighborhoods may suffer even greater impairment, but the mechanisms linking neighborhood poverty and individual outcomes are poorly understood. This study sought to identify mediators of the effect of neighborhood poverty on physical functioning using longitudinal data from a Missouri cancer registry-based sample of 909 female breast-cancer survivors.
Methods
Survivors were recruited one year after diagnosis (Y1) and completed two telephone interviews, at Y1 and one year later (Y2). The association between census-tract-level poverty and physical functioning (RAND SF-36) was tested using a multilevel a priori path model with 19 hypothesized mediators, demographic and socioeconomic confounders, and covariates. Hypothesized mediators included clinical and treatment variables, psychosocial factors (depression, stress, social support), perceived neighborhood characteristics, behavioral risk factors (physical activity, smoking, body mass index, alcohol use), and comorbidity.
Results
In unadjusted analysis, women living in neighborhoods with higher poverty were more likely to report lower physical functioning at Y2 (β= −.19, p<.001). The final mediated model fit the data well (χ2(8)=12.25, p=0.14; CFI=.996; RMSEA=.024). The effect of neighborhood poverty on physical functioning was fully mediated by physical activity and body mass index.
Conclusions
Breast cancer survivors living in neighborhoods with greater poverty reported lower physical functioning, but this effect was fully explained by physical activity and body mass index. Community-based lifestyle interventions sensitive to the unique challenges faced by cancer survivors and the challenges of living in a high-poverty neighborhood are needed to ameliorate neighborhood socioeconomic disparities in physical functioning.
Keywords: Quality of Life, Survivors, Neighborhood, Poverty, Health Behavior, Physical Functioning
INTRODUCTION
In 2007, an estimated 2.7 million American women were alive with a breast cancer history.[1] Breast cancer survivors are a large and growing population, due in large part to advances in early detection and treatment. Consequently, the quality of life (QOL) of this population represents a public health issue of growing significance.
Breast cancer survivors may experience reduced health-related QOL, including poorer physical functioning, compared with non-cancer controls.[2–7] Physical functioning, one of multiple QOL domains, measures the extent to which an individual can walk, climb stairs, run, and participate in activities of daily living. The consequences and costs of reduced physical functioning among survivors are high; both poorer physical function and declines in physical functioning over time are associated with worse prognosis.[8,9] Declines in physical functioning may occur shortly after cancer diagnosis and treatment. Many, but not all breast cancer survivors return to normal physical functioning at some point following treatment.[6,7,10,11] Many factors have been associated with differences in physical functioning and overall health-related QOL, including age, race, socioeconomic status, cancer stage, cancer-related symptoms, comorbidity, physical activity (PA), body mass index (BMI), and psychosocial factors.[6,12–18]
Survivors living in higher poverty neighborhoods may face an increased burden of poorer physical functioning and greater difficulty returning to normal physical function following treatment. Breast cancer survivors living in areas of higher poverty face worse health-related QOL outcomes including increased ambulatory care–sensitive hospitalizations (preventable hospitalizations for conditions such as asthma, diabetes, pneumonia, etc. that represent a breakdown in access to or the processes of primary care) and poorer survival.[19–21] However, the association between neighborhood poverty and physical functioning among breast cancer survivors remains unexplored.
Although mounting evidence has linked neighborhood poverty to a host of poorer health outcomes among cancer survivors[19–21] little is known about the mediating mechanisms underlying these associations. More generally, the mediating mechanisms linking neighborhood poverty to individual health outcomes are not yet known, and consequently, the identification of these mechanisms is considered a key priority for the advancement of the neighborhood effects literature.[22–24] Understanding these mechanisms in breast cancer survivors is an essential step toward targeting and developing effective environmental, health promotion, and/or public policy interventions for this growing population. However, the opportunity to improve the physical functioning of cancer survivors living in high-poverty neighborhoods will remain unrealized until mediating mechanisms are better understood. Therefore, the purpose of this longitudinal study was first, to assess the effect of neighborhood-level poverty on physical functioning among female breast cancer survivors two years post-diagnosis and second, to identify what potentially modifiable factors mediate the effect of poverty on physical functioning. To demonstrate the robustness of identified mediators and to provide greater confidence in our results, we also accounted for the effect of other independent variables by adding both confounders and covariates to our analysis.
MATERIALS AND METHODS
Study sample
Women aged ≥25 diagnosed with a first primary breast cancer between June 1, 2006 and June 30, 2008 were identified from the Missouri Cancer Registry. Women were initially recruited by mail and telephone calls were made to non-respondents. After obtaining informed consent, trained interviewers administered computer-assisted telephone interviews at one (Y1) and two years (Y2) after diagnosis. All data were collected between 2007 and 2010. By protocol guidelines, Y1 interviews occurred between 10–14 months after diagnosis. Women were excluded if, at Y1 or Y2, they scored more than 10 on the Orientation-Memory-Concentration test, a screen for cognitive impairment[25], had changed residence between years, or had residential street addresses that could not be geocoded. This study was approved by Institutional Review Boards at Washington University and the University of Missouri-Columbia.
Measures
We sought to identify the relevant mechanisms linking neighborhood poverty to physical functioning by empirically testing potentially mediating pathways that were identified using both conceptual models and empirical studies as a guide. Specifically, we borrowed from conceptual models of health-related quality of life[26,27] that explicitly demonstrate how characteristics of the individual and environment can contribute to physical functioning. We also drew from previous literature about neighborhood effects on various health outcomes,[23,24,28–32] reviews of quality of life factors in breast cancer survivorship,[6,12–18] and disparities in breast cancer [19–21] when selecting variables and directional pathways in our model. Using this literature as a guide, our hypothesized path model specified a priori pathways that related all measures (mediators, confounders, or covariates) to the predictor (neighborhood poverty) and/or the outcome (physical functioning). Mediators are variables that are hypothesized to be caused or influenced by the predictor that in turn cause or influence the dependent (outcome) variable. Confounders, while also associated with both the predictor and outcome, do not lie in a hypothesized causal pathway, unlike mediators. Covariates are associated with either variable, but not both.
Physical Functioning
We chose Physical Functioning as our QOL outcome because it is a frequently used outcome in other studies and because it is closely linked to both comorbidity and survival. [8,9] Physical Functioning was measured at Y2 using the RAND 36-Item Health Survey 1.0.[33,34] The 10-item scale measures physical aspects of health-related QOL, including walking, climbing stairs, daily maintenance activities such as carrying groceries and bathing oneself, and participation in vigorous (e.g. running) and moderate (e.g. vacuuming) activities. Scores are standardized and range from 0 to 100; higher scores indicate better functioning.[33]
Neighborhood Poverty
The predictor, neighborhood poverty rate, was measured using 2000 U.S. Census data, which was several years prior to the Y1 survey, and was defined as the continuous percentage of the population living below the federal poverty level in the participant’s census tract of residence. Because it is unknown whether the mechanisms linking neighborhood SES and physical functioning differ based on the selected SES indicator, we opted to use a commonly used single item measure. We specifically chose neighborhood-level poverty rate as the optimal single-item indicator of neighborhood SES for our study based on the literature indicating its robust association with a variety of health outcomes in diverse populations, because it is a standard measure that is easily interpreted across places and over time, it has been identified as an upstream determinant of downstream health and social factors in other longitudinal studies, and for its relevance for policy makers.[21,35,36]
Potential Mediators
Multiple hypothesized mediators measured at Y1 were considered, including clinical and treatment variables, comorbidity, perceived neighborhood conditions, psychosocial variables, and behavioral risk factors.
Clinical and treatment variables included a surgical side effects index as well as the following yes/no variables: axillary lymph node removal, chemotherapy, radiotherapy, ever using hormonal therapy (i.e. Tamoxifen, Raloxifene, or aromatase inhibitors), and type of surgery (lumpectomy vs. mastectomy [the 24 women with no surgery were omitted from this comparison]). Self-reported treatment has been shown to be accurate relative to medical record review.[37] For the surgical side effects index, women rated how often each of 5 breast-surgery-associated side effects (e.g. arm weakness, arm lymphedema) affected them in the previous month using a 5-point scale ranging from “not at all” (1) to “all of the time” (5). This measure includes 5 of the 8 items from a previously developed and validated index (alpha=0.74[38]).[38–41]
Cormorbidity was measured using Katz’s validated self-report adaptation of the Charlson comorbidity index,[42,43] which captures the presence and/or history of multiple chronic conditions (e.g. myocardial infarction, diabetes, and other cancers). A weighted score accounts for both the presence and severity of comorbidities.
Three perceived neighborhood conditions were measured. Using 4-point scales (strongly agree to strongly disagree), Physical Disorder/Decay (6 items) and Social Disorder (9 items) captured the extent to which respondents’ perceive physical or social cues in their neighborhoods that signify the breakdown of social control.[44] Collective Efficacy, a 10-item 5-point scale ranging from either very likely to very unlikely and strongly agree to strongly disagree, measured resident’s perceptions of the community’s ability to control residents’ behavior and to organize effectively.[45]
Five psychosocial factors were measured. A score of 9 or greater on the 11-item version of the Center for Epidemiologic Studies Depression (CESD-11) scale[46] was used as an indicator of clinically significant depressive symptoms (yes vs. no). The 4-item Cohen’s Perceived Stress Scale assessed stress using a 5-point response scale (never to very often).[47] The 19-item 5-point Medical Outcomes Study (MOS) scale measured perceived availability of social support (none of the time to all of the time).[48] Finally, two yes/no questions captured community and social involvement: “Do you belong to a church or other religious organization where you meet with others on a regular basis?” and “Do you belong to any clubs or other social organizations where you meet with others on a regular basis?”
Behavioral risk factors were measured using standard questions from the Behavioral Risk Factor Surveillance System and included current smoking status (current, former, never), any participation in leisure time PA in the past month (yes vs. no), BMI (underweight [<18.5 kg/m2], normal weight [18.5–24.9 kg/m2], overweight [25.0–29.9 kg/m2], and obese [>=30.0 kg/m2]), and alcohol use during the past month (≤1 drink per day vs. >1 drink per day).
Potential Confounders
Potential confounding factors measured at Y1 included: age (continuous), race (nonwhite vs. white), current marital status (married vs. unmarried), educational attainment (≤high school, some college, or ≥college graduate), annual household income (≤$24,999, $25–34,999, $35–49,999, $50–74,999, or ≥$75,000), years lived at residence (continuous), having health insurance (yes vs. no), and ability to afford a doctor in the past 12 months (yes vs. no).
Additional potential confounders included each patient’s stage at diagnosis (in situ, localized, or regional/distant) obtained from the Missouri Cancer Registry, and neighborhood (census tract) variables measured using 2000 U.S. Census data, including residential stability (the continuous percentage of the population at same address 5 years ago) and urban/rural status (metropolitan Rural Urban Continuum Area codes 0–3 vs. non-metropolitan codes 4–10).
Potential Covariates
Two potential covariates of the outcome were measured at Y2. Disease progression was defined as having any: contralateral breast cancer, a recurrence of cancer in the same breast, or metastasis (yes vs. no). Current use of hormone therapy was coded yes vs. no.
Data Analysis
Descriptive (STATA 11.0) and multilevel path analyses (Mplus 5.2) were used to describe the sample and test the hypothesized model. Multilevel path analyses accounted for the clustering of individuals within census tracts. Path analyses can accommodate missing data in the predictors and outcomes and do not require listwise deletion.
Direct effect model
The direct effect of neighborhood poverty on physical functioning was examined first.
Multiple confounders and covariates model
To identify variables for inclusion in the subsequent mediation models, we first explored the associations between all hypothesized confounders with physical functioning and neighborhood poverty controlling for the direct effect of neighborhood poverty on physical functioning. If hypothesized confounders were not associated with both the predictor and outcome, they were included in subsequent analyses as covariates (associated with either variable) or were dropped if not associated with either variable. Hypothesized covariates that were not significantly associated with the outcome were dropped from subsequent analyses. Single and multiple mediator models
All hypothesized mediators were tested in separate single mediator models, and then all statistically significant mediators were first included in a multiple mediator model without the inclusion of either covariate or confounder variables from the prior analyses. If two mediators were highly correlated with each other (r≥0.70), one was dropped. If mediators were not significantly associated with both the predictor and outcome, they were included in subsequent analyses as covariates (associated with either variable) or were dropped if not associated with either variable. Additionally, correlations between mediators were estimated. In each model, significant mediation was assessed using MacKinnon et al.’s recommended asymmetric confidence intervals[49] which provide more power than Sobel’s Delta method[50] and may be a more accurate test when using categorical mediators.[51] Statistical significance is indicated when the confidence limits do not include zero.
Next, to examine the robustness of the mediators, we added the previously identified statistically significant confounders and covariates to the multiple mediator model. We retained non-significant mediators as covariates and dropped non-significant covariates from further analyses.
Next, we included the hypothesized mediators found to be associated with only the outcome in the single mediator models as additional covariates in the multiple mediator model, and those that remained statistically significant were retained as covariates.
The final multiple mediator model included all a priori and results-driven covariates and confounders, and correlations between all mediators, covariates, and confounders. All associations in the final model were statistically significant because previous steps in the analysis excluded variables that were not significantly associated with either the predictor or outcome. Overall model fit was assessed with multiple fit indices including chi-square, the comparative fit index (CFI), and the Root Mean Square Error of Approximation (RMSEA) and its 90% confidence intervals (CI). CFI values 0.95 or above suggest good fit[52,53] and RMSEA values <.06 suggest good model fit.[53]
RESULTS
Sample characteristics
Of the 4020 female survivors eligible to participate, 675 could not be contacted after seven attempts. Of the remainder, 1164 (34.8%) completed the Y1 interview. The American Association for Public Opinion Research (AAPOR) response rate (RR1) at Y1 was 29.0%.[54] Compared with participants, nonparticipants were more likely to be older and African American; neighborhood poverty rate did not differ significantly between participants and nonparticipants. Of the 1164, 1037 (88.9%) completed the Y2 interview. Nearly all (98.4%) completed the Y2 interview within 11–14 months following the Y1 interview (range: 9–18 months). After exclusion criteria were applied (n=87 screened positive on the Orientation-Memory-Concentration test in either year; n=45 moved residence between interviews; n=2 were not geocoded), 909 women were included in analyses.
Participants were primarily non-Hispanic White (92.0%), had medical insurance (97.5%), and mean age was 57.9 (range: 27–91) (Table 1). The 909 participants were distributed across 577 census tracts (568 in Missouri; 9 in other states), with an average of 1.6 women per tract (range: 1–8). Most women had lived in the same residence over the study period (870 [95.7%] during their diagnosis in the year prior to the Y1 survey and 622 [68.4%] in the year 2000, when neighborhood poverty rate was measured).
Table 1.
Sample characteristics among 909 breast cancer survivors at Year 1 (baseline).
| N (%) or mean ± SD | |
|---|---|
|
| |
| Y2 Physical functioning (range: 0–100) | 73.7 ± 25.1 |
|
| |
| Neighborhood poverty (range: 0.4–44.0) | 9.7 ± 7.4 |
|
| |
| Age (range: 27–91) | 57.9 ± 11.2 |
|
| |
| Race/ethnicity | |
| Non-Hispanic White | 836 (92.0) |
| Other, multiple | 73 (8.0) |
|
| |
| Education | |
| ≤High school | 315 (34.7) |
| 1–3 years college | 249 (27.4) |
| ≥College graduate | 343 (37.7) |
|
| |
| Household income | |
| ≤24,999 | 140 (15.4) |
| $25–34,999 | 106 (11.7) |
| $35–49,999 | 137 (15.1) |
| $50–74,999 | 194 (21.3) |
| ≥$75,000 | 287 (31.6) |
|
| |
| Marital status | |
| Married or living together | 641 (70.5) |
| Unmarried | 268 (29.5) |
|
| |
| Time lived at residence in years (range: 0–60) | 15.6 ± 12.8 |
|
| |
| Health insurance (Yes vs. No) | 886 (97.5) |
|
| |
| Can afford to see a doctor in the last year (Yes vs. No) | 33 (3.6) |
|
| |
| Residential stability (percent living in home 5 yrs. ago) | 54.8 ± 9.9 |
|
| |
| Urban (vs. Rural) Status | 608 (66.9) |
|
| |
| Stage at diagnosis | |
| In situ | 174 (19.1) |
| Local | 205 (55.2) |
| Regional/Distant | 227 (25.0) |
|
| |
| Y2 Disease progression (Yes vs. No) | 68 (7.5) |
|
| |
| Type of definitive surgery | |
| Lumpectomy | 534 (58.8) |
| Masectomy | 351 (38.6) |
| No surgery | 24 (2.6) |
|
| |
| Surgical side effects index (range: 5–23) | 8.1 ± 3.3 |
|
| |
| Axillary lymph node removal (Yes vs. No) | 709 (78.0) |
|
| |
| Chemotherapy (Yes vs. No) | 402 (44.2) |
|
| |
| Radiotherapy (Yes vs. No) | 653 (71.8) |
|
| |
| Ever received hormone therapy (i.e. Tamoxifen, Raloxifene, or aromatase inhibitors) (Yes vs. No) | 602 (66.2) |
|
| |
| Y2 Currently receiving hormone therapy (i.e. Tamoxifen, Raloxifene, or aromatase inhibitors) (Yes vs. No) | 486 (53.5) |
|
| |
| Comorbidity Index (range: 0–7) | 0.6 ± 1.2 |
|
| |
| Physical Disorder (range: 6–20) | 7.8 ± 2.4 |
|
| |
| Social Disorder (range: 9–27) | 12.6 ± 3.6 |
|
| |
| Collective Efficacy (range: 1.2–4) | 2.0 ± 0.4 |
|
| |
| Clinically significant depressive symptoms (Yes vs. No) | 183 (20.1) |
|
| |
| Perceived stress scale (range: 4–18) | 7.4 ± 3.0 |
|
| |
| Social support scale (range: 1.2–5) | 4.4 ± 0.7 |
|
| |
| Regularly attends church or religious organization (Yes vs. No) | 604 (66.5) |
|
| |
| Regularly attends a club or social organization (Yes vs. No) | 412 (45.3) |
|
| |
| Smoking status | |
| Current smoker | 84 (9.2) |
| Former smoker | 312 (34.3) |
| Never smoked | 512 (56.3) |
|
| |
| Body mass index | |
| Underweight (<18.5 kg/m2) | 9 (1.0) |
| Normal (18.5– 24.9 kg/m2) | 266 (29.6) |
| Overweight (25.0 29.9. kg/m2) | 314 (34.9) |
| Obese (≥30 kg/m2) | 311 (34.6) |
|
| |
| Leisure time physical activity (Yes vs. No) | 693 (76.2) |
|
| |
| >1 Alcoholic drinks/day (vs. ≤1/drinks day) | 470 (51.7) |
All variables measured at Year 1 (data collected 2007–2009) unless specified Y2 (Year 2 data collected 2008–2010).
Direct effect model
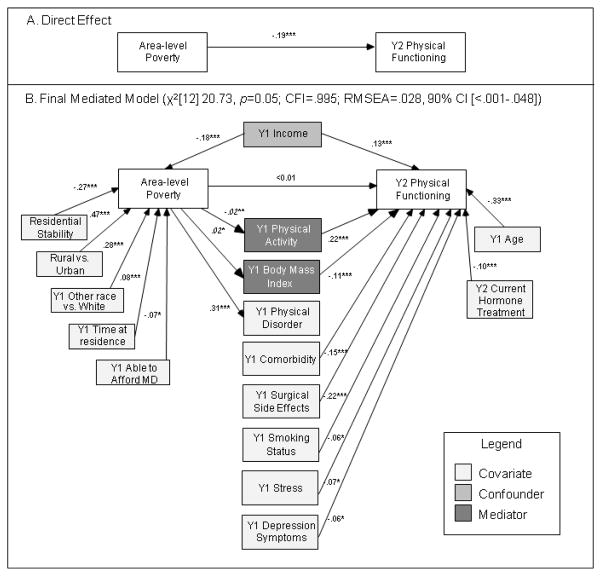
The direct effect of neighborhood poverty on physical functioning was statistically significant, and it accounted for 3.5% of the variance in physical functioning (Figure 1A). Greater neighborhood poverty was associated with lower physical functioning.
Fig. 1.
Direct (A) and mediated (B) effects of neighborhood-level poverty on physical functioning among breast cancer survivors mediated by BMI and physical activity and controlling for covariates and correlations between variables (n=909)
Note: Standardized estimates shown. * p < .05; ** p <.01; *** p < .001; Y1 and Y2 indicate measurement with the Year 1 (data collected 2007–2009) or Year 2 (data collected 2008–2010) survey, respectively. Area-level poverty, residential stability, and urban/rural status were measured using the 2000 U.S. Census. Italicized estimates of association between poverty and categorical mediators are unstandardized probit regression coefficients. Significant correlations between model covariates, confounders, and mediators not shown in Figure 1 are reported in Table 2.
Multiple confounders and covariates model
Only income was a significant confounder (associated with both poverty and physical functioning). Covariates of neighborhood poverty rate included: residential stability, urban/rural status, race, time lived at residence, and ability to afford a doctor in the past 12 months. Hypothesized confounder variables that were unrelated to the predictor and were instead retained as covariates of physical functioning included: age, education, cancer stage at diagnosis, and currently receiving hormone therapy at Y2.
Single mediator models
Nine of the hypothesized mediators were statistically significant in single mediator models: PA, BMI, Physical Disorder, Social Disorder, comorbidity, surgical side effects, smoking status, alcohol use, and Collective Efficacy. Several additional hypothesized mediators were significantly associated with physical functioning but not neighborhood poverty: perceived stress, depressive symptoms, social support, and type of breast cancer surgery and therefore were examined as potential covariates in the multiple mediator model.
Multiple mediator models
An 8 mediator model was examined. Because Physical and Social Disorder were highly correlated (r= 0.76 p<.001), only Physical Disorder was included (similar results were observed with Social Disorder [not shown]). Model fit was good (χ2[1] 1.83, p=0.18; CFI=.999; RMSEA=.030; 90% CI[<.001–.099]) but alcohol use and Collective Efficacy were no longer associated with the outcome and were dropped from further analyses. PA, BMI, Physical Disorder, comorbidity, surgical side effects, and smoking status were significant mediators.
Including all confounders and covariates in a 6 mediator model maintained good fit (χ2[9] 14.63, p=0.10; CFI=.996; RMSEA=.026, 90% CI[<.001–.050]) but comorbidity, smoking status, and surgical side effects were no longer associated with neighborhood poverty and were therefore retained as covariates of physical functioning. Physical Disorder was no longer associated with physical functioning and was retained as a covariate of neighborhood poverty in further analysis. Two covariates, education and cancer stage, were no longer associated with physical functioning and were dropped from further analysis. Two variables remained significant mediators: PA (αβ= −.061, CI: −.075 to −.046) and BMI (αβ= −.050, CI: −.068 to −.033).
The resulting two mediator model was then examined after adding the 4 variables identified in the single mediator analyses as covariates of physical functioning (stress, depression symptoms, social support, and type of surgery). Two of these variables (social support and type of surgery) were no longer significant covariates in the full model and were dropped from further analyses.
The fit of the final two mediator model with one confounder (income) and multiple covariates was very good (χ2[12] 20.73, p=0.05; CFI=.995; RMSEA=.028, 90% CI [<.001–.048]). The effect of neighborhood poverty on physical functioning was fully mediated by PA and BMI (Figure 1B, Table 2). The final model accounted for 48.0% of the variance in physical functioning. Significant associations in the final model between all model variables (including covariates, confounders, and mediators) with the predictor and/or outcome are shown in Figure 1B. Significant correlations between all model covariates, confounders, and mediators not shown in Figure 1B are reported in Table 2 and are organized following the left-to-right layout of Figure 1B.
Table 2.
Significant Correlations between Model Covariates, Confounders, and Mediators in the Final Mediated Model (Not Shown in Figure 1B)
| Variables | Standardized Estimates | |
|---|---|---|
| Income | Rural vs. urban | −.17*** |
| Other race vs. white | −.11*** | |
| Time at residence | −.07* | |
| Able to afford MD | .24*** | |
| Physical activity | .25*** | |
| Body Mass Index (BMI) | −.15*** | |
| Physical disorder | −.22*** | |
| Comorbidity | −.26*** | |
| Surgical side effects | −.13*** | |
| Smoking status | −.17*** | |
| Stress | −.16*** | |
| Depression symptoms | −.17*** | |
| Age | −.30*** | |
|
| ||
| Residential stability | Time at residence | .18*** |
| Surgical side effects | −.08* | |
|
| ||
| Rural vs. urban | Other race vs. white | −.15** |
| Physical disorder | −.11* | |
|
| ||
| Other race vs. white | Time at residence | −.07* |
| Able to afford MD | −.09** | |
| Y2 Hormone treatment | .06* | |
| Physical activity | −.12** | |
| Body mass index (BMI) | .08* | |
| Physical disorder | .12*** | |
| Comorbidity | .13*** | |
| Surgical side effects | .09*** | |
|
| ||
| Time at residence | Able to afford MD | .08* |
| Surgical side effects | −.10** | |
| Smoking status | −.07* | |
| Depression symptoms | −.08* | |
| Age | .39*** | |
| Y2 Hormone treatment | −.08* | |
|
| ||
| Able to afford MD | Physical disorder | −.07** |
| Comorbidity | −.10*** | |
| Surgical side effects | −.11*** | |
| Smoking status | −.13*** | |
| Stress | −.18*** | |
| Depression symptoms | −.19*** | |
| Age | .11** | |
|
| ||
| Physical activity | Body mass index (BMI) | −.24*** |
| Physical disorder | −.17*** | |
| Comorbidity | −.27*** | |
| Surgical side effects | −.16*** | |
| Smoking status | −.12** | |
| Stress | −.19*** | |
| Depression symptoms | −.21*** | |
|
| ||
| BMI | Physical disorder | .08* |
| Comorbidity | .16*** | |
| Smoking status | −.07* | |
| Depression symptoms | .10* | |
|
| ||
| Physical disorder | Comorbidity | .09** |
| Surgical side effects | .15*** | |
| Smoking status | .10*** | |
| Stress | .22*** | |
| Depression symptoms | .21*** | |
|
| ||
| Comorbidity | Surgical side effects | .15*** |
| Smoking status | .08* | |
| Stress | .16*** | |
| Depression symptoms | .18*** | |
| Age | .24*** | |
|
| ||
| Surgical side effects | Smoking status | .13*** |
| Stress | .39*** | |
| Depression symptoms | .39*** | |
| Y2 Hormone treatment | .13*** | |
| Age | −.17*** | |
|
| ||
| Smoking status | Stress | .18*** |
| Depression symptoms | .15*** | |
| Y2 Hormone treatment | .07* | |
|
| ||
| Stress | Depression symptoms | .56*** |
| Age | −.18*** | |
| Y2 Hormone treatment | .07* | |
|
| ||
| Age | Depression symptoms | −.09** |
|
| ||
| Y2 Hormone treatment | Depression symptoms | .09** |
p < .05;
p <.01;
p < .001.
All variables measured at Year 1 (data collected 2007–2009) unless specified Y2 (Year 2 data collected 2008–2010).
In a sensitivity analysis, we repeated the final model analysis with only those women who lived in the same residence since 2000 (n=622): the model fit was largely unchanged (χ2[12] 18.32, p=0.11; CFI=.995; RMSEA=.029 90% CI [<.000–.054]) and PA and BMI remained significant mediators.
DISCUSSION
This study demonstrated that breast cancer survivors living in neighborhoods with higher poverty rates reported lower physical functioning, but this effect was fully mediated by PA and BMI. By identifying potential targets for intervention, these results significantly advance the study of neighborhood conditions and QOL among breast cancer survivors.
Both greater PA and lower BMI are associated with better QOL outcomes and increased survival among cancer survivors.[18,55–63] For example, a recent home-based diet and exercise intervention significantly reduced the rate of declines in physical functioning among older, overweight long-term cancer survivors.[64] Accordingly, weight management and exercise programs are increasingly accepted as critical components of cancer rehabilitation and supportive care.[65] However, while a burgeoning literature has explored optimal individually based interventions[58,66–68] and recommendations for PA among cancer survivors have been issued,[60,69,70] both PA and BMI are suboptimal among cancer survivors.[71,72] In this study, nearly one quarter (24.4%) of survivors reported no leisure time PA whatsoever in the previous month and 69.5% were overweight or obese.
While numerous lifestyle interventions have been developed specifically for cancer survivors, we demonstrate the importance of addressing neighborhood context in the development and delivery of these interventions. However, the feasibility of implementing interventions targeted to both cancer survivor status and neighborhood poverty is uncertain. Interventions targeting community-dwelling cancer survivors living in high-poverty neighborhoods may face serious impediments to recruitment and retention such as competing demands for the participants’ time, residential instability, lack of transportation, inconsistent telephone service, and medical mistrust.[73–78] Other potential barriers include the limited geographic proximity of targeted high-poverty neighborhoods to each other and the relative scarcity of cancer survivors in any given neighborhood. However, because the identified mediators in this study likely underlie physical functioning outcomes in other chronically ill groups (e.g. diabetics), one option would be community-based interventions designed for high-poverty neighborhoods that target all chronically ill residents. Intervention sub-components could be added as needed to address disease-specific issues, such as the unique motives, barriers, and preferences for activity held by cancer survivors.[62] Another practical approach involves adapting existing patient-based cancer survivorship interventions in order to address different neighborhood contexts, particularly the unique challenges faced by residents of high-poverty neighborhoods. For example, exercise recommendations for walking outdoors may not be as relevant for survivors living in high-poverty, high-crime neighborhoods, and could be adapted accordingly. The relevance of the local environment to the success of lifestyle interventions is of paramount importance. In a comprehensive review, the World Health Organization concluded that the most effective strategies to improve diet and PA are multi-component population-level interventions that are adapted to the local context.[79] To date, however, limited data are available to guide interventions for cancer survivors or other chronically ill individuals at a community or systems level.[80–82]
Consistent with previous research among healthy adults,[83,84] this study demonstrated less PA and higher BMI among survivors living in higher poverty neighborhoods. Multiple factors may contribute to these observed differences, including differences in the built environment, such as sidewalks in varying states of disrepair and the prevalence of abandoned buildings, recreational facilities, fast food restaurants, and grocery stores, as well as differences in social norms and perceptions about neighborhood safety or the availability of recreational resources.[85–90]
Several limitations should be noted. First, although we recruited a population-based sample and had a good follow-up response rate (88.9%), we had a low Y1 response rate and higher nonresponse at Y1 by African Americans, potentially limiting generalizability. Second, we used self-reported and several single-item measures and did not measure diet. While the use of a single-item measure of PA is a limitation of our study, it was a significant mediator in our model, providing strong evidence of PA as an important mediator. The use of more objective (e.g., number of steps measured by an accelerometer) and reliable PA measures (e.g. multi-item measures of moderate or vigorous PA per week), could help to monitor compliance with PA recommendations and further elucidate the tested pathways. Third, because Y1 physical functioning was not included, it was not possible to study the effect of neighborhood poverty on change in physical functioning. Notably, however, our longitudinal study allowed for the exploration of the associations of poverty and multiple other factors measured at or before Y1 on Y2 physical functioning. Fourth, women were surveyed only twice. Additional data collection points would allow for more nuanced tests of mediation. However, our use of sensitivity analyses and a time-lagged mediation model (measurement of the predictor, neighborhood poverty, using 2000 Census data preceded the measurement of the Y1 mediators which preceded the Y2 outcome) increase our confidence in the observed meditational pathways.
Despite these limitations, our study confirms the hypothesized effects of neighborhood poverty on physical functioning among breast cancer survivors and that lifestyle factors (PA and BMI) fully mediated this effect. As such, lifestyle interventions that can address both the unique challenges faced by cancer survivors and the challenges of living in a high-poverty neighborhood are needed. Such interventions, if developed with an eye for feasibility and testability, have the potential to reduce observed neighborhood socioeconomic disparities in physical functioning, thereby improving QOL across diverse neighborhoods. Given the documented relationship between health-related QOL and survival [8,9], our findings also provide some insight into the mechanisms driving cancer survival disparities between socioeconomically disadvantaged and more advantaged populations that deserves future study. Accordingly, next steps could include the development and testing of a conceptual framework and a longer causal model that posit testable hypotheses linking neighborhood factors to health-related QOL and ultimately, to survival outcomes. Notably, our use of path analysis with longitudinal data presents several advantages over the more commonly used “black box” regression methods and cross-sectional studies. Particular advantages here included the ability to distinguish between confounders and covariates and the simultaneous estimation of both direct and mediated effects. These methods hold promise for researchers interested in disentangling the mediating mechanisms underlying observed associations between neighborhood factors and individual health outcomes.
Acknowledgments
We thank the staff of the Missouri Cancer Registry and the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri, for the use of the Health Behavior, Communications, and Outreach Core, which is supported in part by the National Cancer Institute Cancer Center Support Grant (P30 CA091842) to the Siteman Cancer Center. This research also was supported in part by grants from the National Cancer Institute (CA112159). Dr. Pruitt was supported by the National Center for Research Resources Clinical and Translational Science Award to Washington University (KL2 RR024994) and a faculty recruitment award from the Cancer Prevention Research Institute of Texas (CPRIT). Dr. McQueen was supported by an American Cancer Society Mentored Research Scholar Grant (CPPB-113766). Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health or the American Cancer Society. The funders did not have any role in the design of the study; the analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG. Edwards BKe SEER Cancer Statistics Review, 1975–2007. National Cancer Institute; Bethesda, MD: 2010. < http://seer.cancer.gov/csr/1975_2007/>, based on November 2009 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003;97(3):674–681. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16 (2):501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 4.Helgeson VS, Tomich PL. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psychooncology. 2005;14(4):307–317. doi: 10.1002/pon.848. [DOI] [PubMed] [Google Scholar]
- 5.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer. 2000;89(11):2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::AID-CNCR5>3.0.CO;2-6. [pii] [DOI] [PubMed] [Google Scholar]
- 6.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 Suppl):2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffe DB, Perez M, Liu Y, Collins KK, Aft RL, Schootman M. Quality of life over time in women diagnosed with ductal carcinoma in situ, early-stage invasive breast cancer, and age-matched controls. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saquib N, Pierce JP, Saquib J, Flatt SW, Natarajan L, Bardwell WA, Patterson RE, Stefanick ML, Thomson CA, Rock CL, Jones LA, Gold EB, Karanja N, Parker BA. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. 2011;20(3):252–259. doi: 10.1002/pon.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. 1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorval M, Maunsell E, Deschenes L, Brisson J, Masse B. Long-term quality of life after breast cancer: comparison of 8-year survivors with population controls. J Clin Oncol. 1998;16 (2):487–494. doi: 10.1200/JCO.1998.16.2.487. [DOI] [PubMed] [Google Scholar]
- 11.Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer. 2008;8:330. doi: 10.1186/1471-2407-8-330. 1471-2407-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashing-Giwa KT, Lim JW. Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Oncol Nurs Forum. 2009;36(1):79–88. doi: 10.1188/09.ONF.79-88. 3X25668M253452P6. [DOI] [PubMed] [Google Scholar]
- 13.Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K, Baumgartner KB, Fetherolf J, Reeve BB, Smith AW, Ganz PA, McTiernan A, Barbash RB. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Predictors of quality of life of breast cancer patients. Acta Oncol. 2003;42 (7):710–718. doi: 10.1080/02841860310017658. [DOI] [PubMed] [Google Scholar]
- 15.Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, Schwartz K, Katz SJ. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 2007;16(9):1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 16.Michael YL, Berkman LF, Colditz GA, Holmes MD, Kawachi I. Social networks and health-related quality of life in breast cancer survivors: a prospective study. J Psychosom Res. 2002;52(5):285–293. doi: 10.1016/s0022-3999(01)00270-7. S0022399901002707. [DOI] [PubMed] [Google Scholar]
- 17.Mols F, Vingerhoets AJ, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. Eur J Cancer. 2005;41(17):2613–2619. doi: 10.1016/j.ejca.2005.05.017. S0959-8049(05)00726-4. [DOI] [PubMed] [Google Scholar]
- 18.Voskuil DW, van Nes JG, Junggeburt JM, van de Velde CJ, van Leeuwen FE, de Haes JC. Maintenance of physical activity and body weight in relation to subsequent quality of life in postmenopausal breast cancer patients. Ann Oncol. 21(10):2094–2101. doi: 10.1093/annonc/mdq151. mdq151. [DOI] [PubMed] [Google Scholar]
- 19.Schootman M, Jeffe DB, Lian M, Deshpande AD, Gillanders WE, Aft R, Sumner W. Area-level poverty is associated with greater risk of ambulatory-care-sensitive hospitalizations in older breast cancer survivors. J Am Geriatr Soc. 2008;56(12):2180–2187. doi: 10.1111/j.1532-5415.2008.02002.x. JGS2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schootman M, Jeffe DB, Lian M, Gillanders WE, Aft R. The role of poverty rate and racial distribution in the geographic clustering of breast cancer survival among older women: a geographic and multilevel analysis. Am J Epidemiol. 2009;169(5):554–561. doi: 10.1093/aje/kwn369. kwn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh GK, Miller BA, Hankey BF, Edwards BK. Area socioeconomic variations in US cancer incidence, mortality, stage, treatment, and survival, 1975–1999. National Cancer Institute; Bethesda, MD: 2003. [Google Scholar]
- 22.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91 (11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55 (1):125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 24.O’Campo P. Invited commentary: Advancing theory and methods for multilevel models of residential neighborhoods and health. Am J Epidemiol. 2003;157 (1):9–13. doi: 10.1093/aje/kwf171. [DOI] [PubMed] [Google Scholar]
- 25.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140 (6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 26.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. J Nurs Scholarsh. 2005;37 (4):336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273 (1):59–65. [PubMed] [Google Scholar]
- 28.Diez Roux AV. Integrating social and biologic factors in health research: a systems view. Ann Epidemiol. 2007;17(7):569–574. doi: 10.1016/j.annepidem.2007.03.001. S1047-2797(07)00096-8. [DOI] [PubMed] [Google Scholar]
- 29.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88 (2):216–222. doi: 10.2105/ajph.88.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macintyre S, Ellaway A. Neighborhoods and health: An overview. In: Kawachi I, Berkman L, editors. Neighborhoods and health. Oxford University Press; 2003. pp. 45–64. [Google Scholar]
- 31.Oakes JM. Commentary: Advancing neighbourhood-effects research--selection, inferential support, and structural confounding. Int J Epidemiol. 2006;35 (3):643–647. doi: 10.1093/ije/dyl054. [DOI] [PubMed] [Google Scholar]
- 32.Robert SA. Neighborhood socioeconomic context and adult health. The mediating role of individual health behaviors and psychosocial factors. Ann N Y Acad Sci. 1999;896:465–468. doi: 10.1111/j.1749-6632.1999.tb08171.x. [DOI] [PubMed] [Google Scholar]
- 33.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2 (3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30 (6):473–483. [PubMed] [Google Scholar]
- 35.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156 (5):471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 36.Franzini L, Caughy M, Spears W, Fernandez Esquer ME. Neighborhood economic conditions, social processes, and self-rated health in low-income neighborhoods in Texas: a multilevel latent variables model. Soc Sci Med. 2005;61(6):1135–1150. doi: 10.1016/j.socscimed.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Schootman M, Jeffe DB, West MM, Aft R. Self-report by elderly breast cancer patients was an acceptable alternative to surveillance, epidemiology, and end results (SEER) abstract data. J Clin Epidemiol. 2005;58(12):1316–1319. doi: 10.1016/j.jclinepi.2005.04.002. S0895-4356(05)00187-3. [DOI] [PubMed] [Google Scholar]
- 38.Schootman M, Deshpande AD, Pruitt SL, Jackson-Thompson J, Jeffe D. Neighborhood foreclosures and self-rated health among breast cancer survivors. Qual Life Res. 2012;21 (1):133–141. doi: 10.1007/s11136-011-9929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins KK, Liu Y, Schootman M, Aft R, Yan Y, Dean G, Eilers M, Jeffe DB. Effects of breast cancer surgery and surgical side effects on body image over time. Breast Cancer Res Treat. 2011;126(1):167–176. doi: 10.1007/s10549-010-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Perez M, Aft RL, Massman K, Robinson E, Myles S, Schootman M, Gillanders WE, Jeffe DB. Accuracy of perceived risk of recurrence among patients with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 19(3):675–680. doi: 10.1158/1055-9965.EPI-09-1051. 1055-9965.EPI-09-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez M, Liu Y, Schootman M, Aft RL, Schechtman KB, Gillanders WE, Jeffe DB. Changes in sexual problems over time in women with and without early-stage breast cancer. Menopause. 17(5):924–937. doi: 10.1097/gme.0b013e3181d5dd26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34 (1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40 (5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 44.Ross CE, Mirowsky J. Disorder and decay: The concept and measurement of perceived neighborhood disorder. Urban Affairs Review. 1999;34 (3):412–432. [Google Scholar]
- 45.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277 (5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 46.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5 (2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24 (4):385–396. [PubMed] [Google Scholar]
- 48.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32 (6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 49.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7 (1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt SE, editor. Sociological Methodology. Jossey-Bass; San Francisco: 1982. pp. 290–312. [Google Scholar]
- 51.MacKinnon DF. Introduction to statistical mediation analysis. Lawrence Earlbaum Associates; New York, NY: 2008. [Google Scholar]
- 52.Hu L, Bentler P. Structural equation modeling. Concepts. issues, and applications. Sage; London: 1995. Evaluating model fit; pp. 76–99. [Google Scholar]
- 53.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6 (1):1–55. [Google Scholar]
- 54.Deerfield, IL: 2008. [Accessed on September 28, 2011]. American Association for Public Opinion Research Standard definitions: Final dispositions of case codes and outcome rates for surveys. Revised 2008. http://www.aapor.org/AM/Template.cfm?Section=Standard_Definitions2&Template=/CM/ContentDisplaycfm&ContentID=3156. [Google Scholar]
- 55.Kendall AR, Mahue-Giangreco M, Carpenter CL, Ganz PA, Bernstein L. Influence of exercise activity on quality of life in long-term breast cancer survivors. Qual Life Res. 2005;14 (2):361–371. doi: 10.1007/s11136-004-1468-5. [DOI] [PubMed] [Google Scholar]
- 56.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. 175/1/34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. S0378-5122(10)00005-8. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. 14/7/1588. [DOI] [PubMed] [Google Scholar]
- 59.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 61.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20 (4):1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 62.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 63.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. 301/18/1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giovannucci EL. Physical Activity as a Standard Cancer Treatment. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs229. [DOI] [PubMed] [Google Scholar]
- 66.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol. 2006;24(32):5125–5131. doi: 10.1200/JCO.2006.06.6175. 24/32/5125. [DOI] [PubMed] [Google Scholar]
- 67.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43(1):32–38. doi: 10.1136/bjsm.2008.053843. bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 68.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. 23/16/3830. [DOI] [PubMed] [Google Scholar]
- 69.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, Andrews KS. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56(6):323–353. doi: 10.3322/canjclin.56.6.323. 56/6/323. [DOI] [PubMed] [Google Scholar]
- 70.Physical Activities Guidelines Advisory Committee. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services; 2008. [Accessed 11-22-2010]. http://www.health.gov/PAGuidelines/guidelines/default.aspx. [Google Scholar]
- 71.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. 26/13/2198. [DOI] [PubMed] [Google Scholar]
- 72.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005;40(6):702–711. doi: 10.1016/j.ypmed.2004.09.011. S0091-7435(04)00462-1. [DOI] [PubMed] [Google Scholar]
- 73.Paskett ED, Reeves KW, McLaughlin JM, Katz ML, McAlearney AS, Ruffin MT, Halbert CH, Merete C, Davis F, Gehlert S. Recruitment of minority and underserved populations in the United States: the Centers for Population Health and Health Disparities experience. Contemporary clinical trials. 2008;29(6):847–861. doi: 10.1016/j.cct.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52 (12):1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 75.Sears SR, Stanton AL, Kwan L, Krupnick JL, Rowland JH, Meyerowitz BE, Ganz PA. Recruitment and retention challenges in breast cancer survivorship research: results from a multisite, randomized intervention trial in women with early stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12 (10):1087–1090. [PubMed] [Google Scholar]
- 76.Stull VB, Snyder DC, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. The Journal of nutrition. 2007;137 (1 Suppl):243S–248S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 77.Giuliano AR, Mokuau N, Hughes C, Tortolero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000;10 (8 Suppl):S22–34. doi: 10.1016/s1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 78.Stevinson C. Ready to Change Lifestyle? The Feasibility of Exercise Interventions in Cancer Patients. In: Saxton J, Daley A, editors. Exercise and Cancer Survivorship. Springer; New York: 2010. pp. 211–221. [DOI] [Google Scholar]
- 79.World Health Organization. [Accessed 11-22-2010];Interventions on Diet and Physical Activity: What Works; Implementation of the Global Strategy on Diet, Physical Activity and Health. 2009 http://www.who.int/dietphysicalactivity/whatworks/en/
- 80.Lajous M, Mozaffarian D, Mozaffarian R, Schrag D, Adami HO. Lifestyle prescriptions for cancer survivors and their communities. Journal of Internal Medicine. 2010 doi: 10.1111/j.1365-2796.2010.02273.x. Epub ahead of print: 23 SEP 2010. [DOI] [PubMed] [Google Scholar]
- 81.Wolin KY, Colditz GA. Implementing chronic disease prevention amongst cancer survivors. J Intern Med. doi: 10.1111/j.1365-2796.2010.02295.x. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization. Technical Report Series. 894. 1997. Obesity. Preventing and managing the global epidemic. Report of a WHO Consultation on obesity. Report on a WHO Consultation. [PubMed] [Google Scholar]
- 83.Turrell G, Haynes M, Burton NW, Giles-Corti B, Oldenburg B, Wilson LA, Giskes K, Brown WJ. Neighborhood disadvantage and physical activity: baseline results from the HABITAT multilevel longitudinal study. Ann Epidemiol. 20(3):171–181. doi: 10.1016/j.annepidem.2009.11.004. S1047-2797(09)00360-3. [DOI] [PubMed] [Google Scholar]
- 84.Wang MC, Kim S, Gonzalez AA, MacLeod KE, Winkleby MA. Socioeconomic and food-related physical characteristics of the neighbourhood environment are associated with body mass index. J Epidemiol Community Health. 2007;61(6):491–498. doi: 10.1136/jech.2006.051680. 61/6/491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fish JS, Ettner S, Ang A, Brown AF. Association of perceived neighborhood safety on body mass index. Am J Public Health. 100(11):2296–2303. doi: 10.2105/AJPH.2009.183293. AJPH.2009.183293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63(4):1011–1022. doi: 10.1016/j.socscimed.2006.03.012. S0277-9536(06)00146–8. [DOI] [PubMed] [Google Scholar]
- 87.Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC. The built environment and obesity. Epidemiol Rev. 2007;29:129–143. doi: 10.1093/epirev/mxm009. mxm009. [DOI] [PubMed] [Google Scholar]
- 88.Wendel-Vos W, Droomers M, Kremers S, Brug J, van Lenthe F. Potential environmental determinants of physical activity in adults: a systematic review. Obes Rev. 2007;8(5):425–440. doi: 10.1111/j.1467-789X.2007.00370.x. OBR370. [DOI] [PubMed] [Google Scholar]
- 89.Joshu CE, Boehmer TK, Brownson RC, Ewing R. Personal, neighbourhood and urban factors associated with obesity in the United States. J Epidemiol Community Health. 2008;62(3):202–208. doi: 10.1136/jech.2006.058321. 62/3/202. [DOI] [PubMed] [Google Scholar]
- 90.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. S0749-3797(08)00838-6. [DOI] [PubMed] [Google Scholar]