Abstract
BACKGROUND & AIMS
Direct evidence to support the airway protective function of aerodigestive reflexes triggered by pharyngeal stimulation was previously demonstrated by abolishing these reflexes by topical pharyngeal anesthesia in normal subjects. Studies have also shown that these reflexes deteriorate in cigarette smokers. Aim of this study was to determine the influence of defective pharyngeal aerodigestive reflexes on airway protection in cigarette smokers.
METHODS
Pharyngoglottal Closure reflex; PGCR, Pharyngo-UES Contractile reflex; PUCR, and Reflexive Pharyngeal Swallow; RPS were studied in 15 healthy non-smokers (24.2 ± 3.3 SD y, 7 males) and 15 healthy chronic smokers (27.3 ± 8.1, 7 males). To elicit these reflexes and to evaluate aspiration, colored water was perfused into the hypopharynx at the rate of 1 mL/min. Maximum volume of water that can safely dwell in the hypopharynx before spilling into the larynx (Hypopharyngeal Safe Volume; HPSV) and the threshold volume to elicit PGCR, PUCR, and RPS were determined in smokers and results compared with non-smokers.
RESULTS
At baseline, RPS was elicited in all non-smokers (100%) and in only 3 of 15 smokers (20%; P < .001). None of the non-smokers showed evidence of laryngeal spillage of water, whereas 12 of 15 smokers with absent RPS had laryngeal spillage. Pharyngeal anesthesia abolished RPS reflex in all non-smokers resulting in laryngeal spillage. The HPSV was 0.61 ± 0.06 mL and 0.76 ± 0.06 mL in non-smokers and smokers respectively (P = .1).
CONCLUSIONS
Deteriorated reflexive pharyngeal swallow in chronic cigarette smokers predispose them to risks of aspiration and similarly, abolishing this reflex in non-smokers also results in laryngeal spillage. These observations directly demonstrate the airway protective function of RPS.
Keywords: Cigarette Smoking, Airway Protection, Reflux, UES
Anatomic contiguity between the pharyngolaryngeal and gastroesophageal pathways can predispose the airways to risks of aspiration during retrograde transit of gastric contents or during antegrade flow of material during swallowing. Several aerodigestive reflexes triggered at various levels have been proposed to protect the airways against aspiration.1–16 For example, distention of the esophagus can enhance upper esophageal sphincter (UES) pressure: called the esophago-UES contractile reflex1–4 and this may prevent entry of esophageal contents into the pharynx. Fluid in the pharynx can enhance UES pressure: called the pharyngo-UES contractile reflex (PUCR),5–8 and this in turn may protect against further esophagopharyngeal reflux. At a larger volume, fluid in the pharynx can also trigger an irrepressible swallow: called the reflexive pharyngeal swallow (RPS),5,8,10 that not only triggers glottal closure, but also clears the pharynx of any fluid. Glottal closure without a swallow also can be triggered by fluid in the pharynx: called the pharyngoglottal closure reflex (PGCR).3,11,14 Although it has been proposed that these aerodigestive reflexes protect the airways against aspiration, thus far there has been no evidence to directly show their role in airway protection.
Previous studies using patients with neurogenic dysphagia as human models have shown that impairment in the mechanisms of airway protection during primary swallow predisposes these patients to aspiration.17–19 However, with the identification of impaired aerodigestive reflexes in healthy subjects (chronic cigarette smokers)10,11 and with the availability of sensitive techniques to evaluate predisposition to aspiration, we now have the opportunity to directly study the airway protection function of these aerodigestive reflexes in subjects without neurologic impairment as compared with other studies performed on those with neurologic dysphagia.
Aerodigestive reflexes triggered by pharyngeal stimulation (PUCR, PGCR, and RPS) are deteriorated in healthy chronic cigarette smokers, they are either not elicitable or require a larger threshold volume for elicitation.10,11 Hence, chronic cigarette smokers can be used as healthy human models to study the direct role of these reflexes in airway protection. The aim of the present study was to determine the role of pharyngeal aerodigestive reflexes in protecting the airways against aspiration by studying these reflexes during simulated pharyngeal reflux events in normal healthy volunteers and in chronic cigarette smokers.
Materials and Methods
This study was approved by the Institutional Review Board of the Medical College of Wisconsin and all subjects gave informed written consent before the study. All subjects underwent unsedated transnasal endoscopy20 to rule out any associated silent upper gastrointestinal disorders because it previously has been shown that despite no symptoms around 20% of subjects have abnormal findings in the upper gastrointestinal tract when screened using unsedated transnasal endoscopy.21 On the day of the study all subjects filled out a questionnaire and underwent a brief history and physical examination.
Fifteen healthy nonsmokers (24.2 ± 3.3 y, 7 men) and 15 healthy chronic smokers (27.3 ± 8.1 y, 7 men) were studied in a semi-inclined position (45°). Nonsmokers were defined as those who never smoked. Smokers were defined as those with a history of smoking one or more packs of cigarettes per day for at least 2 years. Smokers were asked to refrain from smoking for 12 hours before the study. Nonsmokers were studied before and after the pharynx was anesthetized with 4% topical lidocaine (Roxane Laboratories, Columbus, OH). The anesthetic agent was applied to the posterior pharyngeal mucosa using an atomizer as well as spraying additional lidocaine through the biopsy channel of a laryngopharyngoscope (FNL-10AP; Pentax Imaging, Montvale, NJ) positioned just above the hypopharynx. Because previous studies have shown10,11 that these aerodigestive reflexes are defective in cigarette smokers, smokers were studied without using pharyngeal anesthesia.
Aerodigestive reflexes triggered by pharyngeal stimulation (PGCR, PUCR, and RPS) were elicited using the previously described technique of concurrent trans-nasal unsedated videoendoscopy, UES manometry (Dentsleeve, Adelaide, Australia), and submental electromyography.3,8,9,11,14 Resting UES pressure and UES response to pharyngeal water stimulation was monitored using a UES sleeve assembly (Dentsleeve) that incorporated a sleeve device (6 × 0.5 × 0.3 cm) with side-hole recording ports at its proximal and distal ends for manometric positioning. To prevent the possibility of anesthetizing the pharynx, a nonanesthetic jelly was used to lubricate the nasal passages (Surgilube; E. Fougera & Co, Atlanta, Inc, Melville, NY) with a cotton-tipped applicator. The manometric assembly was introduced through the nose and positioned such that the manometric port immediately proximal to the sleeve sensor was positioned 2 cm above the UES high-pressure zone and directed posteriorly. This port was used for eliciting the reflexes by perfusing water at 1 mL/min. The posterior orientation of the perfusion port prevented contact of water with the larynx, which also was monitored with an ultrathin laryngopharyngoscope passed through the other nostril. The injection port, the esophageal ports, and the sleeve sensor were connected to pressure transducers in line with a minimally compliant pneumohydraulic pump (Arndorfer Medical Specialties, Greendale, WI). A MMS Motility System (Medical Measurement Systems, Enschede, The Netherlands) was used to record the onset and offset of water injection and UES pressure responses.
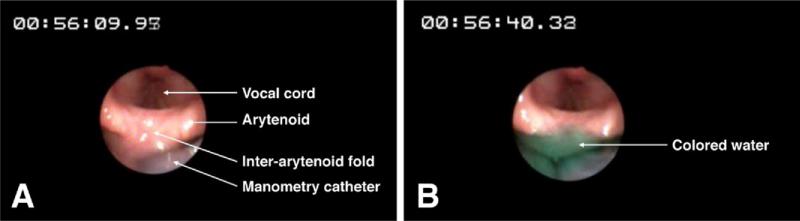
Glottal response (adduction) to pharyngeal water stimulation (PGCR) was monitored by a Pentax FNL-10AP laryngopharyngoscope passed through the other nostril and positioned within the pharynx such that the vocal chords were visualized completely3,11,14 (Figure 1). Greencolored water was used for better endoscopic visualization. Endoscopic images were recorded digitally on a DVD recorder for subsequent analysis in real-time and slow motion (Figure 2A and B). The laryngopharyngo-scope and manometric recording were synchronized using a specially designed timer (Thalner Electronics Labs, Inc, Ann Arbor, MI). After intubation (endoscope and manometry catheter), all subjects were given 15 minutes to adapt before starting the study.
Figure 1.
Endoscopic views of the larynx and the pharynx during water perfusion into the pharynx at 1 mL/min. Perfusion was started at time reading (A) 00:56:09.95 and around (B) 31 seconds later (timer 00:56:40.33) water has risen up to the superior margin of the interarytenoid fold (HPSV). Any further perfusion beyond this point would have resulted in laryngeal spillage of water.
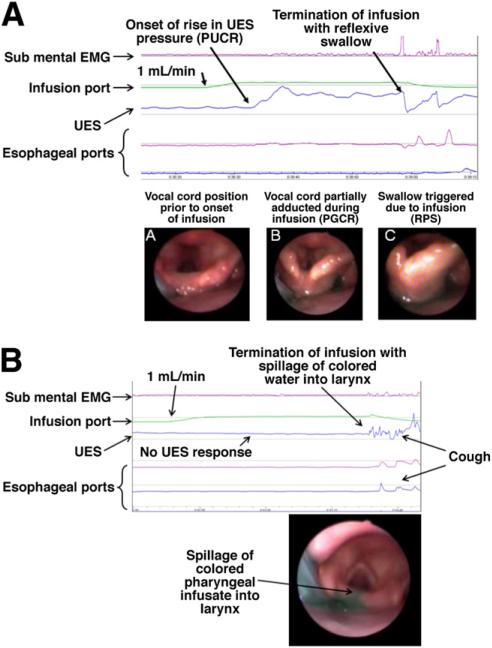
Figure 2.
(A) PUCR, PGCR, and RPS: infusion of water at 1 mL/min into the pharynx of a nonsmoker subject resulted in a more than 100% increase in UES pressure above the baseline (PUCR) after 5.4 seconds (0.1 mL) from the onset of infusion. This was not associated with any submental electromyographic changes. At 37.3 seconds after the onset of water infusion (0.6 mL) an irrepressible swallow was triggered (RPS). Infusion of water into the pharynx also resulted in adduction (incomplete closure) of vocal cords (PGCR). (a) Vocal cord position before pharyngeal infusion of water. (b) Adduction (incomplete closure) of vocal cords during the infusion (PGCR). This response was not associated with any submental electromyographic activity. (c) Onset of swallow. (B) Unlike the nonsmoker subject in A, infusion of water at 1 mL/min into the pharynx of a smoker subject did not induce any UES pressure changes (no PUCR) and did not induce RPS. At 30.6 seconds after onset of pharyngeal infusion, video endoscopic views of the larynx showed colored water rising up to the superior margin of the interarytenoid fold with spillage into the larynx. This resulted in a cough response. EMG, electromyography.
PGCR, PUCR, and RPS were elicited by pharyngeal stimulation with water at room temperature perfused into the pharynx through a dedicated perfusion port at a rate of 1 mL/min using a Harvard infusion pump (model N0975; Harvard Apparatus Co, Inc, Dover, MA) until an irrepressible swallow occurred (RPS). Each injection was started 5–10 seconds after the UES pressure returned to baseline after a swallow, and subjects withheld swallowing as long as they could. Each injection was performed 3 times and subjects were asked to swallow between injections to clear the pharynx of any residual water. After the onset of pharyngeal water perfusion, an increase in UES pressure that was not associated with submental electromyographic activity was considered as PUCR. The average end-expiratory UES pressure at baseline (10-second period before the injection) and after the injection (3 of 3 injections) were recorded. The post-injection pressure was defined as the maximum UES pressure after pharyngeal water injection, excluding the 3-second interval before deglutitive relaxation, if a swallow occurred. This 3-second interval was used to avoid counting the commonly seen pressure increase that is registered by the sleeve immediately before its swallow-induced relaxation. We determined the frequency elicitation response and the smallest volume of injected water in each subject: the threshold volume that in 3 of 3 injections triggered glottal adduction (PGCR), an increase in UES pressure (PUCR), and an irrepressible pharyngeal swallow (RPS). In those patients in whom RPS was absent, occurrence of laryngeal penetration was documented by endoscopically observing colored water rise up to the superior level of the interarytenoid notch and spill into the larynx, at which point perfusion was stopped and the volunteers were asked to swallow to prevent spillage into the trachea (aspiration). Laryngeal penetration was defined as spill-age of water into the laryngeal vestibule but not below the vocal cords into the trachea (aspiration). The maximum volume of fluid that could safely dwell in the hypopharynx before spilling into the larynx, the hypopharyngeal safe volume (HPSV), was measured in the nonsmokers after pharyngeal anesthesia and in those smokers in whom RPS was absent at baseline.
Statistical Analysis
A comparison of the frequency elicitation response of PUCR, PGCR, RPS, and aspiration between smokers and nonsmokers was performed using the Fisher exact test. A comparison of threshold volumes between the 2 groups was performed by unpaired t test. For data that did not pass the normality test, the Mann–Whitney rank sum method was used. Values are presented as mean (±standard error) unless stated otherwise.
Results
All subjects completed the study and no volunteer experienced any adverse effects.
Pharyngoglottal Closure Reflex
Frequency elicitation
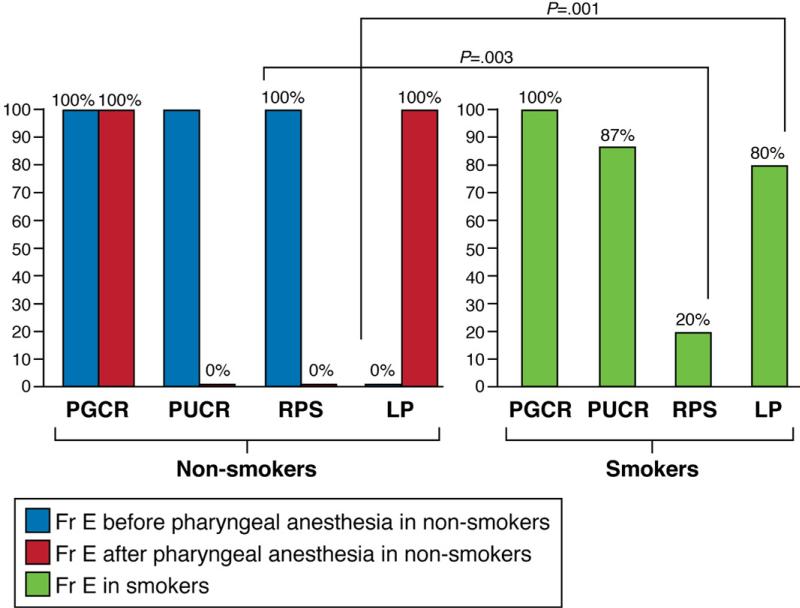
PGCR was elicited in all nonsmokers, both before and after application of topical pharyngeal anesthesia. Similarly, this reflex was preserved in all chronic smokers (Figure 3).
Figure 3.
Frequency elicitation (fr e) of PUCR, PGCR, RPS, and laryngeal penetration. Nonsmokers were studied before and after pharyngeal anesthesia and smokers were studied without pharyngeal anesthesia. PGCR was elicited in all nonsmokers before and after pharyngeal anesthesia and this reflex was preserved in all chronic smokers. PUCR was elicited in 87% of nonsmokers and 87% of chronic smokers. Pharyngeal anesthesia abolished this reflex. RPS was elicited in all nonsmokers and it was absent in 80% of smokers in whom laryngeal penetration was observed. Pharyngeal anesthesia abolished this reflex in all nonsmokers who then showed evidence of laryngeal penetration.
Threshold volume
The threshold volume to elicit PGCR in nonsmokers was 0.20 ± 0.02 mL, which was not significantly different from chronic smokers (0.24 ± 0.08 mL; P = .6) (Table 1). The threshold volume to elicit this reflex after pharyngeal anesthesia in non-smokers was 0.21± 0.03 mL (P = .6, compared with before anesthesia).
Table 1.
Threshold Volumes (mL ± Standard Error) to Trigger PGCR, PUCR, RPS, HPSV, and Volume at Which Laryngeal Penetration Was Observed in Nonsmokers (Before and After Pharyngeal Anesthesia) and in Chronic Smokers
| PGCR | PUCR | RPS | HPSV | Laryngeal penetration | |
|---|---|---|---|---|---|
| Nonsmokers | |||||
| Before pharyngeal anesthesia | 0.20 ± 0.02a | 0.27 ± 0.04b | 0.71 ± 0.07c | N/A | Not observed |
| After pharyngeal anesthesia | 0.21 ± 0.03a | Absent | Absent | 0.61 ± 0.06c,d | Observed |
| Smokers | 0.24 ± 0.08a | 0.31 ± 0.04b | Absent | 0.76 ± 0.06c,d | Observed |
| P value | .6a | .1b | .2c | .1d |
NOTE. P-values comparing “a”, “b” and “b”, “c” and “c”, and “d” and “d”.
PUCR
Frequency elicitation
Pharyngeal injection of water elicited PUCR in 13 of 15 nonsmoker subjects at baseline. In these subjects, this reflex was abolished after pharyngeal anesthesia. PUCR also was elicited in 13 of 15 subjects (86.7%) in the smoker group (Figure 3).
Threshold volume
The threshold volume to trigger UES response in the nonsmokers before anesthesia was 0.27 ± 0.04 mL, which was not significantly different from that in chronic smokers 0.31 ± 0.04 mL (P = .1) (Table 1).
UES pressure
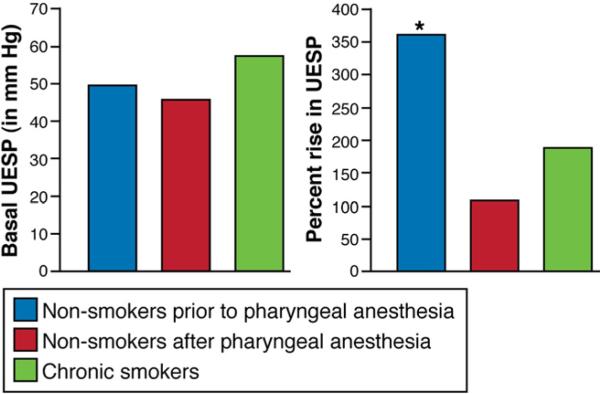
Baseline UES pressure in chronic smokers was 57 ± 4 mm Hg, in nonsmokers before anesthesia it was 50 ± 4 mm Hg, and after pharyngeal anesthesia it was 46 ± 3 mm Hg (P = NS). However, the percentage increase in UES pressure after injection of water into the pharynx was significantly lower in nonsmokers after anesthesia (108% ± 14%) and in chronic smokers (188% ± 11%) as compared with non-smokers before anesthesia (361% ± 48%; P < .001) (Figure 4).
Figure 4.
UES pressure (UESP, in mm Hg) and percentage increase in UESP in nonsmokers before and after pharyngeal anesthesia and in chronic smokers. The percentage increase in UESP was significantly higher in the nonsmokers compared with smokers (*P < .001). Pharyngeal anesthesia resulted in attenuation of an increase in UESP compared with the pre-anesthesia percentage increase in the nonsmoker group (*P < .001). The percentage increase in UESP in chronic smokers was similar to the percentage increase in UESP in nonsmokers after pharyngeal anesthesia.
RPS
Frequency elicitation
RPS was present in all the nonsmokers (100%) compared with being present in only 3 of 15 chronic smokers (frequency elicitation, 20%; P = .003) (Figure 3). After topical pharyngeal anesthesia, this reflex was abolished in all nonsmoker subjects.
Threshold volume
The threshold volume to trigger RPS in 15 of 15 nonsmokers at baseline was 0.71 ± 0.07 mL (Table 1).
Aspiration/HPSV
Close monitoring of the pharyngoglottal region using a transnasal ultrathin endoscope did not show any evidence of laryngeal penetration of the perfused fluid in any of the 15 nonsmokers before pharyngeal anesthesia. After application of topical pharyngeal anesthesia, all the nonsmokers had endoscopic evidence of impending aspiration (perfusion was stopped before the occurrence of aspiration) with fluid spilling into the larynx. Compared with the nonsmokers without application of topical pharyngeal anesthesia, the colored water was seen to rise in the hypopharynx and reach the interarythenoid level and spill over into the larynx in 12 of 15 chronic smokers (80%; P < .001) (Figure 2B). The 12 smoker subjects who had laryngeal penetration were those in whom the RPS could not be elicited.
The volume of water in the smokers resulting in laryngeal penetration averaged 0.76 ± 0.06 mL. This volume in healthy volunteers after their topical pharyngeal anesthesia averaged 0.61 ± 0.06 mL and was not statistically different from those of chronic smokers.
Discussion
The pharynx is a shared pathway for ingestion and respiration and because of its anatomic contiguity with the stomach and larynx its capacity to safely accommodate residue left behind after a swallow or during gastroesophagopharyngeal reflux without spilling over into the airway is of crucial clinical importance. Because of technical limitations and safety concerns, this capacity has not been measured systematically before.
Laryngeal penetration and aspiration of pharyngeal residue is dependent on the volume of material that the pharynx can safely accommodate without spilling into the airway (the HPSV). Pharyngeal reflexes such as RPS and PUCR have been proposed to prevent the pharyngeal content exceeding this volume and therefore protect the airway against aspiration. These and other reflexes triggered by stimulation of the pharynx or the esophagus help protect the airways by different mechanisms. For example, during gastroesophageal reflux episodes, stimulation of the esophagus by the refluxed material can augment the UES pressure (esophago-UES contractile reflex), thereby increasing the pressure barrier against entry of refluxate into the pharynx.1,22 Esophageal dis-tension also can cause reflexive adduction of the vocal cords (esophagoglottal closure reflex), closing the airway transiently to prevent potential aspiration.3,7,22 If the refluxate does enter the pharynx, stimulation of the pharynx also can augment the UES pressure; thereby preventing further entry of refluxate into the pharynx (PUCR).6,9 Additional protection also is provided by glottal adduction induced by pharyngeal stimulation (PGCR).12–14 Contact of pharyngeal content with the laryngeal structures also trigger vocal cord adduction (laryngeal adductor reflex), closing the introitus to the trachea and preventing or limiting aspiration.23 At a larger volume, fluid in the pharynx can trigger an irrepressible swallow that not only lifts and closes the glottis but also clears the pharynx of any residual fluid (RPS).5,8,10 However, as yet there is no direct evidence in human beings to show that these reflexes indeed protect the airway against aspiration because of the lack of a human model with defective aerodigestive reflexes. In our previous studies we have shown that anesthetizing the pharyngeal mucosa can either abolish or increase the threshold volume to elicit PUCR and RPS in healthy volunteers24 and that a larger volume of fluid is required to trigger PGCR, PUCR, and RPS in chronic cigarette smokers compared with healthy nonsmokers.10,11 The present study was undertaken to use these human models of deteriorated aerodigestive reflexes and our technique of concurrent pharyngeal perfusion, pharyngolaryngeal endoscopy, and manometry to directly study the role of these reflexes in protecting the airways.
Similar to our previous studies,10,11 in this study we have again shown that elicitation of RPS is defective in smokers compared with nonsmokers. This defect in smokers (absent RPS during pharyngeal stimulation with water perfused at 1 mL/min) led to accumulation of fluid in the pharynx that was seen endoscopically to spill over into the larynx while the subjects were awake in a sitting position. RPS was preserved in all nonsmokers and none of the healthy controls were noted to have laryngeal penetration. However, abolishing RPS with pharyngeal topical anesthesia in these healthy nonsmokers led to spillage of water into the larynx. These observations show the direct role of RPS in airway protection.
Characteristics of PGCR were similar between smokers and nonsmokers and before and after pharyngeal topical anesthesia. Although by momentarily closing the vocal cords PGCR can prevent aspiration, it will not protect against entry of fluid into the supraglottic larynx (laryngeal penetration). This fluid then potentially can enter the trachea (aspiration) once the vocal cords abduct. During retrograde transit of fluid, entry of fluid into the pharynx can augment the upper esophageal sphincter pressure (PUCR), thereby preventing further entry of fluid from the esophagus into the pharynx. At a larger threshold volume, fluid in the pharynx can trigger an irrepressible swallow (RPS), that not only causes glottal closure but also elevates the larynx and clears the pharynx of any residual fluid. Hence, despite a preserved PGCR, defective PUCR and RPS may predispose to pharyngeal fluid spilling into the larynx. In this study we showed that the percentage increase in UES pressure during PUCR was significantly lower in smokers compared with nonsmokers and absent RPS in the smokers led to laryngeal spillage of water.
As was observed in our previous study,24 in the present study the threshold volume to elicit RPS in healthy individuals before topical anesthesia also was similar to the maximum volume of fluid that can safely dwell in the hypopharynx before spilling into the larynx (HPSV). This finding suggests that receptors triggering RPS may be located at the area near the upper margin of the interarytenoid fold and are not amenable to volitional suppression of swallow as are those receptors located in the rest of the pharyngeal swallow trigger zone such as the posterior pharyngeal wall or tonsillar pillars. This arrangement results in triggering of RPS when the fluid accumulation reaches that area in healthy individuals before pharyngeal anesthesia. After pharyngeal anesthesia or in smokers these receptors are incapable of triggering a swallow, allowing the infused fluid to extend beyond this area and immediately spill over into the airways. Hence, this volume of fluid is similar in volume to the RPS threshold volume in healthy unanesthetized individuals and to the HPSV after pharyngeal topical anesthesia in this group or in chronic smokers without pharyngeal anesthesia. In the smoker group in which no pharyngeal anesthesia was applied, RPS was absent in the majority of the volunteers and water reached the superior margin of the interarytenoid fold in these subjects. The majority of smokers had laryngeal penetration, thereby suggesting that slow accumulation of fluid into the pharynx may predispose smokers to microaspiration. This finding may have further implications in chronic cigarette smokers who also are prone to gastroesophageal reflux disease. For example, cigarette smoking can delay gastric emptying, decrease lower esophageal sphincter pressure, and impair esophageal acid clearance,25–33 all of which may contribute toward predisposition to esophagopharyngeal reflux. Microaspiration is considered to be one of the mechanisms by which gastroesophageal reflux disease can cause chronic pulmonary and laryngeal disorders.34–37 Hence, besides the direct effect of smoking, it is possible that microaspiration secondary to defective aerodigestive reflexes also could contribute to chronic laryngeal and pulmonary disorders in smokers.
Contrary to previous studies,10,11 this study did not show any significant difference in the frequency elicitation and threshold volumes of fluid required to trigger PGCR and PUCR in chronic smokers compared with nonsmokers. This could be secondary to a slower pharyngeal perfusion rate of 1 mL/min used in this study compared with 5.5 mL/min used in previous studies.10,11 A previous study also had shown differences in perfusion rates affecting the frequency elicitation and threshold volumes required to trigger these aerodigestive reflexes.37
The exact mechanism by which cigarette smoking adversely affects the elicitation of the earlier-described reflexes is not known. Recently, it was shown that this effect is not secondary to systemic nicotine when studies were performed using a nicotine patch.38 It is possible that the local effect of cigarette smoke or nicotine on the pharyngeal mucosal sensory nerves could have altered the pharyngeal sensory mechanism, leading to absence of RPS in chronic cigarette smokers. Smoking has been shown to increase keratinization of the mucosa, which is considered to be one of the mechanisms explaining the lower incidence of recurrent aphthous stomatitis in smokers.39 Increased keratinization of the mucosa could result in decreased pharyngeal sensory perception. Studies also have shown nicotine induced oxidative stress to the mucosa secondary to release of free radicals40 and by inhibiting sodium transport.41 It is not known whether the local effect of nicotine on the pharyngeal mucosa also could alter sensory afferent nerve endings.
In summary, this study has shown the direct role of pharyngeal aerodigestive reflexes in protecting the airways. Defective pharyngeal sensory mechanisms resulting in absent RPS seen in the majority of chronic cigarette smokers predispose them to risks of aspiration. Besides the direct effect of cigarette smoking on the airways, this could be an additional mechanism that can account for laryngeal and pulmonary complications in chronic cigarette smokers.
Acknowledgments
Funding
This work was supported in part by National Institutes of Health grants 1P01DK068051-01A1, 5RO1DK025731, and 1UL1RR031973 from the Clinical and Translational Science Award Program of the National Center for Research Resources, National Institutes of Health.
Abbreviations used in this paper
- HPSV
hypopharyngeal safe volume
- PGCR
pharyngoglottal closure reflex
- PUCR
pharyngo–upper esophageal sphincter contractile reflex
- RPS
reflexive pharyngeal swallow
- UES
upper esophageal sphincter
Footnotes
Part of this work was presented at the Neurogastroenterology and Motility meeting, 2008, and at Digestive Diseases Week, 2009.
Part of this work was previously published in abstract form at Digestive Disease Week 2010 Gastroenterology 2010:138:S13.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Enzmann DR, Harell GS, Zboralske FF. Upper esophageal responses to intraluminal distention in man. Gastroenterology. 1977;72:1292–1298. [PubMed] [Google Scholar]
- 2.Freiman JM, El-Sharkawy TY, Diamant NE. Effect of bilateral vagosympathetic nerve blockade on response of the dog upper esophageal sphincter (UES) to intraesophageal distention and acid. Gastroenterology. 1981;81:78–84. [PubMed] [Google Scholar]
- 3.Shaker R, Dodds WJ, Ren J, et al. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology. 1992;102:857–861. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 4.Shaker R, Ren J, Kern M, et al. Mechanisms of airway protection and upper esophageal sphincter opening during belching. Am J Physiol. 1992;262:G621–G628. doi: 10.1152/ajpgi.1992.262.4.G621. [DOI] [PubMed] [Google Scholar]
- 5.Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiology. 1993;79:588–601. doi: 10.1097/00000542-199309000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Medda BK, Lang IM, Layman R, et al. Characterization and quantification of a pharyngo-UES contractile reflex in cats. Am J Physiol. 1994;267:G972–G983. doi: 10.1152/ajpgi.1994.267.6.G972. [DOI] [PubMed] [Google Scholar]
- 7.Shaker R, Ren J, Medda B, et al. Identification and characterization of the esophagoglottal closure reflex in a feline model. Am J Physiol. 1994;266:G147–G153. doi: 10.1152/ajpgi.1994.266.1.G147. [DOI] [PubMed] [Google Scholar]
- 8.Shaker R, Ren J, Zamir Z, et al. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology. 1994;107:396–402. doi: 10.1016/0016-5085(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 9.Shaker R, Ren J, Xie P, et al. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol. 1997;273:G854–G858. doi: 10.1152/ajpgi.1997.273.4.G854. [DOI] [PubMed] [Google Scholar]
- 10.Dua K, Bardan E, Ren J, et al. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut. 1998;43:537–541. doi: 10.1136/gut.43.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dua K, Bardan E, Ren J, et al. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut. 2002;51:771–775. doi: 10.1136/gut.51.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang IM, Dana N, Medda BK, et al. Mechanisms of airway protection during retching, vomiting, and swallowing. Am J Physiol Gastrointest Liver Physiol. 2002;283:G529–G536. doi: 10.1152/ajpgi.00062.2002. [DOI] [PubMed] [Google Scholar]
- 13.Medda BK, Kern M, Ren J, et al. Relative contribution of various airway protective mechanisms to prevention of aspiration during swallowing. Am J Physiol Gastrointest Liver Physiol. 2003;284:G933–G939. doi: 10.1152/ajpgi.00395.2002. [DOI] [PubMed] [Google Scholar]
- 14.Shaker R, Ren J, Bardan E, et al. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology. 2003;49:12–20. doi: 10.1159/000066504. [DOI] [PubMed] [Google Scholar]
- 15.Bajaj JS, Bajaj S, Dua KS, et al. Influence of sleep stages on esophago-upper esophageal sphincter contractile reflex and secondary esophageal peristalsis. Gastroenterology. 2006;130:17–25. doi: 10.1053/j.gastro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lang IM, Haworth ST, Medda BK, et al. Airway responses to esophageal acidification. Am J Physiol Regul Integr Comp Physiol. 2008;294:R211–R219. doi: 10.1152/ajpregu.00394.2007. [DOI] [PubMed] [Google Scholar]
- 17.Kahrilas PJ, Lin S, Rademaker AW, et al. Impaired deglutitive airway protection: a videofluoroscopic analysis of severity and mechanism. Gastroenterology. 1997;113:1457–1464. doi: 10.1053/gast.1997.v113.pm9352847. [DOI] [PubMed] [Google Scholar]
- 18.Clavé P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24:1385–1394. doi: 10.1111/j.1365-2036.2006.03118.x. [DOI] [PubMed] [Google Scholar]
- 19.Rofes L, Arreola V, Romea M, et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil. 2010;22:851–858. doi: 10.1111/j.1365-2982.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- 20.Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc. 1994;40:346–348. doi: 10.1016/s0016-5107(94)70068-0. [DOI] [PubMed] [Google Scholar]
- 21.Dua KHM, Surapaneni S, Tatro L, et al. Prevalence of abnormal upper GI findings in apparently healthy volunteers enrolled for research studies. Gastrointest Endosc. 2009;69:AB350–AB351. [Google Scholar]
- 22.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1246–G1263. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 23.Aviv JE, Spitzer J, Cohen M, et al. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Surapaneni SN, Dua KS, Kuribayashi S, et al. Aerodigestive protective reflexes are triggered before the safe capacity of pharynx is exceeded (abstr). Gastroenterology. 2010;138:S13. [Google Scholar]
- 25.Chattopadhyay DK, Greaney MG, Irvin TT. Effect of cigarette smoking on the lower oesophageal sphincter. Gut. 1977;18:833–835. doi: 10.1136/gut.18.10.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennish GW, Castell DO. Inhibitory effect of smoking on the lower esophageal sphincter. N Engl J Med. 1971;284:1136–1137. doi: 10.1056/NEJM197105202842007. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RD, Horowitz M, Maddox AF, et al. Cigarette smoking and rate of gastric emptying: effect on alcohol absorption. BMJ. 1991;302:20–23. doi: 10.1136/bmj.302.6767.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahrilas PJ, Gupta RR. The effect of cigarette smoking on salivation and esophageal acid clearance. J Lab Clin Med. 1989;114:431–438. [PubMed] [Google Scholar]
- 29.Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4–10. doi: 10.1136/gut.31.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahal PS, Wright RA. Transdermal nicotine and gastroesophageal reflux. Am J Gastroenterol. 1995;90:919–921. [PubMed] [Google Scholar]
- 31.Rattan S, Goyal RK. Effect of nicotine on the lower esophageal sphincter. Studies on the mechanism of action. Gastroenterology. 1975;69:154–159. [PubMed] [Google Scholar]
- 32.Scott AM, Kellow JE, Shuter B, et al. Effects of cigarette smoking on solid and liquid intragastric distribution and gastric emptying. Gastroenterology. 1993;104:410–416. doi: 10.1016/0016-5085(93)90408-5. [DOI] [PubMed] [Google Scholar]
- 33.Stanciu C, Bennett JR. Smoking and gastro-oesophageal reflux. Br Med J. 1972;3:793–795. doi: 10.1136/bmj.3.5830.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt JF, Fang K, Malik R, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 35.Jack CI, Calverley PM, Donnelly RJ, et al. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax. 1995;50:201–204. doi: 10.1136/thx.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruth M, Carlsson S, Mansson I, et al. Scintigraphic detection of gastro-pulmonary aspiration in patients with respiratory disorders. Clin Physiol. 1993;13:19–33. doi: 10.1111/j.1475-097x.1993.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 37.Bajaj JSDK, Bajaj S, Rittmann T, et al. Effect of infusion rate on elicitation of pharyngeal reflexes and development of aspiration among smokers. Neurogastroenterol Motil. 2004;16:672. [Google Scholar]
- 38.Dua KS, Surapaneni SN, Santharam R, et al. Effect of systemic alcohol and nicotine on airway protective reflexes. Am J Gastroenterol. 2009;104:2431–2438. doi: 10.1038/ajg.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers RS. Recurrent aphthous stomatitis: clinical characteristics and associated systemic disorders. Semin Cutan Med Surg. 1997;16:278–283. doi: 10.1016/s1085-5629(97)80017-x. [DOI] [PubMed] [Google Scholar]
- 40.Wetscher GJ, Bagchi D, Perdikis G, et al. In vitro free radical production in rat esophageal mucosa induced by nicotine. Dig Dis Sci. 1995;40:853–858. doi: 10.1007/BF02064991. [DOI] [PubMed] [Google Scholar]
- 41.Orlando RC, Bryson JC, Powell DW. Effect of cigarette smoke on esophageal epithelium of the rabbit. Gastroenterology. 1986;91:1536–1542. doi: 10.1016/0016-5085(86)90212-x. [DOI] [PubMed] [Google Scholar]