Abstract
KRAS mutation is a hallmark of pancreatic ductal adenocarcinoma (PDA), but remains an intractable pharmacological target. Consequently, defining RAS effector pathway(s) required for PDA initiation and maintenance is critical to improve treatment of this disease. Here we demonstrate that expression of BRAFV600E, but not PIK3CAH1047R, in the mouse pancreas leads to pancreatic intraepithelial neoplasia (PanIN) lesions. Moreover, concomitant expression of BRAFV600E and TP53R270H result in lethal PDA. We tested pharmacologic inhibitors of Ras effectors against multiple human PDA cell lines. MEK inhibition was highly effective both in vivo and in vitro, and was synergistic with AKT inhibition in most cell lines tested. We demonstrate that RAF→MEK→ERK signaling is central to the initiation and maintenance of PDA and to rational combination strategies in this disease. These results emphasize the value of leveraging multiple complementary experimental systems to prioritize pathways for effective intervention strategies in PDA.
Keywords: Pancreatic Ductal Adenocarcinoma, Systems Biology, Mouse Models of Cancer
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) poses a major challenge in oncology due to our inability to diagnose the disease early in its progression, its aggressive clinical behavior and the lack of effective systemic chemotherapy (1). The vast majority of PDAs harbor a mutationally activated form of KRAS (2). Moreover, KRAS mutation is an early event in PDA, as evidenced by its high prevalence in pancreatic intra-epithelial neoplasia (PanIN) lesions; thought to be a benign precursor to malignant PDA (3). Furthermore, widespread expression of KRASG12D throughout the developing mouse pancreas leads to multifocal PanIN formation and to PDA with low frequency in adult mice (4). Progression of KRASG12D-induced PanIN lesions to PDA is dramatically accelerated by alterations in tumor suppressor genes such Cdkn2a, Smad4, or Trp53 (5).
Mutationally activated KRAS binds to a multiplicity of effector proteins including: RAF kinases, PI3’-lipid kinases (PI3K), guanine nucleotide exchange factors for RAL and RHO GTPases respectively, among others (6). Since mutationally activated RAS remains an intractable pharmacological target, defining relevant RAS effector pathway(s) in PDA is of tremendous clinical importance. Since potent and specific inhibitors of key components of RAS effector pathways are being clinically deployed in a number of malignancies, it has become crucial to understand how best to implement these drugs in the clinical arena for maximal efficacy while minimizing toxicity. Unlike the scenario in melanoma or colorectal cancer, mutational activation of RAS effectors (e.g. BRAF or PIK3CA) is extremely rare in PDA and therefore uninformative as to the key downstream mediators of RAS signaling (7). This might suggest that numerous RAS effector pathways may be essential for PDA and that effective targeting of cancers maintained by mutationally activated KRAS could require concomitant inhibition of two or more RAS effector pathways (8).
We examined the requirements for the RAF or PI3K effector arms of KRAS signaling in the initiation, progression and maintenance of PDA using genetically engineered mouse cancer models and cancer cell lines derived from human or mouse PDA. Whereas pancreas-specific expression of BRAFV600E led to the rapid formation of multi-focal PanIN lesions, similarly initiated expression of PIK3CAH1047R was without obvious effect. Furthermore, combined expression of BRAFV600E and gain of function TP53R270H uniformly led to lethal PDA in the mouse. We found that oral delivery of MEK inhibitor was effective in inhibiting ERK phosphorylation in vivo in an established, autochthonous model of PDA reported to exclude drugs, and prolonged survival in a novel syngenic model of PDA. Pharmacological inhibition of MEK potently suppressed proliferation in a subset of PDA-derived cell lines in vitro but induced activation of AKT in both KRAS wt and mutant PDA human cell lines. Finally, combined MEK and AKT inhibition demonstrated synergistic interactions between these two agents in most human PDA cells. Overall, our findings demonstrate the potential utility of concerted clinical efforts to completely inhibit the Ras→Raf→MEK→ERK pathway at or below MEK in a subset of patients with PDA, and to develop tolerable combination regimens of MEK and AKT inhibitors in this disease.
RESULTS
Expression of BRAFV600E, but not PIK3CAH1047R, is sufficient for PanIn formation
To test the consequences of activating the RAF→MEK→ERK pathway specifically in the pancreas, we crossed p48Cre mice with BRafCA/CA mice. As described previously, BRafCA encodes normal BRAF but following Cre-mediated recombination is rearranged to encode BRAFV600E (9). p48Cre expresses cre recombinase in place of the Pitf gene. No compound p48Cre; BRafCA/+ progeny were detected at the time of weaning, leading us to conclude that widespread expression of BRAFV600E in the developing mouse pancreas is incompatible with development to adulthood. This lethality contrasts with the viability of p48Cre; KRasLSL-G12D mice (10). To circumvent this lethality, we generated compound Pdx1::CreERT2; BRafCA/+ mice (BC mice hereafter) where expression of BRAFV600E is induced in the adult pancreas under the control of a conditionally active cre recombinase driven by the Pdx1 promoter (11). BC mice were born at normal Mendelian ratios and were healthy and fertile. In parallel, and as a comparator, we generated a cohort of Pdx1::CreERT2; KRasLSL-G12D mice (KC mice). Cohorts of BC and KC mice were treated with tamoxifen at P14 to initiate cre activity and thereby BRAFV600E or KRASG12D expression in the pancreas. Mice were euthanized for analysis around P100 and all mice were healthy at the time of euthanasia.
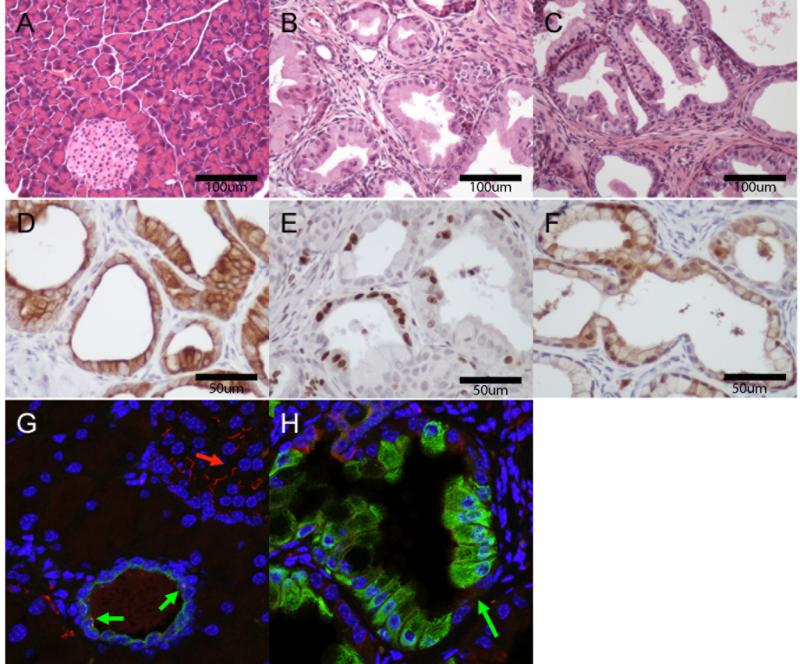
Pancreatic expression of BRAFV600E led to near total replacement of the exocrine pancreas with PanIN lesions (Figures 1A & 1B). These lesions were morphologically indistinguishable from those arising in KC mice and of similar grade although were greater in number (Figure 1C, and not shown). PanINs from BC mice expressed the ductal marker cytokeratin (CK) 19 (Figure 1D), Ki67 (a marker of proliferation) (Figure 1e) and had abundant phosphorylated nuclear ERK1/2 (Figure 1F) indicating activation of the RAF→MEK→ERK pathway. Additionally, whereas primary cilia were observed in both pancreatic islets and normal ducts, PanIN cells from BC mice lacked primary cilia (Figure 1G & 1H), consistent with previous findings in KRASG12D-induced induced PanIN lesions (12). Six BC mice aged to one year age showed no evidence of PDA upon euthanasia (Supplemental Figure 1).
Figure 1.
BrafV600E is Sufficient to Induce PanIN Lesions in the Mouse. H&E staining of tamoxifen induced A) Pdx1::CreERT2 (C) mice, B) BrafCA/+, Pdx1::CreERT2 (BC) mice C) KrasLSL-G12D/+, Pdx1::CreERT2 (KC) mice. PanIns in BC mice express ductal markers: D), CK19, are proliferative: E), Ki67, and show activation of the MAPK pathway F), phospho-ERK). BrafCA/+-induced PanIns lack primary cilia. G) Pdx1::CreERT2 (C) mice (red:acetylated tubulin, blue:DNA, green:CK19): normal islet (red arrow) and duct (green arrow) with cilia. H) BC mice (red:acetylated tubulin, blue:DNA, green:CK19): PanIn (green arrow) without cilia.
To test the ability of activated PI3’-kinase-α to initiate PanIN formation we generated Pdx1::CreERT2; Pik3calat-H1047R (PC) mice. The Pik3calat-H1047R allele encodes normal PI3’-kinase-αprior to cre mediated recombination after which mutationally activated . (PIK3CAH1047R) is expressed from the endogenous Pik3ca locus (13). We used a specific PCR to show that recombination (and thus activation) of the Pik3caH1047R allele in the pancreas occurred (not shown), but found neither detectable PanIN lesions nor any other pancreatic abnormalities in mice PC up to six months after cre induction with tamoxifen. These data indicate that mutationally activated BRAFV600E, but not PIK3CAH1047R, can initiate PanIN formation with an efficiency that at least equals that of KRASG12D.
BRAFV600E cooperates with gain of function TP53R270H for PDA formation
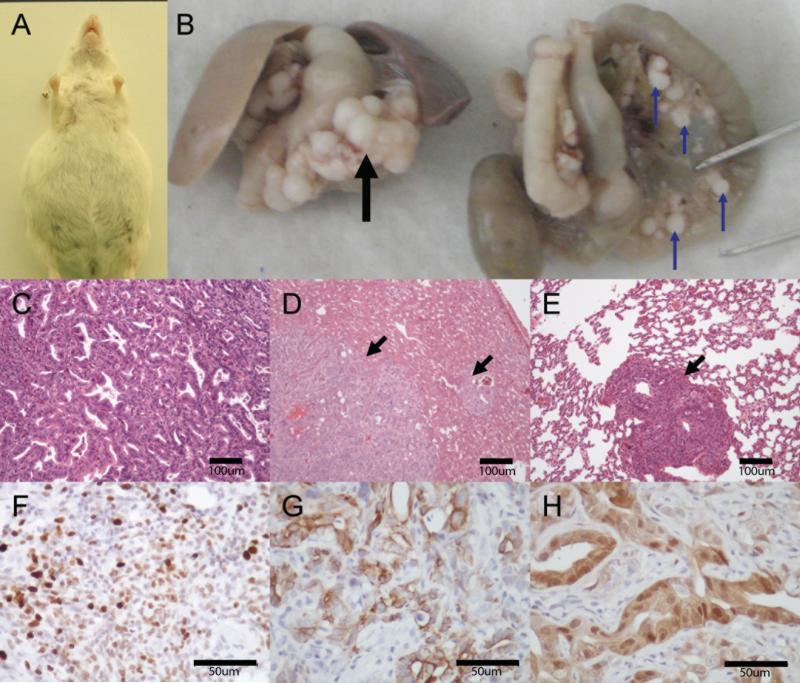
Mutationally activated KRasG12D cooperates with gain of function Tp53R270H to promote development of PDA with high penetrance and striking histological and clinical similarity to the human disease (5). Hence, to test if oncogenic BRAFV600E might display similar cooperation, we generated a cohort of Pdx1::CreERT2; BRafCA/+; Trp53lox-R270H/+ mice (BPC mice hereafter). All such mice required euthanasia at 4.5 to 9 months due to abdominal distention, wasting and substantial loss of body weight (Supplemental Figure 1). At necropsy all BPC mice displayed clear evidence of PDA. Mice typically presented with ascites, and extra-pancreatic spread of metastatic disease (Figures 2A & 2B), most often to the liver (Figure 2D), peritoneal cavity and lung (Figure 2E). Analysis of tumor-derived genomic DNA confirmed recombination of the BRafCA allele and excluded spurious acquisition of activating mutations in either exon one of Kras or exon 20 of Pik3ca (data not shown). Histologic examination of these cancers showed them to be moderately differentiated PDA (Figure 2C) displaying robust proliferation (Ki67, Figure 2F), heterogeneous CK19 expression (Figure 2G) and abundant phosphorylated ERK (Figure 2H). Interestingly, both BRAFV600E-induced PanINs and PDAs displayed abundant stroma and a desmoplasia similar to that seen with in the human disease. We concluded that oncogenic BRAFV600E substitutes for most, if not all, of the oncogenic functions of KRASG12D in the genesis, progression and maintenance of PDA in the mouse.
Figure 2.
BrafV600E and Trp53R270H cooperate to form lethal PDA resembling the human disease. (A) a six month old Pdx1::CreERT2; BRafCA/+; Trp53lox-R270H/+ (BCP) mouse with ascites. (B) Gross images of primary pancreatic tumor (black arrow) and omental metastases (blue arrows). (C) H&E of primary PDA arising in the pancreas of a BCP mouse. H&E staining of (D) liver metastases (black arrows) or (E) lung metastases (black arrow) from same. PDA arising in BCP mice are proliferative (F, Ki67), heterogeneously express ductal markers (G, CK19), and display high levels of MAPK activation (H, phosphoERK).
Bioavailability of MEK1/2 Inhibitor PD325901 in PDA
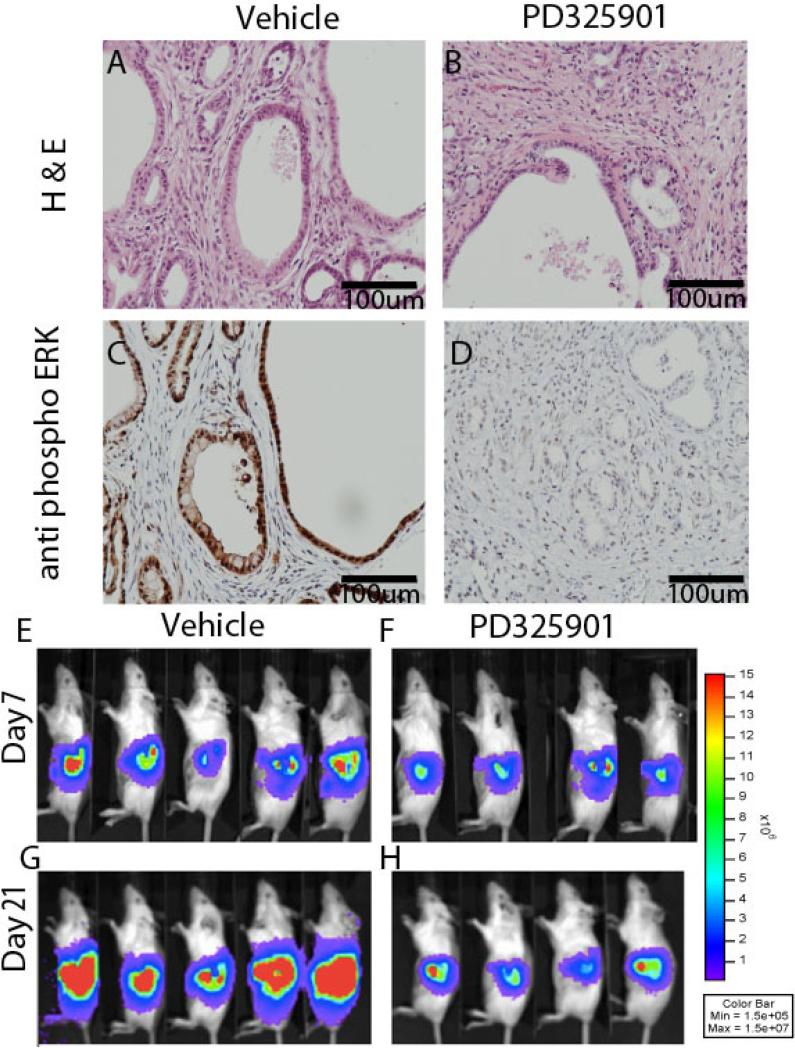
The above results suggested to us that while mutant KRAS can serve many oncogenic functions in cancer, activation of the RAF pathway alone satisfied a genetic sufficiency argument for the PanIN and PDA initiation. To translate this finding into a more clinically testable hypothesis, we next considered the clinical use of MEK inhibitors, and potential barriers to successful trials with these agents in PDA. It has been recently reported that chemotherapeutic agents are excluded from PDA tissue due to poor drug perfusion into the primary tumor, which is in turn attributable to poor tumor vascularization (14), (15). To interrogate if MEK1/2 inhibitor PD325901 was bioavailable to PDA tissue, we utilized an autochthonous KRasLSL-G12D, Trp53lox-R270H/+, p48Cre (KPC) mouse model similar to that previously described to exclude various drugs from PDA and high pressures (14), (15). In this system, two daily treatments with MEK1/2 inhibitor PD325901 led to profound reductions in phosphorylated ERK as detected by immunohistochemistry (Figure 3A-D), suggesting that this agent is bioavailable to PDA cells in vivo at clinically achievable doses. We concluded that sufficient levels of MEK1/2 inhibition may be pharmacologically feasible in PDA, despite the drug delivery challenges posed by hypovascularity and desmoplasia in this disease.
Figure 3.
MEK Inhibition in vivo. H & E (A and B) or phosphoERK (C and D) staining of KPC mice treated with either vehicle (A and C) or MEK1/2 inhibitor PD3258901 (B and D). Day seven (E, F) post-implantation, pre-treatment bioluminescent images of FVBn mice after orthotopic injection of syngenic KrasLSL-G12D/+, Cdnk2aF/+ cells and subsequent treatement with vehicle (G) or MEK1/2 inhibitor PD0325891 (H) for two weeks.
For drug efficacy studies, we next developed two new in vivo, mouse, syngeneic orthotopic models of PDA denoted INK4.1syn_Luc and p53 2.1.1syn_Luc employing previously described mouse PDA-derived cell lines engineered to express luciferase (16). We found that implantation of either line in the pancreas of immune competent FVB/n mice reproducibly lead to PDA with characteristics of the clinical disease including recruitment of activated stroma (Supplemental Figure 2), ascites, cachexia, and bowel obstruction requiring euthanasia at five to six weeks post implantation. The predictable kinetics and quantifiable tumor implantation allowed for relatively economical drug efficacy studies, as compared to the autochthonous model (17). Following orthotopic engraftment, tumor-bearing mice were divided into equal tumor bearing groups (as quantified by bioluminescence), and treated with either vehicle or MEK1/2 inhibitor PD325901 by gavage for 14 days, and monitored clinically daily for disease progression. PD325901 lead to pERK reductions six hours after a single oral gavage (Supplementary Figure 3), indicating that the drug gains access to tumor cells in this model as in the autochthonous model. Treated mice were healthy while receiving drug whereas control treated mice began to decline clinically. The experiment was terminated when the final vehicle treated mouse required euthanasia, as dictated by objective, approved protocols at our center. By this analysis, MEK inhibition resulted in a statistically significant survival advantage in mice bearing either INK4.1syn_Luc (log rank, p=0.043) or p53 2.1.1syn_Luc (log rank, p<0.01) syngeneic, orthotopic xenografts. Despite this survival advantage, we noted that MEK inhibition was mostly cytostatic, as noted in vitro (18), and upon cessation of PD325901, all treated mice progressed clinically (Figure 3 E-H).
RAS pathway dependencies of human PDA cell lines
We next sought to complement our analysis of genetically engineered mouse (GEM) models of PDA by expanding a previously reported panel of human PDA cell lines with the goal of better representing the heterogeneity of PDA (16). To pharmacologically assess dependence of human PDA cells on specific signaling nodes downstream of Ras, we exposed all cell lines to either MEK1/2 inhibitors (GSK1120212 or PD325901), RAF inhibitor (GDC0879) or AKT1/2 inhibitor (GSK690693). In addition, cells were treated with pair-wise combinations of these agents to probe for synergistic growth inhibition by targeting both RAF→MEK and PI3’K→AKT signaling simultaneously in the same cells.
RAF inhibitor GDC0879 lead to only minor inhibitory effects on PDA cell proliferation when used alone. Furthermore, RAF inhibition clearly antagonized the anti-proliferative effects of MEK1/2 inhibition when RAF inhibitor GDC0879 was co-administered with MEK1/2 inhibitor PD325901, (Supplemental Figure 4A). Western blotting for phosphorylated ERK confirmed that Raf inhibitor GDC0879 indeed augmented RAF→MEK→ERK signaling in KRAS mutant Suit2 cells, essentially antagonizing MEK1/2 inhibition (Supplemental Figure 4B). Consequently, neither GDC0879 nor its combinations were pursued further.
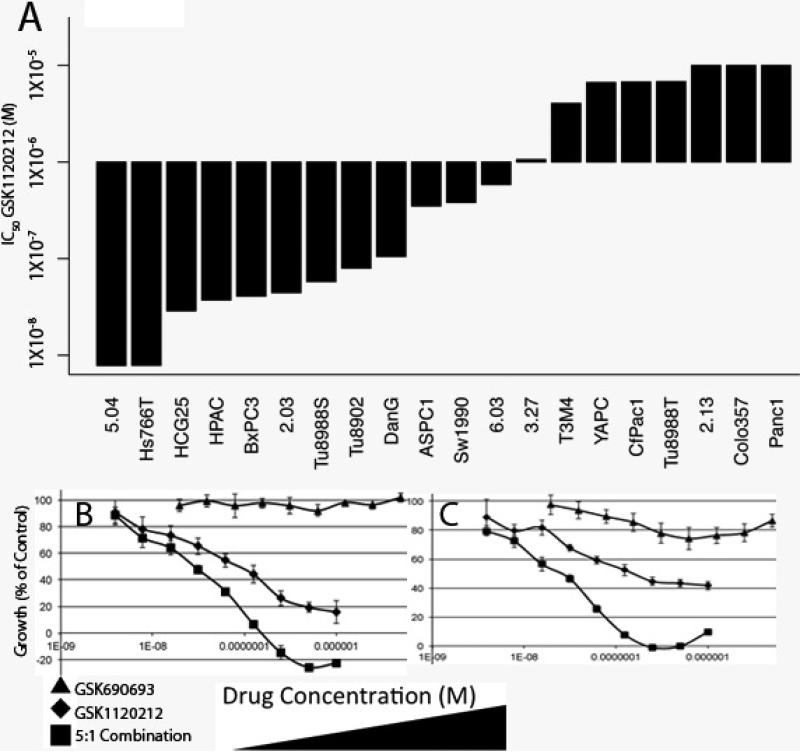
To examine the effects of MEK and AKT inhibition while maximizing translational relevance, we used a MEK inhibitor currently in clinical trials with other agents; GSK1120212 (e.g. NCT00955773, NCT01138085). IC50 values for MEK inhibitor GSK1120212 were dynamic across the cell line panel (Figure 4A). We found that MEK inhibition led to induction of phosphorylated AKT, (a marker of activation of PI3’-kinase→AKT signaling), in several PDA lines (Supplementary Figure 5). Consistent with this effect, we found that while AKT inhibitor monotherapy with GSK690693 had minimal effects on its own, combination treatment with GSK1120212 and GSK690693 lead to statistically significant synergy in most PDA lines tested (Supplemental Figure 6 and Supplemental Table 1) (19). Treatment with GSK1120212 led to a decrease in phosphorylation of ERK, rpS6, and 4EBP1 (Thr37/46). GSK690693 treatment showed the expected increase in AKT phosphorylation and suppressed phosphorylation of the direct downstream target of AKT; PRAS40 (Thr246). The combination of the two inhibitors had a more profound inhibitory effect on both rpS6 and 4EBP1 phosphorylation in most of the PDA cell lines compared to single agent treatments (Supplemental Figure 7). We concluded that while some PDA lines are sensitive to MEK1/2 inhibition alone, the addition of AKT inhibition consistently potentiated responses, as evaluated by formal drug synergy analysis.
Figure 4.
Combined Inhibition of MEK and AKT leads to synergistic effects across a large panel of PDA cell lines. (A) IC50 measurements of human PDA cell lines treated with MEK inhibitor GSK1120212. Cell lines are on the X axis and IC50 (M) is on the Y axis. Representative dose response curves of (B) 3.27 or (C) Sw1990 treated with either GSK690693 (triangles), GSK1120212 (diamonds), or a 5:1M fixed dose combination ratio of GSK1120212:GSK690693 (squares) plotted as the dose of GSK1120212 in the combination. X axis is drug concentration in M. Y axis is percent growth inhibition at 72 hours. Error bars are +/- standard deviation.
DISCUSSION
The strikingly poor prognosis of patients with PDA is largely attributable to late diagnosis and general resistance to conventional cytotoxic or targeted therapeutics. Although mutational activation of KRAS is a signature genetic event of PDA, approaches to directly inhibit constitutively active, GTP-bound RAS proteins are lacking. Consequently, considerable attention has shifted to pharmacologically tractable targets acting downstream of RAS-GTP on its various effector pathways. Chief among these are the RAF→MEK→ERK and the PI3’K→AKT pathways for two reasons. First, the RAF and PI3’K kinases are themselves frequently mutationally activated in human cancer whereas other putative RAS effectors are not. Second, components of these pathways are targeted with available inhibitors in clinical development. In this study we sought to explore the relative importance of these RAS-effector pathways in PDA initiation and maintenance to better prioritize treatment approaches with such pathway inhibitors, and to prospectively define combinations of inhibitors likely to be of benefit, specifically in this lethal disease. A key conclusion of this research is that induced expression of BRafV600E, but not Pik3CAH1047R, signaling can recapitulate the PDA phenotype endowed by mutant KRasG12D in mice. Moreover, pharmacological inhibition of MEK has anti-tumor effects against a subset of PDAs, and broadly synergizes with AKT inhibition in this disease.
Whereas KRAS mutation is nearly universal in PDA, mutational activation of either BRAF or PIK3CA are uncommon. It is perhaps surprising then that BRAFV600E is able to phenocopy the effects of KRASG12D with such efficiency. These data suggest that little more is required of KRASG12D than activation of the RAF MEK ERK axis for PDA initiation, at least in the mouse. In this capacity, activated BRAF (like activated KRAS), appears capable of activating additional pathways (e.g. Myc, NFKappaB, etc.) and processes (inflammation, stromal recruitment, etc.) necessary for tumorigenesis Moreover, the absence of an overt pancreatic phenotype in Pdx1::CreER; Pik3calat-H1047 mice further emphasizes the relative specificity of the RAF MEK ERK pathway in PDA initiation. These results are consistent with GEM models of KRASG12D induced lung tumorigenesis wherein RAF→MEK→ERK signaling is both necessary (20) and sufficient (9) for tumor initiation in lung and agree with the requirement for RAF in RAS-induced skin cancer (21).
There are numerous inhibitors of RAF→MEK→ERK and PI3’-kinase→PDK→AKT signaling currently in drug development. Our findings support the contraindication of RAF inhibitors the treatment of cancers driven by mutationally activated RAS proteins due to their lack of efficacy and possible growth stimulatory characteristics (22). We find that MEK1/2 inhibition has potent anti-tumor activity against human or mouse PDA cell lines and against orthotopically implanted tumors. We observed mostly cytostatic responses to MEK inhibition in vivo and observed induction of AKT signaling in response to MEK inhibition in PDA cells. This suggested a functional feedback loop as observed by others in breast or colorectal cancer lines harboring RAS mutations (23), (24). Indeed, we found that combining inhibition of MEK with inhibition of AKT lead to synergistic effects in the majority of human PDA cell lines tested, similar to findings in lung cancer (25). We interpret our findings with those of others to suggest that PDA cells, while relatively resistant to AKT inhibition as a monotherapy, appear to recruit this important survival pathway in response to MEK inhibition, possibly explaining the synergistic interactions seen with these two classes of agents.
Taken in total these findings emphasize the central role played by RAF→MEK→ERK signaling in both the genesis and maintenance of PDA. These results are important because while KRAS remains an undruggable molecule, there are several potent kinase inhibitors being developed against the downstream effectors of RAS but if or how these inhibitors should be combined remains to be established. We demonstrate that agents currently in clinical trials show potent synergy in PDA treatment. These findings strongly support the further development of combined MEK and AKT inhibition in PDA, and suggest a clear direction for the implementation of pathway-targeted approaches in this disease with tremendous unmet medical need.
Supplementary Material
STATEMENT OF SIGNIFIGANCE.
Pancreatic Ductal Adenocarcinoma is difficult to treat in large part due to recurrent mutations in the KRAS gene. Here we define rational treatment approaches to the disease achievable today with existing drug combinations by thorough genetic and pharmacologic dissection of the major KRAS effector pathways; RAF→MEK→ERK and PI3’K→AKT.
Acknowledgments
We thank Katie Bell, for help with staining, Grace Kim for pathologic review, Donghui Wang, Don Hom, Paul Phojanakong, Nesrine Affara, and Jeff Chang for assistance with animal modeling.
Financial Support: E.A.C is supported by NIH/NCI K08 CA137153. Funds were provided by the Noren Fund for Pancreatic Cancer Research (to M.M.). W.A.P. is supported by project grants from the National Health and Medical Research Council of Australia. This research was supported under J.W.G. by NIH, National Cancer Institute (NCI) Grants P50 CA 58207, U54 CA 112970, and NHGRI U24 CA 126551; by the Department of the Army, Award W81XWH-07-1-0663.
Abbreviations
- PDA
pancreatic ductal adenocarcinoma
- PanIN
pancreatic intraepithelial neoplasia
References Cited
- 1.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes & development. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 2.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 3.Yanagisawa A, Ohtake K, Ohashi K, Hori M, Kitagawa T, Sugano H, et al. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993;53:953–6. [PubMed] [Google Scholar]
- 4.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 5.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Young A, Lyons J, Miller AL, Phan VT, Alarcon IR, McCormick F. Ras signaling and therapies. Adv Cancer Res. 2009;102:1–17. doi: 10.1016/S0065-230X(09)02001-6. [DOI] [PubMed] [Google Scholar]
- 7.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science (New York, NY. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes & development. 2007;21:379–84. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiser PW, Cano DA, Landsman L, Kim GE, Kench JG, Klimstra DS, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 12.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–30. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinross KM, Montgomery KG, Kleinschmidt M, Waring P, Ivetac I, Tikoo A, et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. The Journal of clinical investigation. 2012;122:553–7. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–93. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 18.Gysin S, Lee SH, Dean NM, McMahon M. Pharmacologic inhibition of RAF-->MEK-->ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res. 2005;65:4870–80. doi: 10.1158/0008-5472.CAN-04-2848. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 20.Karreth FA, Frese KK, Denicola GM, Baccarini M, Tuveson DA. C-Raf is required for the initiation of lung cancer by K-Ras. Cancer Discovery. 2011;1:128–36. doi: 10.1158/2159-8290.CD-10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrenreiter K, Kern F, Velamoor V, Meissl K, Galabova-Kovacs G, Sibilia M, et al. Raf-1 addiction in Ras-induced skin carcinogenesis. Cancer Cell. 2009;16:149–60. doi: 10.1016/j.ccr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 23.Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, et al. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565–72. doi: 10.1158/0008-5472.CAN-08-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. The Journal of clinical investigation. 2011;121:4311–21. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng J, Dai B, Fang B, Bekele BN, Bornmann WG, Sun D, et al. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS ONE. 2010;5:e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.